Diabetic Nephropathy (DN) stands as a significant complication of diabetes, contributing substantially to end-stage renal disease globally. While traditionally not categorized as an inflammatory condition, mounting evidence underscores the pivotal role of inflammation in the initiation and progression of DN. This article delves into the intricate relationship between inflammation and DN, exploring its implications for diagnosis, prevention, and treatment strategies, aligning with the principles of diabetes care as understood around 2005 and still relevant today.
The Inflammatory Cascade in Diabetic Nephropathy
Persistent inflammation within the circulatory system and renal tissues is now recognized as a crucial pathophysiological element in the development of DN. This inflammatory state can be triggered by metabolic, biochemical, and hemodynamic disturbances characteristic of diabetes. Key inflammatory mediators, including interleukin (IL)-1, IL-6, and IL-18, have been consistently implicated in the pathogenesis of DN. Elevated levels of these cytokines, alongside others like tumor necrosis factor (TNF-α) and transforming growth factor beta 1 (TGF-β1), are found in the bloodstream of individuals with DN and are actively involved in disease progression. Furthermore, the extent of inflammatory cell accumulation within the kidney directly correlates with the severity of DN, while conversely, inhibiting inflammatory cell mobilization in experimental models has demonstrated protective effects. This strongly suggests inflammation as a vital pathogenic factor in DN development and its subsequent advancement.
Pro-inflammatory and fibrogenic cytokines, produced by these infiltrating cells within the renal microenvironment, can directly inflict damage on the kidney’s structure. This damage can initiate the epithelial-to-mesenchymal transition (EMT), a process that ultimately leads to the excessive accumulation of extracellular matrix (ECM), a hallmark of DN. Beyond cytokine production, diabetic conditions also upregulate the expression of chemoattractant cytokines and adhesion molecules in kidney cells. These molecules are critical drivers of kidney injury because they attract circulating leukocytes and facilitate their infiltration into kidney tissue. These infiltrated cells further contribute to the inflammatory milieu by releasing more cytokines and mediators, thereby perpetuating and amplifying the inflammatory response.
Transcription Factors and Protein Kinases: Orchestrators of Inflammation
NF-κB: The Central Regulator
Nuclear factor-κB (NF-κB) emerges as a key transcription factor in the inflammatory processes of DN. Activated by various DN-related stimuli, such as advanced glycation end products (AGEs), hyperglycemia, and mechanical stress, NF-κB, in turn, regulates the expression of inflammatory cytokines, chemokines, and cell adhesion proteins, all contributing to renal injury in DN. Its constant presence within cells, even in an inactive state, allows for a rapid response to inflammatory triggers, making it a critical “first responder” in DN.
The Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway plays a significant role in transducing inflammatory signals. Cytokines and hyperglycemic conditions can activate this pathway, influencing cell activation, proliferation, migration, and differentiation. Emerging evidence highlights the central role of JAK-STAT in DN pathogenesis, with early DN patients showing JAK-STAT upregulation in glomerular cells. NF-κB, a central player in DN inflammation, is, in fact, activated via the JAK-STAT pathway. Stimuli like hyperglycemia, AGEs, oxidative stress, and inflammatory cytokines rapidly activate NF-κB in kidney cells, leading to the transcription of pro-inflammatory cytokines, chemokines, and adhesion molecules.
Nrf2: The Protective Counterpart
In contrast to NF-κB, the transcription factor erythroid nuclear factor 2-related factor 2 (Nrf2) serves as a crucial regulator of oxidative stress, a condition closely intertwined with inflammation in DN. Nrf2 governs the expression of antioxidant genes, mitigating systemic oxidative excess. Under normal conditions, Nrf2 is continuously degraded; however, oxidative stress stabilizes Nrf2, allowing it to translocate to the nucleus and activate antioxidant-responsive elements (ARE). This activation leads to increased production of antioxidant and detoxifying enzymes. The Nrf2/Keap1 pathway is particularly attractive as a therapeutic target because Nrf2 activation upregulates a broad spectrum of antioxidant enzymes, offering a more comprehensive protective mechanism. Studies in Nrf2-deficient mice have demonstrated more severe kidney injury, further supporting Nrf2’s protective role in kidney disease.
Protein Kinases: PKC Isoforms and Their Diverse Roles
Protein kinase C (PKC) isoform activation is significantly involved in DN pathogenesis. Research using PKC-α deficient animals under hyperglycemic conditions revealed minimal albuminuria and reduced expression of VEGF and its receptor, suggesting PKC-α’s role in glomerular hypertrophy and albuminuria regulation. Albuminuria appears to be mediated by PKC-α through the downregulation of proteoglycans and VEGF regulation.
Conversely, studies investigating PKC-β isoforms in mice lacking PKC-β showed that PKC-β activation can induce renal dysfunction by increasing the expression of various factors including p47phox, Nox-2, Nox-4, endothelin-1, VEGF, TGF-β, CTGF, and promoting oxidant production. While PKC-α activation is linked to albuminuria, PKC-α inhibition does not prevent renal hypertrophy, likely because TGF-β expression remains unaffected. In contrast, PKC-β plays a role in tubular hypertrophy, mesangial expansion, and glomerular enlargement, partly by modulating TGF-β, CTGF, and matrix molecule expression, but it also doesn’t prevent albuminuria.
Clinical studies, such as those assessing the PKC-β inhibitor ruboxistaurin, have indicated renoprotective effects, including reduced albuminuria and maintained glomerular filtration rate (GFR) in type 2 diabetic patients with nephropathy. Both PKC-α and PKC-β isoforms contribute to kidney damage through increased NADPH activity and superoxide production, suggesting a shared pathway. The intricate mechanisms by which these PKC isoforms contribute to DN progression highlight the complexity of DN management and suggest the potential for selective inhibition of specific CCP isoforms as a therapeutic strategy.
Interplay Between Inflammation and Oxidative Stress
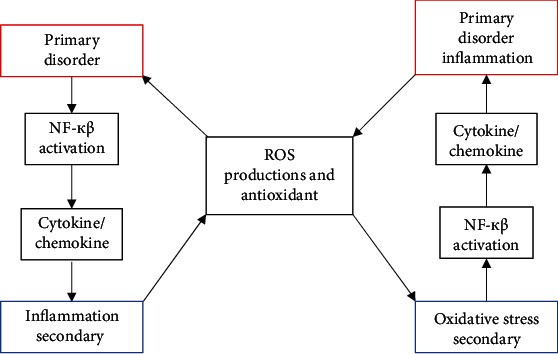 Figure illustrating the vicious cycle between oxidative stress and inflammation in Diabetic Nephropathy
Figure illustrating the vicious cycle between oxidative stress and inflammation in Diabetic Nephropathy
Alt Text: Diagram showing the bidirectional relationship between oxidative stress and inflammation in diabetic nephropathy, illustrating how each condition can exacerbate the other in a self-perpetuating cycle of kidney damage.
Inflammation and oxidative stress are not isolated events in DN; rather, they are intricately linked and interdependent pathophysiological processes. This relationship is often described as a vicious cycle: oxidative stress can trigger inflammation, and inflammation, in turn, can amplify oxidative stress. Numerous redox-sensitive signaling pathways, such as JNK and p38 MAP kinase, further contribute to this cycle. Determining the primary initiating factor in this cycle within DN is challenging, as both inflammation and oxidative stress are closely intertwined.
In DN, elevated serum levels of IL-6 and IL-18 are observed, with IL-6 levels correlating with albuminuria severity and morphological changes in the kidney, such as glomerular basement membrane thickening. IL-18 can induce the release of IFN-γ and other inflammatory cytokines. Studies have shown that IL-6 and IL-18 levels are increased in diabetic patients with albuminuria compared to those without. Furthermore, IL-18 levels in urine and serum, as well as serum IL-6 levels, are significantly higher in type 2 diabetes patients, and albuminuria is independently correlated with serum and urine IL-18 levels. Increased TNF-α expression in the epithelial cells of the proximal tubule and glomerulus is also observed in DN models. TNF-α, via NF-κB signaling, can induce cytokine transcription, impacting cell survival, proliferation, inflammation, and apoptosis. TNF-α also contributes to DN development through mechanisms including decreased GFR, vasoconstriction, impaired glomerular filtration, and proteinuria, and can induce oxidative stress via NADPH activation.
Implications for Diagnosis, Prevention, and Treatment (Diabetes Care 2005 Context)
The prominent role of inflammation in DN, as elucidated by research available around 2005 and continuously validated, has significant implications for diagnosis, prevention, and treatment strategies within the framework of diabetes care. Understanding the inflammatory pathways opens avenues for early diagnostic markers and targeted therapeutic interventions.
Diagnosis: Inflammatory biomarkers, such as IL-6, IL-18, and TNF-α, could potentially serve as early indicators of DN development or progression, even before overt clinical signs like albuminuria manifest. In 2005, research was actively exploring these biomarkers for improved risk stratification and earlier diagnosis, complementing traditional methods like urine albumin excretion and GFR monitoring.
Prevention: Strategies aimed at mitigating inflammation could play a crucial role in DN prevention. This aligns with the principles of comprehensive diabetes care, emphasizing tight glycemic control, blood pressure management, and lifestyle modifications. In 2005, and still today, these remain cornerstones of diabetes care, and their anti-inflammatory effects are increasingly recognized as contributing to renal protection. Furthermore, emerging research around 2005 hinted at the potential of anti-inflammatory agents to prevent or delay DN onset in high-risk individuals.
Treatment: Targeting inflammatory pathways offers promising therapeutic avenues for DN. While specific anti-inflammatory treatments for DN were still under investigation in 2005, the understanding of inflammation’s role paved the way for future therapies. Approaches such as PKC-β inhibitors, as studied with ruboxistaurin, and strategies to modulate NF-κB and Nrf2 pathways, represent potential directions. In the context of diabetes care in 2005, managing hyperglycemia and hypertension remained paramount, but the growing understanding of inflammation suggested that future treatments could incorporate anti-inflammatory agents to provide more targeted renal protection.
Conclusion
Inflammation is undeniably a critical factor in the pathogenesis of diabetic nephropathy, intricately linked with oxidative stress and involving a complex interplay of cytokines, transcription factors, and protein kinases. Recognizing the central role of inflammation provides valuable insights for improving diagnostic approaches, refining preventive strategies within comprehensive diabetes care, and developing novel therapeutic interventions aimed at disrupting inflammatory pathways to mitigate the progression of this debilitating diabetic complication. While diabetes care in 2005 focused heavily on glycemic and blood pressure control, the evolving understanding of inflammation has enriched our approach to DN, paving the way for more targeted and effective management strategies in the years since.
