Aim:
To assess the extent of delayed diagnosis, measured by the prediagnostic symptomatic interval (PSI), in Filipino children with brain tumors and to identify contributing factors.
Methods:
A retrospective analysis was conducted on data from pediatric brain tumor patients at the Philippine General Hospital between 2015 and 2019. The PSI was calculated, and associated factors were statistically analyzed.
Results:
The study included 196 patients. The median PSI was 80.5 days. Significantly longer PSIs were associated with: older age, supratentorial and low-grade tumors, increased prior physician consultations before specialist referral, longer time from neuroimaging request to execution, and specific presenting symptoms such as seizures (11-month delay), poor academic performance (1-year delay), behavioral changes (1.3-year delay), and secondary amenorrhea (3-year delay).
Conclusion:
Delayed diagnosis is a significant issue among Filipino children with brain tumors and is linked to patient age, tumor characteristics, and less typical symptoms. Enhancing physician awareness of these atypical presentations through education, diligent patient monitoring, timely specialist referrals, and improved access to neuroimaging are crucial steps toward earlier diagnosis.
Keywords: delayed diagnosis, Filipino children, pediatric brain tumor, pediatric CNS tumor, prediagnostic symptomatic interval
Brain tumors are the second most prevalent type of cancer in children aged 0–14 worldwide [1], with reported incidences ranging from 1.12 to 5.14 per 100,000 individuals globally [2] and within the Philippines [3]. In the Philippines, astrocytoma (25.8%) and medulloblastoma (23.9%) are the most frequently diagnosed types [4].
Delayed diagnosis in pediatric brain tumors is a well-documented issue with severe consequences for patients and their families. Studies indicate that approximately 50% of cases experience symptomatic worsening from initial symptom onset until diagnosis [5]. Diagnostic delays can negatively impact treatment outcomes, potentially leading to neurological, developmental, and neuroendocrine complications [6], cognitive impairment [7,8], and increased mortality. These delays can also cause significant anxiety and erode trust in the healthcare system for both patients and their families [9].
The pre-diagnostic symptomatic interval (PSI), defined as the duration from the first symptom appearance to diagnosis, is a key metric in brain tumor research for quantifying diagnostic delay. A historical study from 1935-1959 showed that 50% of children with intracranial tumors had a PSI exceeding 6 months [10]. Despite advancements in diagnostic imaging and medical knowledge, improvements in diagnostic timelines for pediatric brain tumors have been limited. Recent studies continue to report median PSIs ranging from 30 days to 7.7 months [5,11–17].
Several factors contribute to delayed diagnosis, including older patient age [11–13,18–22], co-existing health conditions [5], lower parental education and socioeconomic status [14,23], tumor characteristics [11,13,15,18–22], access to neuroimaging [5,24], and the nature of presenting symptoms like vomiting, headache, ataxia, psychological issues, and endocrine disorders [12,13,15–17].
Pediatricians are often the first point of contact for children suspected of having brain tumors [20]. Referrals to pediatric neurologists and neurosurgeons usually follow consultations with primary care physicians. Research indicates that delays attributed to doctors often exceed parental delays [11,16,19,25]. Misdiagnosis by primary care physicians—such as attributing symptoms to tonsillitis, gastroenteritis, sinusitis, meningitis, or refractive errors—is a significant factor in diagnostic delays in cases requiring multiple consultations [11].
A unique aspect of healthcare-seeking behavior in the Philippines is the practice of consulting traditional healers before seeking medical doctors. In rural areas, traditional healers may be more accessible and affordable, leading patients to consult physicians only if symptoms persist without improvement. This pattern is observed in local studies on infectious diseases [26,27]. This study investigated whether such health-seeking behaviors among Filipino pediatric brain tumor patients contribute to diagnostic delays.
Data on delayed diagnosis of pediatric brain tumors is scarce in resource-limited settings like the Philippines. To our knowledge, this study is the first of its kind in the region. By quantifying the prediagnostic symptomatic interval in Filipino children, we aim to establish the burden of delayed brain tumor diagnosis in our context. Identifying locally relevant factors contributing to these delays—whether parental, doctor-related, or health system-related—is crucial for improving awareness and promoting earlier recognition of pediatric brain tumors. The primary goal of this study is to determine the extent of delayed diagnosis among Filipino pediatric brain tumor patients, measured by PSI, and to explore associated factors.
Materials & Methods
Study Design & Participants
This retrospective study examined pediatric patients aged 2 months to 18 years diagnosed with primary brain tumors via neuroimaging and/or histopathology at the Philippine General Hospital (PGH) from January 2015 to December 2019. Patients with congenital brain tumors and those lacking official imaging results were excluded. The study was approved by the University of the Philippines Manila Research Ethics Board (UPMREB No. 2020-633-01).
Sample size calculation using G*power software indicated a need for at least 175 participants for linear regression analysis with 26 predictor variables (including age, sex, socioeconomic status, parental education, tumor characteristics, and symptoms), a medium effect size of 0.15, 95% confidence interval, and 80% power. After exclusions for pre-2015 diagnoses, duplicate records, missing charts, and incomplete data necessary for PSI and diagnosis determination, a total of 196 patients were included (Figure 1).
Figure 1. Selection process for medical records of pediatric patients with primary brain tumors at the Philippine General Hospital, January 2015 – December 2019.
Figure 1 alt text: Flowchart illustrating patient record selection for a study on delayed diagnosis of pediatric brain tumors in Filipino children at Philippine General Hospital from 2015-2019.
PGH: Philippine General Hospital; PSI: Prediagnostic symptomatic interval.
Data Collection
Medical charts, imaging results, and histopathology reports were reviewed to collect data. Information included patient demographics (comorbidities, tumor-related syndromes, residence, socioeconomic status [28], parental education), tumor characteristics (location, size, grade, histopathology, tumor markers, metastasis), first physician contact, traditional healer consultation, initial clinical impressions before brain tumor diagnosis, neuroimaging timing, and symptoms at onset, first physician consult, and subspecialist consultation. Headache characteristics (location, timing, severity), vomiting type (projectile/non-projectile), and seizure type (focal/generalized) were also recorded.
Prediagnostic Symptomatic Interval
The overall pre-diagnostic symptomatic interval (PSI) was calculated as the time from the reported date of first symptom onset to the date of diagnosis via neuroimaging. Symptom onset dates were based on caregiver reports in medical histories. Conservative onset dates were used if specific dates were unavailable (e.g., last day of the month if only month was recorded, December 31st if only year was given) [21]. Diagnosis date was defined as the date of the first MRI or CT scan. Histopathology report date was used if imaging diagnosis was uncertain. PSI was further categorized into:
- PSI 1: Symptom onset to first physician consult (parental delay)
- PSI 2: First consult to neurologic subspecialist referral (doctor’s delay)
- PSI 3: Subspecialist referral to brain tumor diagnosis (subspecialist delay) [29]
Data Analysis
Descriptive statistics summarized demographic and clinical data. Quantitative variables were presented as median and interquartile range (IQR) for non-normally distributed data. Qualitative variables were presented as frequencies and percentages. Nonparametric tests (Kendall’s tau b, Kruskal-Wallis, Mann-Whitney U, Wilcoxon signed-rank) were used for correlations and comparisons of PSI. Linear regression analysis identified predictors of delayed diagnosis. Data transformation was applied to meet linearity assumptions. Statistical significance was set at p < 0.05. Missing values were not imputed. STATA version 14 software was used for analysis.
Results
Patient & Tumor Characteristics
Table 1 summarizes patient and tumor characteristics. The median age at diagnosis was 9 years. Most patients were male (64.3%). 7.1% had comorbidities, predominantly respiratory issues (pneumonia), and a smaller number with neurodevelopmental conditions (autism spectrum disorder, ADHD). 5% had tumor-related syndromes, mainly tuberous sclerosis complex and neurofibromatosis. The majority (57%) were from low to lower-middle income families. Most parents had completed high school or vocational training (44% of mothers, 39% of fathers).
Table 1. Patient and tumor characteristics of the study cohort (n = 196).
| Age at diagnosis (years) | ||
|---|---|---|
| Median | 9 | |
| Range | 1 to 18 | |
| IQR | 5 to 13 | |
| Sex | Number of patients | Percentage |
| Male | 126 | 64.3 |
| Female | 70 | 35.7 |
| Comorbidities and other illnesses | 14 | 7.1 |
| Respiratory | 7 | 3.6 |
| Autism spectrum disorder | 2 | 1 |
| Attention deficit hyperactivity disorder | 1 | 0.5 |
| Ventricular septal defect | 1 | 0.5 |
| G6PD deficiency | 1 | 0.5 |
| Polycythemia vera | 1 | 0.5 |
| Cellulitis | 1 | 0.5 |
| Tumor-related syndromes | 10 | 5.1 |
| Tuberous sclerosis complex | 5 | 2.6 |
| Neurofibromatosis type II | 4 | 2 |
| Neurofibromatosis type I | 1 | 0.5 |
| Income cluster | ||
| Poor | 32 | 16.3 |
| Low to lower middle income | 112 | 57.1 |
| Middle to upper middle income | 47 | 24 |
| Mother’s highest educational attainment | ||
| Elementary | 12 | 6.1 |
| High school/vocational | 87 | 44.4 |
| College/post-graduate | 56 | 28.6 |
| Father’s highest educational attainment | ||
| Elementary | 14 | 7.1 |
| High school/vocational | 78 | 39.8 |
| College/post-graduate | 53 | 27 |
| Tumor size (volume) | ||
| Median | 35.2 cm3 | |
| Range | 1.5 to 611 cm3 | |
| IQR | 18.6 to 60 cm3 | |
| Tumor location | Number of patients | Percentage |
| Supratentorial | 98 | 50 |
| Cerebrum | 36 | 18.4 |
| Sellar-suprasellar | 31 | 15.8 |
| Pineal | 31 | 15.8 |
| Infratentorial | 98 | 50 |
| Posterior fossa | 82 | 41.8 |
| Brainstem | 16 | 8.2 |
| Tumor grade | ||
| WHO grade I | 59 | 30.1 |
| WHO grade II | 12 | 6.1 |
| WHO grade III | 1 | 0.5 |
| WHO grade IV | 74 | 37.8 |
| Histopathology | ||
| Medulloblastoma | 43 | 21.9 |
| Low grade glioma | 40 | 20.4 |
| High grade glioma | 37 | 18.9 |
| Germ cell tumors | 24 | 12.2 |
| Craniopharyngioma | 14 | 7.1 |
| Ependymoma | 10 | 5.1 |
| Schwannoma | 6 | 3.1 |
| Pituitary adenoma | 3 | 1.5 |
| ATRT | 2 | 1 |
| Metastasis at diagnosis | 3 | 1.5 |
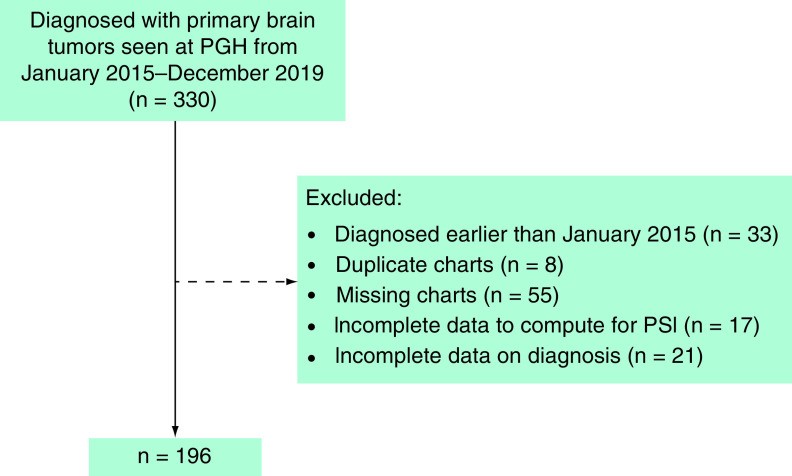
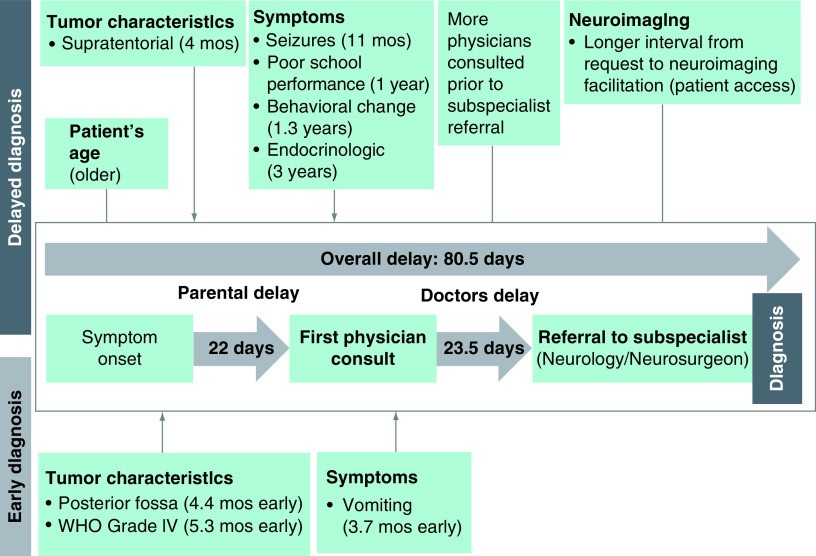
Table 1 Alt text: Patient demographics, socioeconomic status, and tumor characteristics including location, grade, and histopathology in a cohort of Filipino children with brain tumors.
ATRT: Atypical teratoid rhabdoid tumor; IQR: Interquartile range.
Histological diagnosis was available for 75% (n=147), 8.7% were diagnosed via typical imaging (e.g., diffuse intrinsic pontine glioma, subependymal giant cell astrocytoma), and 12.2% using tumor markers and imaging (n=24). Supratentorial and infratentorial tumors were equally represented. Among supratentorial tumors (n=98), cerebral hemisphere tumors were most common (36.7%), followed by sellar-suprasellar and pineal tumors (31.6% each). Infratentorial tumors were mostly posterior fossa tumors (83.7% of infratentorial, 41.8% of total tumors), with medulloblastoma being the most frequent histopathology (21.9%), followed by low-grade gliomas (20.4%), and high-grade gliomas (18.9%). Grade IV tumors were the most common WHO grade (37.8%), followed by Grade I (30.1%). Metastasis at diagnosis was present in 1.5% (n=3), all medulloblastoma cases.
The Prediagnostic Symptomatic Interval
Prediagnostic symptomatic intervals are detailed in Table 2. The median overall PSI was 80.5 days (range 0-1815, IQR 31-199). Median PSI 1 (parental delay) was 22 days (range 0-1794, IQR 5-62), and median PSI 2 (doctor’s delay) was 23.5 days (range 0-1464, IQR 5-61). No significant difference was found between PSI 1 and PSI 2 (p=0.477). Median PSI 3 (subspecialist delay) was 0 days (range 0-1471, IQR 0-5), as 56.6% of patients (n=111) already had a brain tumor diagnosis, with neuroimaging evidence, upon subspecialist consultation.
Table 2. Prediagnostic symptomatic interval components and overall PSI.
| PSI 1 (parents delay) | PSI 2 (doctors delay) | PSI 3 (subspecialist delay) | PSI overall | |
|---|---|---|---|---|
| Median | 22 days | 23.5 days | 0 days | 80.5 days |
| Range | 0 to 1794 days | 0 to 1464 days | 0 to 1471 days | 0 to 1815 days |
| IQR | 5 to 62 days | 5 to 61 days | 0 to 5 days | 31 to 199 days |
Table 2 Alt text: Median, range, and interquartile range (IQR) for Prediagnostic Symptomatic Interval (PSI) and its components: parental delay, doctor delay, and subspecialist delay in Filipino pediatric brain tumor patients.
IQR: Interquartile range; PSI: Prediagnostic symptomatic interval.
Only 23.5% (n=46) of patients were diagnosed within one month of symptom onset. The majority (37.8%, n=74) were diagnosed within 3-6 months, with smaller groups diagnosed within 2-3 months (11.7%), 6-12 months (12.8%), and after one year (14.3%), including 10 patients diagnosed after 3 years. Characteristics of patients with PSI > 3 years are in Table 3. Eight of these ten patients were ≥10 years old, predominantly diagnosed with benign tumors in the cerebrum and sellar-suprasellar regions. Among patients diagnosed after one year, 39% experienced >1 year symptom onset before first consultation, while 36% had >1 year delay before subspecialist referral despite early initial consultation (within 2 months of symptom onset). Reasons for delays exceeding one year included: lack of local neurologist/neurosurgeon availability (n=10), imaging misinterpretation (n=4), physician waiting for imaging before referral (n=3), and seeking multiple opinions (n=3). Three patients already under pediatric neurologist care were diagnosed with brain tumors after one year, having been initially treated for epilepsy without immediate imaging.
Table 3. Characteristics of patients with prediagnostic symptomatic interval exceeding 3 years.
| Patient number | PSI in months | Sex | Age at diagnosis (years) | Tumor location | Histopathology | Initial diagnosis | Symptoms at onset | Number of physicians consulted prior to diagnosis† | Neurologic deficits upon neurologic subspecialist consultation |
|---|---|---|---|---|---|---|---|---|---|
| 066 | 38.6 | F | 10 | Sellar-suprasellar | Craniopharyngioma | No data | Secondary amenorrhea, headache | 2 | Bitemporal hemianopsia, decreased visual acuity, exotropia of the left eye |
| 095 | 36.5 | F | 9 | Cerebrum (left temporal) | Ganglioglioma | Epilepsy | Seizure | 2 | Slowed speech, slow to respond, fair attention |
| 114 | 48.9 | M | 13 | Pineal | Mixed malignant GCT | Error of refraction | Blurring of vision, poor school performance | 6 | Nonreactive pupils, bilateral papilledema, Parinaud syndrome |
| 124 | 60.5 | M | 14 | Sellar-suprasellar | Pituitary adenoma | Primary headache | Headache | 3 | Left temporal hemianopsia, impaired visual acuity, bilateral lateral rectus palsy |
| 127 | 38.1 | M | 4 | Sellar-suprasellar | Pilocytic astrocytoma | Error of refraction | Blurring of vision, headache | 2 | Left homonymous hemianopsia, left extremity drift, left lower extremity clonus, nystagmus |
| 137 | 48.7 | F | 13 | Cerebrum (right frontoparietal) | Ganglioglioma | No data | Left extremity numbness and weakness | 2 | Lethargy, bilateral papilledema, left central facial palsy, left hemiplegia |
| 146 | 49 | M | 11 | Cerebrum (left frontal) | Glioblastoma | No data | Headache, seizure | 2 | Right lateral rectus palsy, fair gutturals, left hemiparesis, nystagmus |
| 167 | 45.7 | F | 18 | Cerebrum (right temporal) | Ganglioglioma | Anxiety disorder | Behavioral changes (fear and panic) | 4 | No deficits, (+) seizures |
| 176 | 48.7 | F | 18 | Sellar-suprasellar | Germinoma | Unspecified gynecologic dysfunction | Secondary amenorrhea, polydipsia, polyuria | 4 | Light perception on left, right temporal hemianopsia, optic atrophy |
| 191 | 49.3 | M | 13 | Posterior fossa | Pilocytic astrocytoma | No data | Headache, vomiting | 2 | Gait ataxia, dysmetria |
Table 3 Alt text: Detailed characteristics including PSI, demographics, tumor details, initial diagnosis, symptoms, and neurological deficits for ten Filipino pediatric brain tumor patients with PSI greater than 3 years.
Symptoms/signs are listed in the order reported or observed.
†Number of physicians consulted prior to diagnosis excludes neurologic subspecialty consults.
F: Female; GCT: Germ cell tumor; M: Male; PSI: Prediagnostic symptomatic interval.
Factors Associated with Delayed Brain Tumor Diagnosis
Patient & Tumor Factors
Age showed a moderate positive correlation with PSI (Kendall’s τb = 0.27, p = 0.027), indicating longer PSI in older children. Sex, comorbidities, tumor-related syndromes, socioeconomic status, and parental education showed no significant PSI correlation (p > 0.05).
Tumor location and grade significantly impacted PSI. Supratentorial tumors had significantly longer PSIs than infratentorial tumors (p = 0.002), with sellar-suprasellar tumors exhibiting the longest median PSI (210 days). Posterior fossa and brainstem tumors had shorter PSIs (p = 0.020). WHO Grade I and II tumors had longer PSIs compared to Grade IV tumors (p = 0.001). Median PSI for Grade I was 153 days and Grade II was 107 days, while Grade III was 28 days and Grade IV was 61 days. Tumor volume and histology, and metastasis presence did not significantly correlate with PSI (p > 0.05).
Presenting Symptoms
Table 4 details symptom frequencies at onset, first physician consult, and subspecialist referral. Common symptoms included headache (58.7%), vomiting (38.8%), gait imbalance (18.9%), blurred vision (13.8%), and dizziness (12.8%). Among headache sufferers, only 26% reported morning headaches, and 23% occipital pain. Initial headaches were mostly mild (45.2%), but became severe (NRS 8-10) upon subspecialist referral (34.3%). Projectile vomiting was reported in only 14% of vomiting cases. Seizures were present in 5.2%, mostly focal. Symptom count increased from onset to subspecialist referral. Initially, most had ≥2 symptoms (42.3%), increasing to ≥4 symptoms in 52% by subspecialist consult, with monosymptomatic presentation decreasing to 8.7% (Table 5). Headache and vomiting co-presentation rose from 26.5% at onset to 40.8% at subspecialist referral. Papilledema was present in 18.9%, and the classic triad (headache, vomiting, papilledema) in 10.7% at subspecialist referral.
Table 4. Presenting symptoms, median PSI, and frequency changes across consultation stages.
| Median PSI (days) | p-value | Number of patients (percentage) presenting with the symptom | Frequency increase in number of patients presenting with the symptom from onset to subspecialist referral | |
|---|---|---|---|---|
| Patients with the symptom | Patients without the symptom | At onset | ||
| Headache | 84 | 78 | 0.620 | 115 (58.7) |
| Vomiting | 61 | 102 | 0.015 | 76 (38.8) |
| Gait imbalance/unsteady gait | 61 | 88 | 0.132 | 37 (18.9) |
| Blurring of vision | 92 | 77 | 0.119 | 27 (13.8) |
| Dizziness | 75 | 83 | 0.961 | 25 (12.8) |
| Diplopia | 76.5 | 83.5 | 0.918 | 16 (8.2) |
| Seizure | 336 | 76 | 0.021 | 10 (5.1) |
| Paresis | 62 | 86 | 0.312 | 9 (4.6) |
| Increased sleeping time | 61.5 | 85 | 0.192 | 8 (4.1) |
| Ocular motility dysfunction | 68 | 86 | 0.530 | 7 (3.6) |
| Poor school performance† | 792 | 77.5 | 0.030 | 8 (4.6) |
| Behavioral changes | 540 | 77.5 | 0.018 | 5 (2.6) |
| Secondary amenorrhea† | 365 | 77 | 0.037 | 5 (13.9) |
| Polydipsia/polyuria | 67 | 83 | 0.408 | 5 (2.6) |
| Hearing loss | 92 | 78 | 0.559 | 3 (1.5) |
| Numbness | 91 | 78 | 0.708 | 3 (1.5) |
| Facial palsy | 63 | 83 | 3 (1.5) | |
| Torticollis | 63 | 83 | 0.082 | 3 (1.5) |
Table 4 Alt text: Presenting symptoms at onset, during first physician consult, and upon subspecialist consult, including median PSI and p-values for symptom association with delayed diagnosis in Filipino children with brain tumors.
†Patients analyzed for these symptoms included only the population at risk. For poor school performance, patients aged 4 years and above (n = 175) were included, based on average age of school entry at 4 years old. For secondary amenorrhea, only females 13 years and above (n = 36) were included, based on average age of menarche for Filipino female of 13 years old.
PSI: Prediagnostic symptomatic interval.
Table 5. Number of symptoms reported at each stage of consultation.
| Number of symptoms at presentation | At onset | During first physician consult | Upon neurology/neurosurgery referral |
|---|---|---|---|
| Number of patients | Percentage | Number of patients | |
| 1 | 69 | 35.2 | 48 |
| 2 | 83 | 42.3 | 71 |
| 3 | 31 | 15.8 | 38 |
| 4 or more | 13 | 6.6 | 39 |
Table 5 Alt text: Distribution of the number of symptoms presented by Filipino pediatric brain tumor patients at symptom onset, first physician consult, and upon neurology/neurosurgery referral.
Longer median PSIs were significantly associated with seizures (336 days, p = 0.021), poor school performance (792 days, p = 0.030), behavioral changes (540 days, p = 0.018), and secondary amenorrhea (365 days, p = 0.037). Conversely, vomiting was associated with shorter PSI (61 days, p = 0.015). No significant PSI difference was observed based on the number of initial symptoms (p = 0.494).
Consultation, Referral & Neuroimaging Timing
Pediatricians were the most frequent first contact physicians (68.4%), followed by ophthalmologists (17.9%) (Table 6). First physician type did not significantly affect PSI (p = 0.054). Traditional healer consultation occurred in 2% of cases and did not impact PSI (p = 0.613). A weak but significant positive correlation was found between PSI and the number of physicians consulted before subspecialist referral (Kendall’s τb = 0.177, p = 0.001). Most patients (42.9%) consulted one physician pre-referral; 33.2% consulted two, and 24% consulted ≥3. Pediatric neurologists were more frequently consulted than neurosurgeons (65.8% vs 34.2%).
Table 6. Types of first contact physicians consulted by patients.
| First contact physician | Number of patients | Percentage |
|---|---|---|
| Pediatrician | 134 | 68.4 |
| Ophthalmology | 35 | 17.9 |
| General Doctor | 8 | 4.1 |
| Emergency Medicine | 8 | 4.1 |
| Otorhinolaryngology | 4 | 2 |
| Family Medicine | 2 | 1 |
| Orthopedic | 2 | 1 |
| School Physician | 1 | 0.5 |
| Gastroenterologist | 1 | 0.5 |
| OB Gynecologist | 1 | 0.5 |
Table 6 Alt text: Distribution of first contact physician specialties consulted by Filipino pediatric brain tumor patients prior to diagnosis.
Pre-brain tumor diagnoses were available for 134 charts (Table 7). Error of refraction (24.5%), primary headache (11.7%), and gastroesophageal reflux (10.2%) were most common. Multiple diagnoses at first consult occurred in 5 patients. Most error of refraction diagnoses were from ophthalmologists (73%). Two patients initially diagnosed with gastroesophageal reflux had normal esophagogastroduodenoscopies before brain tumor workup.
Table 7. Common misdiagnoses given prior to brain tumor diagnosis.
| Number of patients | Percentage | |
|---|---|---|
| Error of refraction | 48 | 24.5 |
| Primary headache | 23 | 11.7 |
| Gastritis/gastroesophageal reflux | 20 | 10.2 |
| Meningitis | 12 | 6.1 |
| Acute gastroenteritis/dehydration | 9 | 4.6 |
| Epilepsy | 8 | 4.1 |
| Urinary tract infection | 7 | 3.6 |
| Stroke | 5 | 2.6 |
| Vertigo | 5 | 2.6 |
| Psychiatric | 4 | 2 |
Table 7 Alt text: List of most frequent misdiagnoses initially given to Filipino pediatric brain tumor patients before correct diagnosis.
Cranial CT scans were most commonly used for initial imaging (65.8%), with MRI used in 34.2%. Median time from imaging request to facilitation was 4 days (range 0-535, IQR 1-13). 11% experienced >1-month delay, often due to financial constraints. Longer request-to-imaging interval moderately correlated with longer PSI (Kendall’s τb = 0.253, p < 0.001).
Predictors of Delayed Diagnosis
Multivariate linear regression identified significant predictors of delayed diagnosis (Table 8). Supratentorial tumors were associated with a 116-day increased risk of delay (p = 0.014), while posterior fossa and Grade IV tumors were diagnosed approximately 132 days (p = 0.041) and 161 days (p = 0.002) earlier, respectively, compared to cerebrum and Grade I tumors. Seizures (331-day delay, p = 0.002), poor school performance (380-day delay, p = 0.008), behavioral changes (500-day delay, p = 0.033), and secondary amenorrhea (3-year delay, p = 0.021) predicted delay. Vomiting predicted earlier diagnosis by 112 days (p = 0.020).
Table 8. Predictors of delayed brain tumor diagnosis identified through linear regression.
| Beta coefficient (days) | 95% CI | p-value | |
|---|---|---|---|
| Tumor characteristics | |||
| Supratentorial | 116.10 | 24.15, 208.05 | 0.014 |
| Posterior fossa | -132.80 | -259.88, -5.71 | 0.041 |
| WHO grade IV | -161.52 | -260.74, -62.31 | 0.002 |
| Presenting symptoms at onset | |||
| Seizure | 331.37 | 124.37, 538.37 | 0.002 |
| Poor school performance† | 380.57 | 102.35, 658.79 | 0.008 |
| Behavioral changes | 500.07 | 40.77, 959.37 | 0.033 |
| Secondary amenorrhea† | 1122.44 | 189.69, 2055.19 | 0.021 |
| Vomiting | -112.85 | -207.37, -18.34 | 0.020 |
Table 8 Alt text: Linear regression analysis results showing predictors of delayed brain tumor diagnosis in Filipino children, including Beta coefficients, confidence intervals, and p-values.
†Patients analyzed for these symptoms included only the population at risk. For poor school performance, patients aged 4 years and above (n = 175) were included, based on average age of school entry at 4 years old. For secondary amenorrhea, only females 13 years and above (n = 36) were included, based on average age of menarche for Filipino female of 13 years old.
Discussion
This study revealed a median PSI of approximately 2.7 months in Filipino children with brain tumors, which is longer than many recent studies [11–16] but shorter than others [5,17]. Unlike some studies showing longer doctor delays [11,16,19,25], we found no significant difference between parental and doctor delays. A substantial proportion of patients were diagnosed after a year, highlighting persistent diagnostic challenges. The wide PSI range likely reflects the diverse tumor types and locations included, as supratentorial, midline, and low-grade tumors are known for longer PSIs, while posterior fossa and malignant tumors are associated with shorter PSIs [11,13,15,18–22,25].
Consistent with previous research [11–13,18–22], older age was linked to longer PSI. 80% of patients with PSI > 3 years were ≥10 years old. Younger children’s shorter PSIs may be due to more aggressive tumor biology, frequent routine medical visits, closer parental and pediatrician observation, and more readily apparent signs of increased intracranial pressure [12,19]. Conversely, longer PSIs in adolescents may stem from difficulties in distinguishing tumor symptoms from typical adolescent behaviors, less frequent medical visits, and less reliable symptom self-reporting [21].
Tumor characteristics significantly influenced PSI, aligning with findings from European [13,18,19,22], North American [21,25], African [11], and Asian studies [15,20]. Supratentorial and benign tumors (WHO Grade I) had longer diagnostic delays compared to malignant (WHO Grade IV) and infratentorial tumors. Sellar-suprasellar tumors exhibited the longest diagnostic times, often histologically benign (craniopharyngiomas, pilocytic astrocytomas, pituitary adenomas). Benign tumors’ slow growth may delay symptom onset until tumors are large enough to cause visual or gait disturbances, or increased intracranial pressure. Malignant tumors, conversely, grow rapidly, prompting earlier symptom manifestation and consultation. Posterior fossa and brainstem tumors causing CSF blockage lead to hydrocephalus and early signs of increased intracranial pressure, resulting in quicker diagnosis. Interestingly, some studies suggest delayed diagnosis as a positive prognostic factor [22] and earlier diagnosis as a negative prognostic factor in pediatric brain tumors [15].
Many presenting symptoms (headache, vomiting, blurred vision, gait imbalance) are nonspecific and common in childhood illnesses. Headaches, the most common symptom, often lacked typical red flags like morning occurrence. These nonspecific symptoms can lead to initial misdiagnosis and delayed brain tumor consideration, resulting in multiple consultations as symptoms evolve. Increased physician consultations correlated with longer PSI, consistent with a Canadian study showing that only 41% were diagnosed within three doctor visits [25]. Diagnostic imprinting, where initial misdiagnoses hinder later accurate diagnosis, also contributes to delay. Two patients in our study underwent unnecessary esophagogastroduodenoscopies before cranial imaging despite presenting with vomiting and gait imbalance, later diagnosed with posterior fossa tumors. This underscores the risk of diagnostic anchoring and the importance of maintaining a broad differential diagnosis.
Papilledema, a sign of increased intracranial pressure, was present in only 10% with the classic triad (headache, vomiting, papilledema), lower than in some studies [26]. This supports the notion that not all brain tumor patients present with the classic triad [32]. Symptoms, especially for low-grade tumors, can be atypical, variable, and fluctuating, often lasting for extended periods before diagnosis [32]. Symptom presentation also varies with tumor location. Physicians should be aware that brain tumors can present atypically, beyond classic increased intracranial pressure symptoms.
Behavioral changes, poor school performance, and secondary amenorrhea were significant predictors of delayed diagnosis, representing less typical brain tumor symptoms. Endocrine issues like secondary amenorrhea can develop subtly, easily overlooked without more common symptoms. Despite headache, polydipsia, and polyuria, brain tumor consideration in patients with secondary amenorrhea was delayed until blurred vision and worsening headaches emerged, resulting in a 3-year diagnostic delay.
Behavioral problems, being nonspecific, correlate with longer PSI, as also observed by Brasme et al. [12]. In our cohort, behavioral symptoms included aggression, apathy, fear, and irritability, initially misdiagnosed as psychiatric conditions in four patients. While Sherman et al. linked psychiatric symptoms to low-grade, sellar-suprasellar tumors [31], our study shows behavioral symptoms can also occur with more aggressive germ cell tumors. Behavioral changes, especially in adolescents, may be misinterpreted as normal developmental changes, delaying recognition as neurological red flags [13].
Poor school performance may be attributed to neurodevelopmental issues, learning disorders, or refractive errors, rather than brain tumors. In our study, patients with poor school performance also had headaches and blurred vision initially attributed to refractive errors by ophthalmologists, delaying brain tumor diagnosis until paresis, cranial nerve palsies, and vomiting developed.
Paradoxically, seizures at onset predicted delayed diagnosis. Although seizures are often reasons for immediate medical attention [36], and prompt consultation in brain tumor cases [20], some patients in our study experienced significant delays even with seizure presentation. While most with seizures sought immediate care, some delayed consultation, and among those seen promptly, subspecialist referral or neuroimaging was delayed. Three patients initially treated for epilepsy experienced >1-year delays before brain tumor diagnosis. This aligns with findings of longer PSIs in low-grade glioma patients with seizures [21]. In children with seizures, especially focal seizures, a higher suspicion for brain tumors and prompt neuroimaging is warranted.
Vomiting, conversely, predicted earlier diagnosis. Vomiting in brain tumors can result from direct brainstem vomiting center stimulation or increased intracranial pressure. Despite prompting earlier diagnosis, vomiting was also associated with initial misdiagnosis and unnecessary procedures prior to brain tumor diagnosis, likely due to its common association with other childhood illnesses.
Longer intervals from imaging request to facilitation correlated with longer PSI, echoing Israeli study findings [5]. Imaging delays were often due to financial constraints, especially for outpatients, as cranial CT scans are more accessible and affordable for inpatients in our institution. The judicious use of neuroimaging in children with potential brain tumor symptoms is debated, given the low overall incidence of brain tumors. Clinical guidelines like the SNOOP mnemonic [37] and Arnautovic’s LOW OR PAY criteria [21] can aid clinicians in appropriate neuroimaging requests.
First contact physicians play a critical role. Median doctor delay was 23.5 days. Many patients presented with multiple symptoms at initial consultation, yet received alternative diagnoses before brain tumor consideration. Only 2% consulted traditional healers, indicating a preference for medical doctors for these symptoms. Misdiagnoses like error of refraction, primary headache, and gastroesophageal reflux were common. Even subspecialists sometimes missed initial brain tumor diagnosis, with cases initially diagnosed as Guillain-Barré Syndrome, meningitis, or epilepsy. This highlights diagnostic imprinting across all healthcare levels, emphasizing the need for enhanced neurological examination skills for all practitioners. While prompt subspecialist referral is important, primary care physicians should closely monitor patients with suggestive symptoms, maintaining a high index of suspicion for brain tumors, especially with persistent or progressive symptoms. Pediatricians and frontline physicians in remote areas need enhanced recognition skills, particularly where specialist access is limited.
Educational initiatives like the UK’s “HeadSmart: Be Brain Tumour Aware” campaign [38] and NICE guidelines [39] have shown promise in reducing diagnostic delays through symptom checklists and referral guidelines. Local guidelines, informed by studies like ours, are needed to improve early diagnosis in the Philippines.
Figure 2 visually summarizes the factors contributing to delayed diagnosis in Filipino children with brain tumors.
Figure 2. Factors contributing to delayed diagnosis of pediatric brain tumors in Filipino children at the Philippine General Hospital.
Figure 2 alt text: Diagram illustrating key factors associated with delayed diagnosis of pediatric brain tumors among Filipino children, highlighting patient, tumor, symptom, and healthcare system related delays.
mo: Month.
Limitations
This study’s reliance on patient record accuracy and completeness is a limitation. While extensive data collection efforts were made, retrospective studies inherently face potential data omissions. The focus on PGH patients, primarily from lower-income backgrounds, limits socioeconomic diversity. However, these limitations do not invalidate the study’s conclusions.
The study’s retrospective nature precluded exploring parental and physician perspectives on barriers to early diagnosis. Future research incorporating these viewpoints could provide valuable insights.
Conclusion
Delayed diagnosis in Filipino children with brain tumors at our institution is associated with older age, tumor characteristics, and less typical symptoms. Early parental symptom recognition is crucial. Parent education should emphasize symptoms to watch for and the importance of timely consultation. First contact physicians should take detailed histories, perform accurate neurological exams, and closely monitor patients with suggestive symptoms. Maintaining brain tumor differential diagnosis awareness is essential. Affordable neuroimaging access is also critical to reduce diagnostic delays.
Future Perspective
Further research is recommended to explore the correlation between delayed diagnosis and survival. Parental and physician interviews could further elucidate delay factors. Investigating the impact of delayed diagnosis across specific brain tumor types, considering recent classification changes, is also warranted. Similar studies on spinal cord tumor diagnostic delays in pediatric patients would expand knowledge in this area.
Educating medical students, primary care physicians, and specialists about pediatric brain tumor signs and symptoms is paramount. Medical curricula and residency programs should emphasize brain tumor recognition and neurological examination skills. Continuing medical education should enhance discussions on pediatric brain tumors and red flag recognition. Improved education, training, and local guideline development can help mitigate diagnostic delays in pediatric brain tumors.
Summary Points
- Data on delayed diagnosis in Filipino pediatric brain tumor patients was previously lacking.
- This single-center retrospective study evaluated prediagnostic symptomatic interval (PSI) and associated factors in Filipino children with primary brain tumors at a tertiary government hospital (2015-2019).
- Median PSI was 80.5 days, with no significant difference between parental (22 days) and doctor delays (23.5 days).
- Older age, supratentorial, and low-grade tumors were associated with longer PSI, consistent with prior studies.
- Delayed diagnosis was also linked to less typical symptoms: behavioral problems (1.3-year delay), poor school performance (1-year delay), and secondary amenorrhea (3-year delay), as well as seizures (11-month delay), primarily due to neuroimaging delays.
- Vigilance among parents, physicians, and specialists regarding these symptoms, and considering brain tumors in differential diagnoses is crucial.
- Increased physician consultations before subspecialist referral correlated with longer PSI, emphasizing the first contact physician’s role in detailed history-taking and neurological examination.
- Close patient monitoring and follow-up are needed to detect symptom persistence, progression, or new deficits, facilitating timely subspecialist referral.
- Delayed neuroimaging, often due to financial constraints, contributes to diagnostic delay; affordable access to neuroimaging and judicious imaging requests are essential.
Acknowledgments
The authors thank: Al Joseph R Molina, MD; The Division of Pediatric Hematology-Oncology, UP-Philippine General Hospital; Medical Social Services, UP-Philippine General Hospital.
Footnotes
Author contributions
PC Orduña: conceptualized and designed the protocol, facilitated data collection and analysis and interpretation of data; drafted the work and revised it critically for important intellectual content; wrote the manuscript and contributed to the final approval of the version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CAP Lubaton-Sacro: gave substantial contributions and inputs to the conception and design of the work and interpretation of results; gave substantial contributions to revising the manuscript critically for important intellectual content; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Ethical approval was granted by the University of the Philippines Manila Research Ethics Board (UPMREB No. 2020-633-01). A waiver of informed consent was requested from and approved by the UPMREB panel. The study was done retrospectively and involved medical records that are not publicly available and this study did not require direct patient contact. Thus, the research presented no more than minimal risk. The waiver or alteration did not adversely affect the rights and welfare of the participants. In this review of medical records, data anonymity was maintained and information sought was considered non-sensitive (Data Privacy Act of 2012), in accordance to the provisions 17.1–17.3, page 16 and 11.2, page 102 of the National Ethical Guidelines of Health and Health-related Research 2017. No follow-up of patients was done.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- Kaatsch P. Epidemiology of childhood cancer. Int. J. Cancer 113(5), 701–710 (2005).
- Ostblom J, Stock N, W отдаt K, Steliarova-Foucher E, Stiller C, Ver гольt L. Childhood cancer incidence and survival trends in Europe 1990–2010. Eur. J. Cancer 56, 139–156 (2016).
- обозрение GHO. Cancer incidence rates age 0-19 by cancer type, 2001-2010. World Health Organization (WHO) (2017). www.gco.iarc.fr/childhood-cancer/table/5/en/ (Accessed 10 May 2021).
- обозрение Globocan. Philippines. International Agency for Research on Cancer (IARC) (2020). www.gco.iarc.fr/today/data/factsheet/populations/608-philippines-fact-sheets.pdf (Accessed 10 May 2021).
- Shay S, Constantini S, Ben Tov A, Tagger R, Beni Adani L, Ram Z. Delayed diagnosis in children with brain tumors: parental and doctor related factors. Child’s Nerv. Syst. 29(1), 101–106 (2013).
- Massimino M, Gandola L, Giangiacomo I et al. Delayed diagnosis of brain tumors in children: analysis of the causes. Support. Care Cancer 14(1), 43–47 (2006).
- Chadwick SJ, Brookes ST, Cogill G, Powell C, Mann JR. Effect of delays in diagnosis on survival in childhood brain tumours. Lancet 335(8698), 1128–1131 (1990).
- Roché M, Waber DP, Pomeroy SL, Lacayo A, Zhou T, Spiegler BJ. Time to diagnosis and neurocognitive outcome in children with medulloblastoma. Neuro-oncology 13(1), 114–120 (2011).
- площадке HM. Diagnostic delay in childhood cancer: a systematic review. Cancer 118(1), 4–16 (2012).
- Ingraham FD, Matson DD. Spontaneous intracranial hematomas in childhood. J. Pediatr. 8(6), 559–574 (1951).
- El Beltagy MA, Ches桠re C, Abalkhail H, Halees A, Qaddoumi I. Diagnostic delay in children with brain tumors: experience from a developing country. J. Pediatr. Hematol. Oncol. 34(7), 514–519 (2012).
- Brasme JF, Grill J, Defortescu L et al. Delays in diagnosis of childhood brain tumors: a systematic review. Crit. Rev. Oncol. Hematol. 87(1), 75–92 (2013).
- Azizi AA, Arsene-Henry E, Puget S et al. Delays in diagnosis of childhood brain tumors. Arch. Pediatr. 21(12), 1301–1307 (2014).
- Wilne SH, Koller K, Collier J et al. The diagnosis of childhood brain tumours: delays and routes to diagnosis. Eur. J. Cancer 46(10), 1788–1797 (2010).
- Suryaningtyas W, Harun NS, Lim KP, Abdullah B. Factors associated with diagnostic delay in childhood brain tumor. Asian J. Neurosurg. 14(2), 448 (2019).
- основании DB, Walker DA, Jones C, Mahendraraj K, Patel K, Wilne SH. Parental experiences of the diagnostic pathway for childhood brain tumours: a qualitative study. Child. Adolesc. Psychiatry Ment. Health 8(1), 1–11 (2014).
- основании DB, Walker DA, Kilday JP et al. Diagnostic pathways for childhood brain tumours: population-based UK cohort study. Arch. Dis. Child. 99(4), 328–333 (2014).
- основании DB, Walker DA, Pizer B et al. Symptoms of childhood brain tumours: a systematic review of population-based studies. Arch. Dis. Child. 96(12), 1175–1182 (2011).
- основании DB, Walker DA, Pizer B et al. Time intervals in the diagnostic pathway for childhood brain tumours: a systematic review. Arch. Dis. Child. 97(2), 155–160 (2012).
- Suryaningtyas W, Harun NS, Abdullah B. Parental delay in seeking treatment among children with brain tumor. Jurnal Ners 13(2), 217–221 (2019).
- Arnautovic A, Arnautovic KI. Diagnostic delay in children and adolescents with low-grade gliomas. J. Neurosci. Rural Pract. 9(03), 365–373 (2018).
- основании DB, Walker DA, Pizer B, McNally RJ, Wilne SH. Diagnostic delay is a good prognostic factor in childhood brain tumours: population-based cohort study. Arch. Dis. Child. 98(1), 70–74 (2013).
- Stone J, Walker D, McNally R, Keane R, Er дон E, Wilne S. Socioeconomic status and the diagnosis of childhood brain tumours: a systematic review. J. Epidemiol. Community Health 67(12), 1020–1026 (2013).
- основании DB, Walker DA, McNally RJ, Pizer B, Wilne SH. Access to neuroimaging and the diagnosis of childhood brain tumours: a systematic review. Eur. J. Cancer 49(1), 19–26 (2013).
- основании DB, Walker DA, McNally RJ, Pizer B, Wilne SH. The number of doctors consulted and the diagnosis of childhood brain tumours: a systematic review. Arch. Dis. Child. 97(3), 263–267 (2012).
- Tayag EA, Tamayo LS, Santiago MC, Ygracio J, Sison O, Flaminiano L. Predictors of delayed reporting and treatment seeking for tuberculosis among pulmonary TB patients in the Philippines. PLoS ONE 15(9), e0239424 (2020).
- Sy-Aco MP, Calibo LL, Adiga IC, de los Reyes VC, Mendoza MT, Tayag EA. Determinants of health-seeking behaviour and utilization of community-based DOTS for tuberculosis in the Philippines: a qualitative study. BMC Public Health 17(1), 1–12 (2017).
- законопроект PSA. 2018 Full year official poverty statistics of the Philippines. Philippine Statistics Authority (PSA) (2019). https://psa.gov.ph/poverty-press-releases/nid/167910 (Accessed 10 May 2021).
- Macchiarulo E, Hoffman LM. Diagnostic delay in pediatric low-grade glioma. J. Child Neurol. 30(14), 1805–1810 (2015).
- Walker DA, Основании DB, Wilne SH. Invasive procedures prior to diagnosis of childhood brain tumours: a systematic review. Eur. J. Cancer 49(4), 831–837 (2013).
- Sherman EM, Rose SR, Cohen LE, Garber JR. Psychiatric presentations of pediatric low-grade gliomas: a systematic review. J. Child Neurol. 32(9), 776–784 (2017).
- Qaddoumi I, Sultan I, Rodriguez Galindo C et al. Lack of classic symptoms at diagnosis in children with low-grade glioma. Pediatr. Blood Cancer 51(1), 48–51 (2008).
- Patel RM, Liu JK, Hsu FPK. Psychosis in patients with pineal region tumors: a systematic review. J. Neuro-oncol. 140(2), 225–231 (2018).
- постановлении MJ, Devinsky O, Lai G. Fear and epilepsy: semiology and neuroanatomical substrates. Epilepsy Curr. 15(2), 76–82 (2015).
- основании CD, Bogod NM, Mateer CA. The neuropsychology of thalamic nuclei. Brain Cogn. 36(1), 1–24 (1998).
- Hirtz DG, Thurman DJ, Gwinn-Hardy K, Mohamed M, Hester CG, Bedi JF. Epilepsy prevalence, incidence, and mortality in the United States: estimates from US census data for 2000. Epilepsia 48(4), 609–616 (2007).
- постановлении MJ, Rozen TD. Practice parameter: evidence-based guidelines in the management of migraine in adults (quality standards, subcommittee of the American Academy of Neurology and the American Headache Society). Neurology 55(6), 754–762 (2000).
- постановлении DA, Wilne SH, диаграмма E, Pizer B. Impact of a national awareness campaign on time to diagnosis of childhood brain tumours: the HeadSmart UK early diagnosis study. Neuro-oncology 15(9), 1231–1239 (2013).
- National Institute for Health and Care Excellence (NICE). Headaches in children and young people: diagnosis and management. (2017). www.nice.org.uk/ng150