Introduction
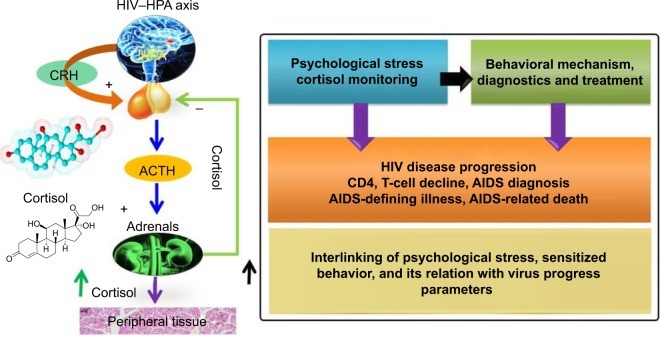
The progression of Human Immunodeficiency Virus (HIV) infection to Acquired Immunodeficiency Syndrome (AIDS) is significantly influenced by the patient’s immune system. Psychological stress, indicated by biomarkers like cortisol, plays a crucial role in this progression and overall diagnosis.1–7 HIV infection triggers a cascade of hormonal and immune responses, starting with the hypothalamus releasing corticotropin-releasing hormone. This leads to cytokine release, an inflammatory response against the viral infection, further stimulating the hypothalamus–pituitary–adrenal (HPA) axis. This complex interplay results in psychological stress and activation of the corticotropic axis. Studies have shown that HIV patients often exhibit increased glucocorticoid-receptor expression and reduced substrate-binding affinity, leading to elevated plasma cortisol levels that correlate with adrenocorticotropic hormone levels, impacting adrenal function.1,2 The hypothesized relationship between cortisol secretion and behavioral changes in HIV-infected patients is visually represented in Figure 1.8
Figure 1.
Figure 1: HPA Axis Dysregulation in HIV Progression. This diagram illustrates the impaired adrenal reverse and increased peripheral glucocorticoid levels characteristic of HIV infection. Understanding this mechanism is crucial for developing point-of-care diagnostics for stress biomarkers like cortisol.
Abbreviations: HPA, hypothalamus–pituitary–adrenal; HIV, human immunodeficiency virus; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; AIDS, acquired immunodeficiency syndrome.
While the precise correlation between cortisol levels and HIV-infection stages remains under investigation,9 research indicates that untreated HIV-positive patients tend to have higher plasma cortisol levels compared to those undergoing treatment.5 Furthermore, a negative correlation between CD4 cell count and plasma cortisol levels has been reported, suggesting that cortisol may contribute to the suppression of beneficial Th-1 cytokines, favoring Th-2 cytokines and potentially accelerating HIV progression.2,5,8 The significance of cortisol detection in relation to various diseases is increasingly recognized.3,7,10–13 Monitoring diurnal cortisol levels, both in laboratory settings and at the point of care (POC), is vital for understanding and managing stress-related behavioral changes.11,12 Disruptions in the diurnal cortisol rhythm can weaken the immune system and are linked to conditions like Cushing’s disease, Addison’s disease, and posttraumatic stress disorder.11,12
Enzyme-linked immunosorbent assays (ELISA) are considered the gold standard for cortisol detection. However, ELISA’s limitations, including long measurement times, complex procedures, need for skilled personnel, and reduced sensitivity at lower concentrations, restrict its use primarily to laboratory settings. The need for rapid and accessible cortisol detection at the clinic or POC is clear, especially for understanding daily behavioral patterns and stress levels in individuals.14
Electrochemical cortisol immunosensors are emerging as a promising alternative due to their rapid, selective, and sensitive detection capabilities, even at picogram per milliliter (pg/mL) levels.11–13 Advancements in nanotechnology, microelectronics, and miniaturized transducers are enhancing the performance of these devices.11,12,15–19 Integrating cortisol sensors with microfluidic systems and miniaturized potentiostats (M-P) is paving the way for compact and portable cortisol monitoring systems.11,18,20 Current research is focused on developing these systems for POC applications, aiming towards personalized health monitoring.14,18,21–23 While electrochemical immunosensors using self-assembled monolayers (SAMs) and nanostructures for cortisol detection exist, their practical application in real-world sample analysis remains limited.16–18 Therefore, establishing a robust electrochemical sensing protocol for cortisol detection in bodily fluids like plasma, blood, saliva, urine, and interstitial fluid at the POC is critically important.
This study presents a novel electrochemical sensing device capable of cyclic voltammetry (CV) for detecting plasma cortisol levels in HIV-positive patients. We validated this method against the ELISA technique, achieving a strong correlation within 2%–5% variation. Our findings suggest that this method holds significant potential for stress management, particularly in personalized healthcare monitoring for HIV-positive patients, leveraging a low-cost miniaturized potentiostat for point-of-care diagnosis.
Materials and Methods
Electrochemical Immunosensor Fabrication
We utilized Dithiobis(succinimidyl propionate) (DTSP), sodium borohydride (NaBH4), monoclonal anticortisol antibody (anti-Cab), cortisol, and other chemicals sourced from Sigma-Aldrich, used without further purification. A phosphate-buffered saline (PBS) solution (10 mM, pH 7.4) was prepared and used to dilute anti-Cab (1 mg/mL) and cortisol to desired concentrations.
The detailed procedure for fabricating the electrochemical cortisol sensor is available in our previously published works.18,24 Briefly, interdigitated gold microelectrodes (IDE-Au; ~5 μL chamber volume, 10 μm electrode width and gap), electrochemically cleaned, were treated with a 2 mg/mL DTSP solution (reduced with NaBH4 [10 mg/mL in DI water]) for 2 hours to form SAMs. After washing with acetone and DI water to remove unbound DTSP, 5 μL of anti-Cab (1 mg/mL) was covalently immobilized onto the DTSP-SAM/IDE-Au electrode for 2 hours via amide bond formation. The resulting Anti-Cab/DTSP-SAM/IDE-Au bioelectrodes were washed with PBS (pH 7.4, 10 mM). To block nonspecific binding sites, 5 μL of ethanolamine (EA, 2 mg/mL) was applied for 10 minutes, followed by DI water washing. The final EA/anti-Cab/DTSP/IDE-Au immunoelectrodes were stored at 4°C for future use.
LTCC-Based Microfluidic Manifold Design
A low-temperature co-fired ceramic (LTCC) microfluidic manifold was designed, featuring two microchannels connected to a reaction chamber directly above the biosensor chip. These channels facilitated sample introduction and waste removal using a three-way solenoid fluidic valve. Computational fluid dynamics, using Comsol Multiphysics® software, optimized the fluid flow profiles for efficient washing, crucial for preventing contamination. Detailed design optimization of the LTCC microfluidic manifold is described in our earlier publication.18
The LTCC microfluidic chip comprised three layers of green tape (DuPont 951). The bottom layer formed the reaction chamber (5 mm diameter, 10 μL volume) interfacing with the sensor. The middle layer contained inlet and outlet microchannels, and the top layer enclosed the microchannels and provided ports for fluidic connectors. Green tapes were patterned using a computer-controlled CO2 laser (10.6 μm, 35 μm spot size), aligned, and laminated using an isostatic hot press (Phi-Tulip) at 150°C and 3,000 psi for 15 minutes. Laminated layers were then fused to LTCC by heating at 850°C in oxygen. Tygon tubing connected the microfluidic manifold’s inlet and outlet ports via polydimethylsiloxane (Dow Corning). Fluids were delivered using a programmable two-syringe pump system (New Era Pumps). The microfluidic device, integrated with the biosensor chip, was mounted in a portable acrylic fixture and connected to the M-P for electrochemical measurements.18
During measurement, 10 μL of sample was introduced at 10 μL/min, incubated on the immunosensor for 30 minutes, and then washed with 30 μL of PBS (pH 7.4) at 10 μL/min for 3 minutes. For electrochemical experiments, 5 μL of measurement buffer (PBS, pH 7.4) with 5 mM Fe(II)/Fe(III) redox moieties was introduced into the reaction chamber. Solenoid three-way valves (Lee) controlled fluid flow.
Miniaturized Potentiostat Design
Figure 2 illustrates the electrochemical cortisol immunosensing protocol, immunosensor preparation, LTCC microfluidic manifold architecture, the reconfigured M-P chip (LMP9100) for electrochemical measurement, and a comparison of CV curves obtained with the M-P and a conventional potentiostat. The M-P preparation details are available in our previous publication.14 In brief, an LMP91000 board from Texas Instruments was reconfigured to perform full-range CV using a three-electrode system. An external central processing unit with SensorAFE Designer software and a USB connection was used for CV measurements with the LMP91000. A BeagleBone microcontroller unit was added for device portability. The microcontroller controlled the LMP91000EVM via the I2C interface.
Figure 2.
Figure 2: Integrated System for Point-of-Care Cortisol Detection. This schematic showcases the step-by-step fabrication and integration of the electrochemical cortisol immunosensor with the LTCC microfluidic manifold and the miniaturized potentiostat, enabling cortisol detection in HIV-infected patients at the point of care.
Notes: A BeagleBone microcontroller interfaces with the reconfigured LMP91000EVM for full-range CV using three-electrode systems. Data is stored on the BeagleBone SD card and accessed via SSH. Reproduced from Cruz AFD, Norena N, Kaushik A, Bhansali S. A Low-cost Miniaturized Potentiostat For Point-of-care Diagnosis. Biosens Bioelectron. 2014;62:249–254.16 The immunosensor is created by immobilizing anti-Cab on a SAM-modified IDE-Au electrode. The LTCC microfluidic chip is fabricated by laser-cutting and fusing green tapes at 780°C. The fully assembled LTCC microfluidic manifold is integrated with a cortisol biosensor chip.18 This system automates sample handling and electrochemical measurements using a reconfigured M-P for full-range CV. Modifications to the LMP9100 chip included removing a two-wire jumper and repositioning the J_MENB jumper for three-electrode measurements and manual configuration. The ADC converts the analog output from the potentiostat chip to a digital signal for the microcontroller via the LMP91000’s I2C interface.14 This electrochemical immunosensing method is used to detect cortisol and plasma cortisol, providing a valuable tool for stress management and personalized therapeutics.
Abbreviations: LTCC, low temperature co-fired ceramic; M-P, miniaturized potentiostat; HIV, human immunodeficiency virus; CV, cyclic voltammetry; anti-Cab, anticortisol antibody; SAM, self-assembled monolayer; IDE, interdigitated electrode; ADC, analog digital converter; SPI, serial peripheral interface; ADC. analog to digital converter; SAM. self-assembled monolayer; SSH. secure shell.
For three-electrode measurements, a two-wire jumper pin was removed, and a J_MENB jumper was connected for manual operation. The reconfigured LMP91000EVM output voltage, proportional to the working electrode current, was acquired by the microcontroller via the ADC161S626 analog-digital converter on the LMP91000EVM’s serial peripheral interface. Optimized programming enabled the LMP91000EVM to perform CV across a −0.6 V to 0.6 V potential range, varying the scan rate. The output voltage, proportional to cell current, was calculated using equation (1), considering the transimpedance-amplifier transfer function and CV measurement parameters:
| Iout=Vout−VINT−ZTIAgain | (1) |
|---|

The M-P’s CV performance on Au-IDE was validated by comparing it to results from a Metrohm Autolab potentiostat, demonstrating comparable performance.16
Electrochemical Measurement Protocol
CV-based electrochemical measurements were performed using the M-P at a 50 mV/s scan rate in 5 μL of PBS (pH 7.4) containing 5 mM (Fe[CN]6)3−/4− redox moieties, within a −0.6 to 0.6 V potential range. This characterized sensor fabrication and cortisol concentration detection. PBS pH 7.4 was chosen to maintain biomolecule activity, optimal for immunosensor electrochemistry. The EA/anti-Cab/DTSP-SAM/IDE-Au electrochemical immunosensor was used to detect cortisol in HIV-positive patient plasma samples.
HIV Patient Plasma Sample Collection
Ethical approval was obtained from the Florida International University (FIU) institutional review board, consistent with FIU and National Institutes of Health (NIH) policies. Informed consent was obtained from all blood donors recruited from the Borinquen Health Care Center, Miami. The study included both male and female HIV-infected patients. Clinical data, including race, sex, drug therapy, and CD4 levels (n=10), were collected and anonymized (Table 1).
Table 1.
Demographics of HIV-Positive Patient Cohort
| Patient identification | Age (years) | Sex (ethnicity) | HIV status (year of diagnosis) | Antiretroviral drugs | CD4 level |
|---|---|---|---|---|---|
| 358 | 26 | Male (African-American) | Positive (1998) | Yes | 363 |
| 261 | 35 | Male (African-American) | Positive (2010) | Yes | 624 |
| 398 | 41 | Male (Caucasian) | Positive (2004) | Yes | 235 |
| 377 | 44 | Male (African-American) | Positive (2005) | Yes | 134 |
| 250 | 45 | Male (African-American) | Positive (1996) | Yes | 671 |
| 256 | 46 | Male (African-American) | Positive (2010) | Yes | 326 |
| 486 | 48 | Male (African-American) | Positive (1999) | Yes | 700 |
| 202 | 50 | Male (African-American) | Positive (1995) | Yes | 237 |
| 487 | 43 | Female (African-American) | Positive (2002) | Yes | 654 |
| 405 | 49 | Female (African-American) | Positive (1993) | Yes | 608 |
Table 1: Patient Demographics and Clinical Characteristics. This table summarizes the demographic and clinical information of the HIV-positive patients participating in the study, crucial for understanding the context of cortisol level measurements.
Abbreviation: HIV, human immunodeficiency virus.
Blood samples for plasma were collected in K2 ethylenediaminetetraacetic acid (BD) tubes and centrifuged at 5,000 g for 15 minutes at 4°C. Plasma supernatants were stored at −20°C and thawed to room temperature before cortisol detection using both electrochemical immunosensors and ELISA (Cortisol (human) ELISA kit, Abnova Assays), following the standard ELISA protocol. For ELISA, 50 μL of 1:3 PBS diluted plasma samples were used.
Results and Discussion
Electrochemical Cortisol Sensor Performance
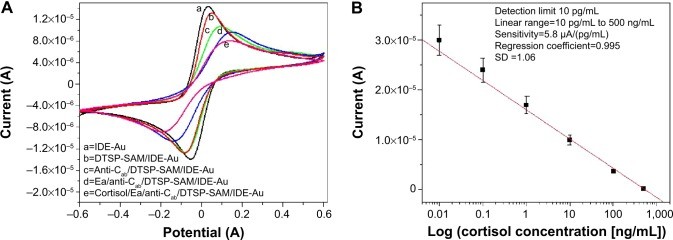
The reconfigured LMP91000-based M-P chip was used to assess the electrochemical immunosensor’s fabrication and cortisol sensing capabilities. Figure 3A displays the CV responses for each sensor fabrication step: IDE-Au electrode (curve a), DTSP-SAM/IDE-Au electrode (curve b), anti-Cab/DTSP-SAM/IDE-Au immunoelectrode (curve c), EA/anti-Cab/DTSP-SAM/IDE-Au immunoelectrode (curve d), and the response change after cortisol addition (curve e). Detailed explanations of sensor fabrication, characterization, and parameter optimization are available in our prior publications.18,24
The decrease in electrochemical response current after DTSP-SAM formation on IDE-Au (curve b) is due to the SAM’s insulating properties hindering electron transfer. Further reduction in current after anti-Cab immobilization (curve c) confirms antibody binding and increased resistance to electron transport. Blocking non-specific sites with EA (curve d) further decreased the current. Scan-rate dependent CV studies indicated a linear relationship between current magnitude and the square root of the scan rate, confirming a diffusion-controlled process at the electrode surface.
Figure 3.
[
Figure 3: Electrochemical Characterization and Calibration of the Cortisol Immunosensor. (A) Stepwise CV response during sensor fabrication, demonstrating the impact of each modification on electron transfer. (B) Calibration curve showing the sensor’s response to varying cortisol concentrations, essential for quantitative point-of-care diagnostics.
Notes: (A) CV response curves for IDE-Au (curve a), DTSP-SAM/IDE-Au (curve b), anti-Cab/DTSP-SAM/IDE-Au (curve c), EA/anti-Cab/DTSP-SAM/IDE-Au (curve d), and cortisol detection (curve e). (B) Calibration curve of the electrochemical cortisol sensor for cortisol concentrations from 10 pg/mL to 500 ng/mL (logarithmic scale).
Abbreviations: CV, cyclic voltammetry; IDE, interdigitated electrode; DTSP, dithiobis(succinimidyl propionate); SAM, self-assembled monolayer; EA, ethanolamine; anti-Cab, anticortisol antibody; SD, standard deviation.
Electrochemical cortisol immunosensing using the EA/anti-Cab/DTSP-SAM/IDE-Au sensor was conducted across cortisol concentrations from 10 pg/mL to 500 ng/mL using the M-P. A 30-minute incubation time ensured optimal antibody-cortisol binding. Unbound cortisol was removed by washing with PBS before electrochemical measurement. All measurements were performed in triplicate. A decrease in electrochemical response current was observed with increasing cortisol concentration, attributed to the insulating immunocomplex formation hindering electron transport.
The calibration curve (Figure 3B) shows a linear relationship between current response and the logarithm of cortisol concentration, described by the equation:
| Y=1.6×10−5+5.8×10−6⋅log(Cortisol concentration):R=0.995 | (2) |
|---|
This electrochemical cortisol immunosensor exhibited a wide linear range (10 pg/mL to 500 ng/mL), a low detection limit (10 pg/mL), high sensitivity [5.8 μA (pg mL)−1], and a regression coefficient of 0.995. Selectivity and stability studies, detailed in previous publications,18,24 confirmed high selectivity for cortisol over other biomarkers and stability for at least 28 days.
Electrochemical Immunosensing of Plasma Cortisol
The EA/anti-Cab/DTSP-SAM/IDE-Au immunosensor was used to measure plasma cortisol concentrations in HIV-positive patients. Plasma samples (5 μL) were incubated for 30 minutes, washed, and measured. The calibration curve (Figure 3A) was used to estimate cortisol concentrations. Triplicate measurements were averaged (Table 2), and validated against ELISA results (Table 2).
Table 2.
Comparison of Plasma Cortisol Detection by ELISA and Electrochemical Methods
| Patient identification | ELISA | Electrochemical |
|---|---|---|
| B%/Bo | Cortisol concentration (1:3) (ng/mL) | Final concentration(ng/mL) |
| 358 | 19.48 | 151 |
| 261 | 42.00 | 71 |
| 398 | 19.48 | 151 |
| 377 | 18.77 | 160 |
| 250 | 27.84 | 114 |
| 256 | 22.21 | 132 |
| 486 | 13.00 | 204 |
| 202 | 22.87 | 120 |
| 487 | 22.31 | 134 |
| 405 | 41.17 | 70 |
Table 2: Plasma Cortisol Levels in HIV Patients: Electrochemical vs. ELISA. This table compares cortisol concentrations in HIV-positive patient plasma samples, measured using both ELISA and our low-cost miniaturized potentiostat-based electrochemical method, demonstrating the comparable performance of the POC diagnostic tool.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; B%/Bo. mean absorbance of standards/means absorbance of negative control.
ELISA-Based Plasma Cortisol Detection
ELISA was performed using a 96-well plate with known cortisol concentrations (20, 50, 100, 200, 400, and 800 ng/mL) to create a calibration curve (Figure 4A). The ELISA kit achieved a detection limit of 1.5 ng/mL, a detection range of 20–8,000 ng/mL, sensitivity of 50 ng/mL (Iab), a regression coefficient of 0.989, and a standard deviation of ±1.06. This calibration curve was used to measure plasma cortisol in HIV-positive patient samples (Table 2). Figure 4B compares plasma cortisol levels measured by ELISA and electrochemical immunosensor.
Figure 4.
Figure 4: ELISA Calibration and Method Comparison for Plasma Cortisol Quantification. (A) ELISA calibration curve for plasma cortisol detection. (B) Comparative analysis of plasma cortisol concentrations in HIV-positive patients, measured using ELISA and the CV method, highlighting the agreement between laboratory standard and point-of-care methods.
Notes: (A) ELISA calibration plot for plasma cortisol detection in HIV-positive patients. (B) Comparison of plasma cortisol levels in HIV patients using ELISA and CV methods.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; CV, cyclic voltammetry; B%/Bo. mean absorbance of standards/means absorbance of negative control; au, arbitrary unit.
Table 3.
Performance Comparison: Electrochemical Miniaturized Potentiostat vs. ELISA for Cortisol Detection
| Detection technique | Detection limit | Detection range | Sensitivity | Measurement time |
|---|---|---|---|---|
| Electrochemical (Miniaturized Potentiostat) | 10 pg/mL | 10 pg/mL to 500 ng/mL | 5.8 μA (pg/mL) | 35 minutes |
| ELISA | 1.5 ng/mL | 20–8,000 ng/mL | 50 Iab (ng/mL) | ~12 hours |
Table 3: Performance Metrics of Cortisol Detection Techniques. This table summarizes and compares the key performance characteristics of the electrochemical method using a miniaturized potentiostat and the conventional ELISA method, emphasizing the advantages of the point-of-care approach in terms of speed and sensitivity.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Iab, intensity of absorption.
The different transduction principles of ELISA (absorbance intensity, Iab) and electrochemical immunosensors (current, A) result in different output magnitudes. To compare the techniques, electrochemical cortisol concentrations were normalized by a factor of 2.4. Both methods showed comparable results, with 2%–5% variation, validating the electrochemical cortisol immunosensing protocol. A normalization factor of 2.2 was previously used for salivary cortisol validation,24 the slight difference potentially due to physiological variations between saliva (free cortisol) and plasma (bound and free cortisol).
This validated electrochemical immunosensing protocol enables accurate plasma cortisol quantification and has potential for detecting cortisol in other biological fluids, facilitating broader point-of-care applications.
Conclusion
This study successfully demonstrated plasma cortisol detection in HIV-positive patient samples using an electrochemical immunosensing device. The device, integrating a miniaturized potentiostat chip with an LTCC microfluidic manifold, achieved pg/mL level cortisol detection with a sensitivity of 5.8 μA (pg mL)−1. Plasma cortisol levels were also validated using ELISA, showing good correlation (2%–5%) with the electrochemical sensor results, confirming the method’s validity.
Future efforts will focus on expanding patient sample volumes and collaborating with clinics and hospitals to study diurnal cortisol variations in HIV patients and their correlation with disease progression. This information can be integrated with clinical diagnostics for personalized health monitoring and optimized therapeutic strategies, leveraging the capabilities of low-cost point-of-care diagnostics.
Acknowledgments
This work was supported by NIH grants RO1-DA027049, R21-MH 101025, RO1-MH085259, and RO1-DA 034547, and partially supported by NSF-NERC (1160483). We thank Dr. Abhay Vasudev (Intel process engineer) for system design assistance and Prof. Dinesh Sood for scientific discussions.
Footnotes
Disclosure
The authors declare no conflicts of interest.