INTRODUCTION
Retroperitoneal masses represent a diverse group of lesions originating within the retroperitoneal space, posing a considerable diagnostic challenge for radiologists and clinicians alike.[1] The majority of these masses are malignant, with approximately 75% being of mesenchymal origin.[2-4] While more commonly observed in adults, retroperitoneal masses can manifest across all age groups.[2]
When these masses do not arise from specific retroperitoneal organs such as the kidneys, adrenal glands, pancreas, or bowel loops, they are classified as primary retroperitoneal masses. These primary masses are further categorized as solid or cystic based on their imaging characteristics, as outlined in [Table 1].[5, 6] Solid retroperitoneal masses are broadly grouped by origin into mesenchymal, neural, germ-cell, and lymphoproliferative types.[3] Among cystic lesions, lymphangiomas and cystic mesotheliomas are the most frequently encountered.[3, 7, 8] It’s also important to consider non-neoplastic conditions in the Differential Diagnosis Of Retroperitoneal Masses, including retroperitoneal fibrosis, Erdheim-Chester disease (a non-Langerhans histiocytosis), and extramedullary hematopoiesis.
Table 1. Categories and Subcategories of Predominantly Solid and Predominantly Cystic Retroperitoneal Lesions in Adults.
| Category | Solid | Cystic |
|---|---|---|
| Neoplastic | Lymphoma | Lymphangioma |
| Liposarcoma | Cystadenoma | |
| Malignant Fibrous Histiocytoma | Cystadenocarcinoma | |
| Leiomyosarcoma | Cystic Mesothelioma | |
| Neurogenic Tumors | Mature Teratoma | |
| Germ-Cell Tumors | ||
| Non-Neoplastic | Retroperitoneal Fibrosis | Epidermoid Cyst |
| Extramedullary Hematopoiesis | Non-Pancreatic Pseudocyst | |
| Erdheim-Chester Disease | Bronchogenic Cyst | |
| Lymphoceles, Urinomas, Hematomas |
Clinical presentations of retroperitoneal masses are often nonspecific, varying with lesion location and relationship to adjacent anatomical structures.[9] Computed tomography (CT) and magnetic resonance imaging (MRI) are the cornerstone imaging modalities for evaluating these lesions. Imaging features are pivotal in guiding the differential diagnosis of retroperitoneal masses, as well as tumor staging, surgical planning, and biopsy guidance.[1, 5, 10, 11] While imaging findings can overlap and histopathological analysis remains the gold standard for definitive diagnosis, specific imaging characteristics are highly suggestive of certain lesions and can effectively guide clinical management. Treatment of retroperitoneal masses is often complex due to their anatomical location, size, potential for vascular encasement, and involvement of surrounding organs.[12, 13]
This article provides a detailed review of retroperitoneal anatomy and presents a practical, imaging-based approach to the differential diagnosis of primary retroperitoneal masses in adults, aiming to enhance clinical decision-making.
RETROPERITONEAL ANATOMY
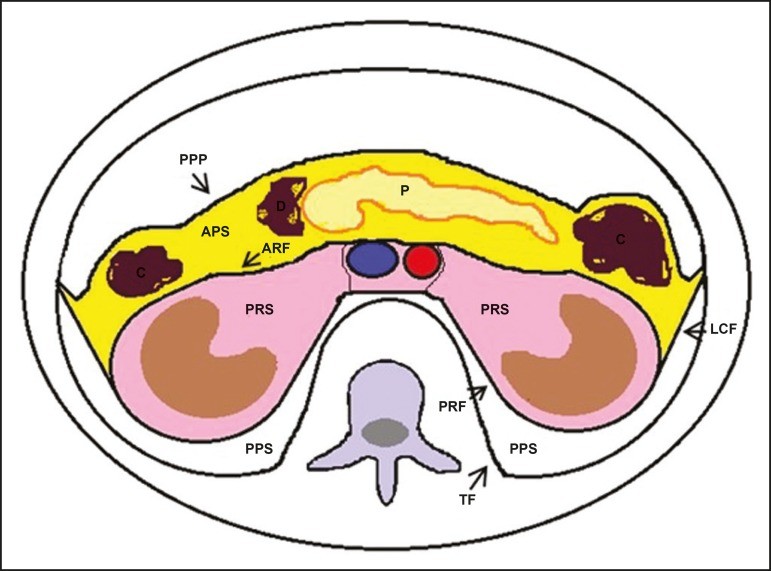
The retroperitoneal space is a complex anatomical region extending from the diaphragm to the pelvis. It is defined posteriorly by the transversalis fascia and anteriorly by the posterior parietal peritoneum, and is further subdivided into distinct compartments,[11, 14, 15] as illustrated in Figure 1. The anterior pararenal space is bordered anteriorly by the posterior parietal peritoneum, posteriorly by the anterior renal fascia, and laterally by the lateroconal fascia. This space houses the pancreas, the second portion of the duodenum, and the ascending and descending colon. The posterior pararenal space, primarily composed of fat, is situated anterior to the transversalis fascia and posterior to the posterior renal fascia. The perirenal space, enclosed between the anterior and posterior renal fasciae, contains the kidneys and adrenal glands. Centrally, the retroperitoneum includes the aorta and inferior vena cava, along with lymphatic chains and nerve plexuses.
Figure 1. Retroperitoneal Anatomy and Compartments.
Diagram illustrating the retroperitoneal anatomy, highlighting the anterior pararenal space (APS), posterior pararenal space (PPS), perirenal space (PRS), central region, and their respective boundaries: posterior parietal peritoneum (PPP), anterior renal fascia (ARF), posterior renal fascia (PRF), lateroconal fascia (LCF), and transversalis fascia (TF). Key organs within each space are labeled: Pancreas (P), Duodenum (D), Colon (C).
RADIOLOGICAL EVALUATION FOR DIFFERENTIAL DIAGNOSIS OF RETROPERITONEAL MASSES
Initial radiological assessment of a retroperitoneal mass requires confirming its retroperitoneal location and identifying the specific compartment involved, such as the anterior pararenal space.[3] Anterior displacement of abdominal structures like the aorta or colon, or retroperitoneal organs such as the kidneys, can provide clues to the lesion’s origin. However, significant anatomical distortion caused by large masses can make precise localization challenging.[1, 3, 5, 16] In such instances, detailing the involvement of retroperitoneal spaces becomes crucial.
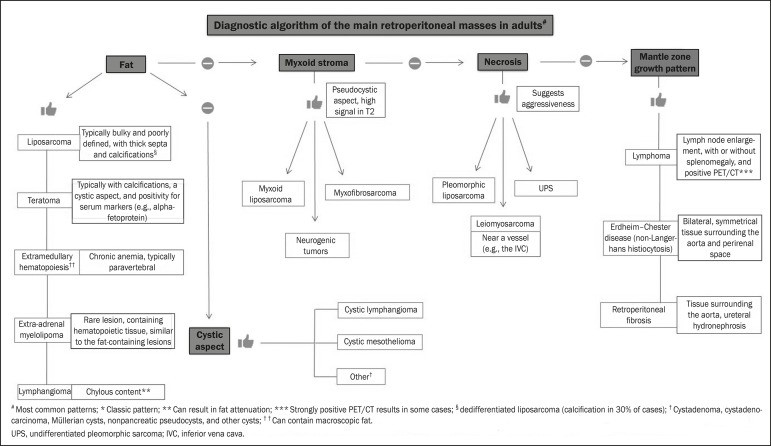
To classify a retroperitoneal mass as primary, it’s essential to exclude origin from major retroperitoneal organs. Subsequent characterization involves categorizing the mass as solid or cystic, evaluating key imaging features (including macroscopic fat, calcifications, myxoid stroma, necrosis, and cystic components), and describing its relationship with adjacent structures. Radiological signs like the crescent sign, embedded organ sign, and phantom organ sign can aid in differentiating primary retroperitoneal masses.[1] A comprehensive evaluation of these findings is crucial for narrowing the differential diagnosis and guiding therapeutic strategies.[1, 3, 5, 14]
Fat-Containing Retroperitoneal Masses
The presence of intralesional fat significantly refines the differential diagnosis, primarily pointing towards lesions with specific biological behaviors like liposarcoma, teratoma, and extramedullary hematopoiesis.[17]
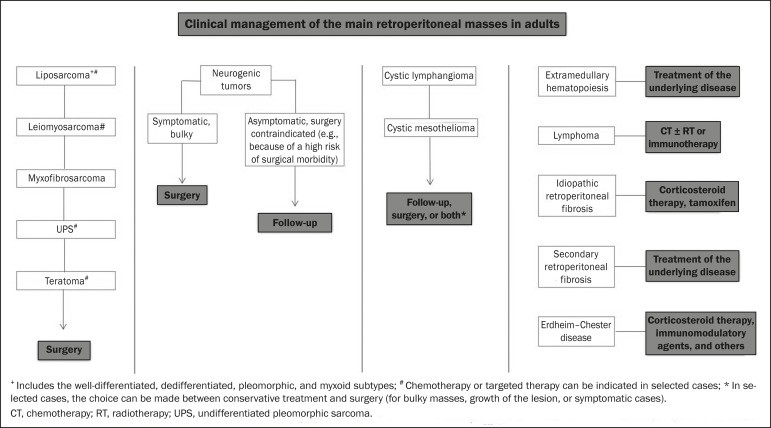
Liposarcoma: Liposarcoma is the most prevalent retroperitoneal sarcoma, accounting for roughly 30% of all retroperitoneal sarcomas. It typically affects individuals in their fifth and sixth decades of life. Liposarcomas are histologically diverse, classified into well-differentiated (with or without dedifferentiated components), myxoid, round cell, and pleomorphic subtypes, each with distinct clinical and radiological features. The perirenal space is a common location for liposarcomas.
Well-differentiated liposarcoma, the most frequent subtype, contains mature adipose tissue and is characterized by infiltration of adjacent structures.[3, 4, 14, 18] Imaging features suggestive of malignancy, differentiating it from benign lesions, include size greater than 10 cm, thick septa (> 0.2 cm), and nodular enhancement.[3, 19, 20] However, definitive differentiation often requires histopathological analysis, aided by molecular markers such as anti-CDK4 and anti-MDM2 antibodies. Surgical resection with wide negative margins is the preferred treatment to minimize local recurrence.[21, 22] Emerging targeted therapies showing promise for well-differentiated and dedifferentiated liposarcomas are being explored.[23, 24]
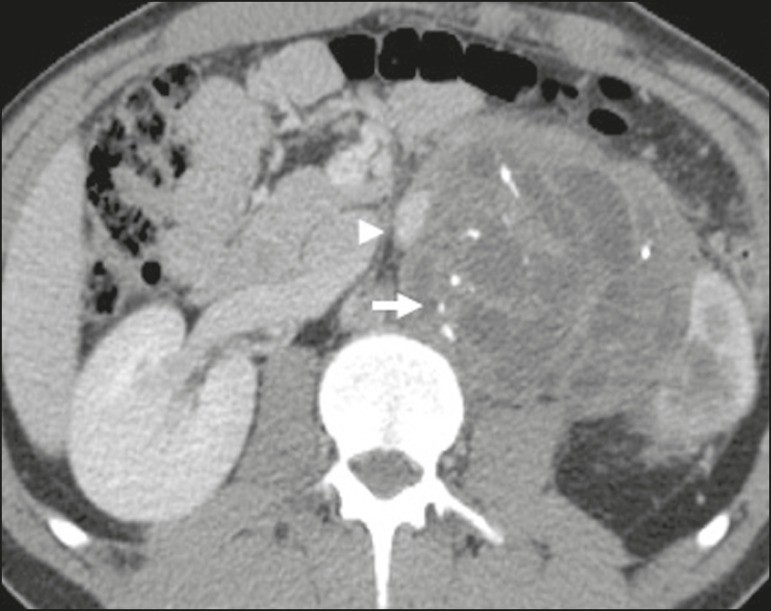
Retroperitoneal Teratoma: Retroperitoneal teratomas are germ cell tumors derived from embryonic layers and can present with elevated serum levels of tumor markers, including alpha-fetoprotein, CEA, CA-19-9, and β-hCG.[6, 25, 26] Imaging characteristics include macroscopic fat, cystic areas, calcifications, and a fat-fluid level, along with heterogeneous contrast enhancement,[26, 27] as shown in Figure 2. Surgical excision is the primary treatment.[6, 25] In male patients, it’s crucial to consider secondary retroperitoneal involvement of gonadal origin and thoroughly investigate the testes.[5]
Figure 2. Mature Retroperitoneal Teratoma.
CT scan of a 23-year-old female patient showing a retroperitoneal mass indicative of mature teratoma, characterized by fat components, cystic regions, and calcifications (arrow). The anterior displacement of the aorta relative to the vertebral body (arrowhead) is an indirect sign of retroperitoneal location. Diagnosis confirmed via percutaneous biopsy.
Extramedullary Hematopoiesis: Extramedullary hematopoiesis is a compensatory mechanism for reduced bone marrow hematopoiesis, resulting in hematopoietic tissue deposits in organs of mesenchymal origin (spleen, liver) and paravertebral tissues.[16] Imaging may reveal multiple, homogeneous, bilateral masses with minimal contrast enhancement, and potentially macroscopic fat.[5] Clinical and laboratory findings of chronic anemia support the diagnosis.
Extra-adrenal Myelolipoma: Extra-adrenal myelolipoma is a rare, typically unilateral, benign mesenchymal lesion containing adipose and hematopoietic tissue. It can occur in the retroperitoneum, often in the presacral region, and commonly affects women aged 50-70. Most patients are asymptomatic, with lesions found incidentally, although some may present with abdominal pain from hemorrhage, infarction, or compression. Differential diagnoses include retroperitoneal teratoma, extramedullary hematopoiesis, and liposarcoma, with the latter being almost indistinguishable on imaging. Biopsy is often necessary for definitive diagnosis, confirmed by the presence of hematopoietic components. Management ranges from imaging surveillance for small, asymptomatic lesions to surgery for symptomatic, large, or growing lesions, or when diagnostic uncertainty persists.[21, 28-30]
Cystic Lymphangioma: Cystic lymphangiomas may demonstrate lipid content on imaging due to chylous ascites.[8]
Key Point: Identifying fat within a retroperitoneal lesion significantly narrows the differential diagnosis to a specific subset of lesions with distinct biological behaviors.
Calcifications in Retroperitoneal Masses
Calcifications can be seen in solid retroperitoneal lesions such as neurogenic tumors, dedifferentiated liposarcomas, teratomas, and undifferentiated pleomorphic sarcomas.[5, 18]
Dedifferentiated Liposarcoma: Calcifications are present in approximately 30% of dedifferentiated liposarcomas. When coupled with heterogeneously enhancing nodules (Figure 3), they are highly suggestive of tumor dedifferentiation and a poorer prognosis.[2, 18, 31, 32] 18F-FDG PET/CT can be valuable in evaluating tumor grade, staging, and postoperative follow-up in liposarcomas, particularly in dedifferentiated and pleomorphic subtypes, by quantifying FDG uptake in solid components.[18, 22, 32] Some studies suggest a correlation between tumor grade and glycolysis rate.[22]
Figure 3. Dedifferentiated Liposarcoma Imaging.
(A) CT scan of a 58-year-old male showing an infiltrative lesion with solid and fat components (arrow), displacement of the right kidney (arrowhead) indicating posterior pararenal space location, and a calcification focus (circle) suggestive of tumor dedifferentiation. (B, C) Contrast-enhanced T1-weighted MRI (fat saturation) and 18F-FDG PET/CT of a 44-year-old female. MRI shows a fat-containing infiltrative lesion (signal loss) with nodules infiltrating the abdominal wall (arrow in B). PET/CT shows hypermetabolism (arrow in C), corresponding to dedifferentiation foci, confirmed by ultrasound-guided biopsy.
Retroperitoneal Teratoma: Retroperitoneal teratomas, particularly mature teratomas, are characterized by predominantly cystic lesions containing fat and calcifications (Figure 2). Immature teratomas are less common and indistinguishable from benign variants based solely on imaging.[3, 5, 33]
Extragastrointestinal Stromal Tumor (GIST): Extragastrointestinal stromal tumors are mesenchymal neoplasms originating from interstitial cells of Cajal, typically outside the GI tract, rarely involving the retroperitoneum. They are characterized by c-kit gene mutations and are histologically similar to gastrointestinal stromal tumors. Imaging reveals homogeneous or heterogeneous masses with central necrosis and calcifications. Sarcomas are a primary differential diagnosis.[18]
Key Point: Calcification is a common finding in various retroperitoneal lesions. When combined with other imaging features like fat, it significantly refines the differential diagnosis. In liposarcoma, calcification, enhancing nodules, and FDG uptake are suggestive of dedifferentiation.
Myxoid Stroma in Retroperitoneal Masses
Myxoid stroma, composed of a mucopolysaccharide-rich matrix, exhibits a hyperintense signal on T2-weighted MRI and delayed contrast enhancement.[1] Lesions with myxoid stroma include neurogenic tumors, myxoid liposarcoma, and myxofibrosarcoma.[2, 31] Myxoid liposarcoma and neurogenic tumors, which can arise from neural sheaths, paraganglion systems, or sympathetic ganglia,[3] are particularly relevant.
Neurogenic Tumors: Neurogenic tumors originate from neural sheaths, encompassing schwannomas and neurofibromas.
Schwannomas: Schwannomas are the most common benign neural sheath tumors, prevalent in young adults, and can be associated with neurofibromatosis.[1, 10, 11, 34] Imaging shows circumscribed, oval or spherical masses with heterogeneous enhancement, typically in the paravertebral region.[3, 11] MRI signal intensity varies with tissue type, with myxoid stroma correlating with hyperintensity on T2-weighted images.[1, 10] Schwannomas can compress adjacent structures depending on size and location.[10, 34] Treatment options range from surgical removal for large or symptomatic lesions to monitoring for asymptomatic cases or those with high surgical risk.[3, 4, 13]
Neurofibromas: Neurofibromas involve proliferation throughout nerve structure elements, causing diffuse nerve enlargement.[10] MRI reveals soft-tissue attenuation masses, hypointense on T1-weighted and variably intense on T2-weighted images.[3] Myxoid degeneration, the target sign, and contrast enhancement are characteristic features.[3, 27] They often present with spindle cell morphology along the nerve trajectory or neural foramen enlargement when originating from spinal nerve roots.[3] Retroperitoneal plexiform neurofibromas are typically bilateral, symmetrical, following the lumbosacral plexus distribution, and almost exclusively linked to neurofibromatosis type I.[3, 11] Malignant peripheral nerve sheath tumors (MPNSTs), including malignant schwannomas and neurofibrosarcomas, account for 10% of soft tissue sarcomas, affecting individuals aged 20-50, and are strongly associated with neurofibromatosis type I.[35, 36]
Paragangliomas: Retroperitoneal paragangliomas, neurogenic tumors from the paraganglion system, originate from extra-adrenal chromaffin cells. The retroperitoneum is the most common extra-adrenal site, often arising from the organ of Zuckerkandl near the inferior mesenteric artery origin.[3, 11, 14] They are characterized by heterogeneous masses with hemorrhage, central necrosis (40% of cases), punctate calcifications, and fluid-fluid levels. MRI shows hyperintense or heterogeneous T2-weighted signals and intense contrast enhancement.[3, 5, 11] Catecholamine hypersecretion can occur.[2, 3, 33] Approximately 40% of retroperitoneal paragangliomas are malignant, often leading to necrosis and distant metastases.[27, 33, 37] Functional imaging, including MIBG scintigraphy, 18F-FDG PET/CT (Figure 4), and 68Ga-DOTATATE PET/CT (superior for lesion detection),[38, 39] is useful for tumor localization and metastasis detection. Surgical resection is the primary treatment, even in metastatic cases, improving survival.[3, 37]
Figure 4. Retroperitoneal Paraganglioma Imaging.
CT and 18F-FDG PET/CT scans ((A) and (B) respectively) of a 24-year-old male with retroperitoneal paraganglioma, showing hypervascular nodular lesions with hyperglycolysis adjacent to the aortoiliac bifurcation.
Ganglioneuromas: Neurogenic tumors from sympathetic ganglia include ganglioneuromas, neuroblastomas, and ganglioneuroblastomas, with the latter two more common in pediatric patients.[27] Ganglioneuromas are benign, slow-growing tumors along the paravertebral sympathetic plexus, typically in the posterior mediastinum or retroperitoneum. They manifest as well-defined masses, less attenuating than muscle, containing myxoid stroma and punctate calcifications,[1, 3] as shown in Figure 5. MRI reveals homogeneous hypointense T1-weighted and variable T2-weighted signals. Functional ganglioneuromas, producing catecholamines and androgens, occur in half of cases.[2, 11] Diagnosis is pathology-based, and complete surgical resection is the preferred treatment, particularly for symptomatic patients.[40] However, imaging follow-up is advocated by some specialists, requiring caution due to potential poorly differentiated components.[40, 41]
Figure 5. Retroperitoneal Ganglioneuroma.
CT scan of a 31-year-old female showing a retroperitoneal ganglioneuroma with a pseudocystic appearance (arrow).
Myxoid Liposarcoma: Myxoid liposarcoma affects individuals aged 40-60, more common in extremities, particularly thigh deep soft tissues, but can occur retroperitoneally.[11, 31] It contains adipocytic and non-adipocytic myxoid components, creating a pseudocystic appearance on imaging with slow, progressive contrast enhancement.[3, 11, 18] Adipose content is typically less than 10% of tumor volume.[31]
Myxofibrosarcoma: Formerly myxoid malignant fibrous histiocytoma, myxofibrosarcoma affects individuals with a mean age of 65, retroperitoneal origin in about 8% of cases. Imaging findings are nonspecific due to myxoid stroma, fibrous tissue, cellular tissue, hemorrhage, and necrosis, with or without fluid-fluid levels or pseudocapsules.[31]
Key Point: Myxoid stroma is a common feature in a wide range of retroperitoneal tumors, characterized by a pseudocystic appearance and hyperintense T2-weighted MRI signal. Anatomical relationships, like nerve structures or the organ of Zuckerkandl, and associated genetic syndromes aid in narrowing the differential diagnosis.
Necrosis in Retroperitoneal Masses
Necrosis is a hallmark of high-grade neoplasms, typically indicating poor prognosis and often seen in the most common malignant retroperitoneal tumors.[1, 3]
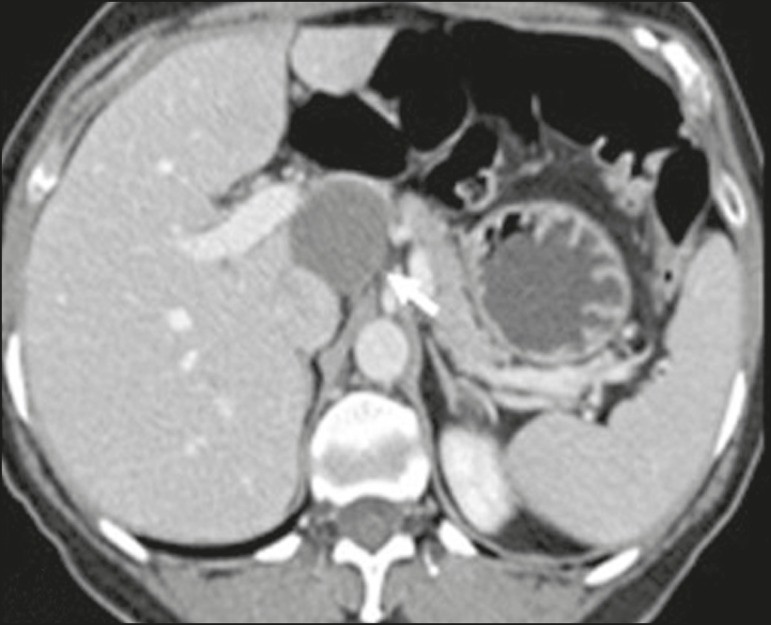
Leiomyosarcoma: Leiomyosarcoma is the second most common primary retroperitoneal tumor in adults aged 50-60, originating from retroperitoneal smooth muscle tissue, vascular walls, or Wolffian duct remnants.[11, 14, 18] Imaging shows bulky masses with necrosis, hemorrhage, and frequent vascular structure involvement (Figure 6). Calcifications and intralesional fat are uncommon.[3, 18, 32] Three vascular growth patterns are described: extraluminal, intraluminal, and mixed.[11, 19] Surgical treatment is complex but remains the primary option. Recurrence is common.[19, 42] Chemotherapy is a palliative therapeutic option.[13, 22, 43]
Figure 6. Retroperitoneal Leiomyosarcoma.
CT scan of a 56-year-old female with retroperitoneal leiomyosarcoma, showing a bulky lesion with heterogeneous enhancement and necrotic foci. Note the extensive contact with the inferior vena cava (arrow).
Undifferentiated Pleomorphic Sarcoma (UPS): Formerly malignant fibrous histiocytoma, reclassified as UPS by the WHO in 2013,[31] it is the third most common retroperitoneal tumor, typically affecting individuals aged 50-70.[11, 31] Imaging shows bulky, infiltrative masses with heterogeneous enhancement and areas of necrosis, hemorrhage, fibrosis, and calcifications (20% of cases), with or without pseudocapsules.[4, 5, 18, 31] Surgical resection is the treatment of choice, and prognosis is linked to tumor grade, size, and metastatic status.[19]
Pleomorphic Liposarcoma: Pleomorphic liposarcoma is an aggressive, heterogeneous, and least common liposarcoma subtype. Imaging shows necrosis and lacks adipose components, making it indistinguishable from other solid retroperitoneal tumors.[3, 14, 18]
Key Point: Liposarcoma, leiomyosarcoma, and undifferentiated pleomorphic sarcoma are the most common malignant mesenchymal retroperitoneal tumors. Necrosis is a shared aggressive feature.
Cystic Retroperitoneal Masses
Some retroperitoneal tumors present with a cystic appearance. Lymphangioma is the primary differential consideration for cystic retroperitoneal masses.
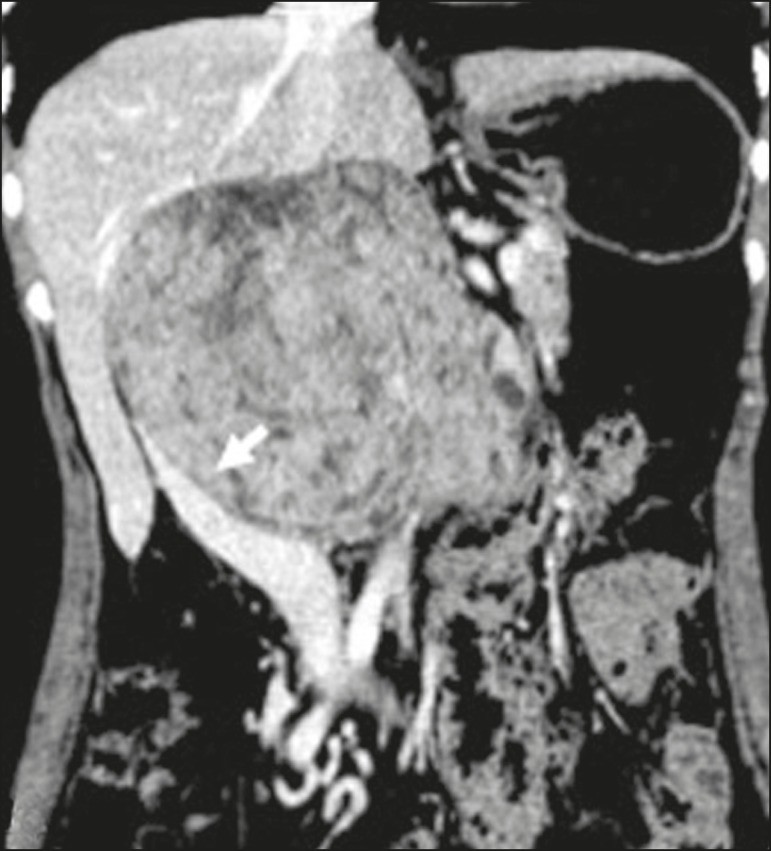
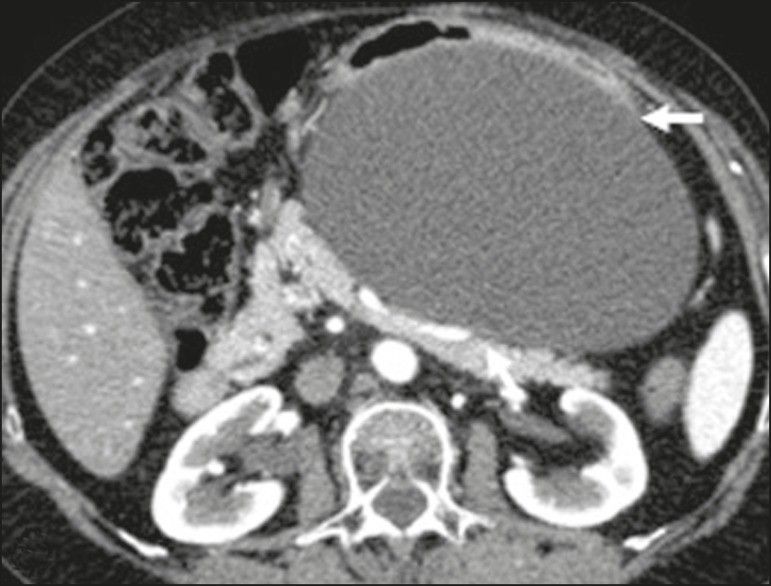
Cystic Lymphangioma: Cystic lymphangioma is a benign, slow-growing lymphatic malformation from failure of retroperitoneal lymphatic channel communication with the main lymphatic system.[33] It may contain serous, chylous, hemorrhagic, or mixed contents, characterized by thin-walled, unilocular or multilocular cystic masses with variable attenuation based on content (Figure 7). Surgical resection is preferred for bulky, rapidly growing, or symptomatic lesions. Conservative management and imaging follow-up are options for others.[3, 4, 8]
Figure 7. Retroperitoneal Cystic Lymphangioma.
CT scan of a 37-year-old male showing a retroperitoneal cystic lymphangioma (arrow), displacing bowel loops anteriorly and in contact with the pancreas. Pathology post-surgical resection confirmed lymphangioma, with dilated lymphatic vessels and no atypia.
Cystic Mesothelioma: Cystic mesothelioma is a rare benign mesothelial neoplasm characterized by thin-walled cysts of varying sizes, indistinguishable from other retroperitoneal cystic masses.[14, 33] While malignant transformation is reported, some argue against its malignant potential.[44] Periodic imaging follow-up is recommended for non-surgical candidates. Less common cystic lesions include Müllerian cysts, epidermoid cysts, nonpancreatic pseudocysts, cystadenomas, and cystadenocarcinomas.[1, 14, 33] Schwannomas and paragangliomas can also be entirely cystic and should be in the differential diagnosis.[33, 34]
Key Point: Management of cystic retroperitoneal lesions is clinically challenging, with options including percutaneous biopsy, clinical follow-up, and surgical excision for symptomatic cases.[33]
Vascularization of Retroperitoneal Masses
Tumor vascularization aids in retroperitoneal mass characterization. Paragangliomas are typically hypervascular. Leiomyosarcomas, myxofibrosarcomas, and other sarcomas are moderately hypervascular. Low-grade liposarcomas, lymphomas, and most benign tumors are hypovascular.[1]
Mantle Growth Pattern in Retroperitoneal Masses
The growth pattern, extent, and relationship with adjacent structures are crucial for differential diagnosis. Mantle growth pattern refers to lesions involving adjacent structures without clear infiltration signs.
Lymphoma: Lymphoma is the most common malignant retroperitoneal neoplasm and small round cell tumor, typically presenting as para-aortic or pelvic masses, involving adjacent structures with a homogeneous and hypovascular appearance.[1, 3-5] Necrosis and calcifications are uncommon before treatment.[3] 18F-FDG PET/CT is standard for diagnosis, staging, and in-treatment evaluation. However, FDG avidity varies with histology, grade, and tumor proliferation.[45, 46] Low-grade lymphomas are less FDG-avid than aggressive lymphomas like large B-cell lymphoma and nodular sclerosing Hodgkin lymphoma.[45, 47] Treatment includes chemotherapy with or without radiotherapy or immunotherapy, depending on the subtype.[46] Metastatic lymph node enlargement is a major differential diagnosis with similar imaging features.[4, 5]
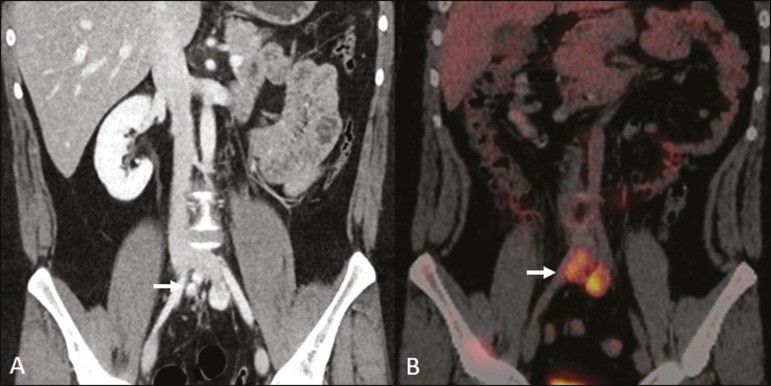
Erdheim-Chester Disease (ECD): ECD, a rare non-Langerhans cell histiocytosis of unknown origin, involves multiorgan inflammation composed of histiocytes, commonly affecting males aged 40-60. Retroperitoneal involvement occurs in 29% of cases, characterized by homogeneous soft-tissue attenuation on CT, hypointense to isointense T1-weighted and hyperintense T2-weighted MRI signals.[3, 5, 48, 49] Circumferential aortic involvement and bilateral symmetrical perirenal space involvement are characteristic. Ureters can also be involved (Figure 8). Diagnosis is based on specific histopathology in the clinical and radiological context. Bone changes are common; biopsy is usually needed for confirmation, even with high clinical suspicion.[48] Treatment is based on corticosteroids, immunomodulatory agents, cytotoxic agents, and other drugs due to the rarity of the disease and limited randomized studies.[48, 49]
Figure 8. Erdheim-Chester Disease with Mantle Zone Growth.
CT scans ((A, B)) of a 62-year-old male with Erdheim-Chester disease showing perirenal solid tissue (mantle zone growth pattern) surrounding the renal hilum (arrow in (B)). No bilateral hydronephrosis was observed.
Retroperitoneal Fibrosis (RPF): RPF is a rare condition, more common in males, classified as idiopathic or secondary.[3, 4, 50] Idiopathic RPF is two-thirds of cases. Secondary RPF is linked to neoplasms, infections, trauma, and other conditions. Imaging shows homogeneous soft-tissue attenuation surrounding the abdominal aorta and iliac arteries, potentially involving ureters and the inferior vena cava, leading to ureteral obstruction and venous thrombosis.[4, 50] MRI shows intense T2-weighted signals and variable enhancement depending on disease activity.[3] Idiopathic RPF treatment is corticosteroids, with tamoxifen effective in some cases. Secondary RPF treatment focuses on the underlying cause.[3, 4, 50]
Key Point: Retroperitoneal lesions with a mantle growth pattern can be benign or malignant (e.g., lymphoma). Percutaneous biopsy is crucial in initial clinical management as treatment is often medical or chemotherapeutic.
DIAGNOSTIC ALGORITHM AND CLINICAL MANAGEMENT
Figure 9 presents a diagnostic algorithm to aid in the differential diagnosis of primary retroperitoneal masses based on key imaging features. Figure 10 outlines the clinical management of primary retroperitoneal lesions.
Figure 9. Algorithm for Imaging-Based Differential Diagnosis of Retroperitoneal Masses.
Flowchart illustrating a practical approach to retroperitoneal mass evaluation based on key imaging characteristics: presence of fat, cystic appearance, myxoid stroma, necrosis, and mantle zone growth pattern, guiding towards differential diagnoses like liposarcoma, teratoma, lymphangioma, neurogenic tumors, leiomyosarcoma, lymphoma, and others.
Figure 10. Clinical Management Algorithm for Retroperitoneal Masses.
Algorithm outlining the clinical management pathway for retroperitoneal masses, from initial imaging and differential diagnosis to biopsy considerations, treatment strategies (surgical resection, chemotherapy, radiotherapy, targeted therapy), and follow-up protocols for various lesion types including sarcomas, lymphomas, neurogenic tumors and benign conditions.
CONCLUSION
Primary retroperitoneal lesions are rare entities that present significant diagnostic challenges due to overlapping imaging features. A thorough understanding of key imaging characteristics is essential for narrowing the differential diagnosis of retroperitoneal masses and guiding effective clinical decision-making.