Introduction
Heart failure (HF) represents a significant clinical syndrome characterized by a cluster of symptoms such as dyspnoea, orthopnoea, and peripheral edema, alongside clinical signs like elevated jugular venous pressure and pulmonary congestion. These manifestations often stem from structural and/or functional cardiac abnormalities, leading to reduced cardiac output and/or elevated intracardiac pressures [1••]. The impact of HF is substantial, affecting not only patient morbidity and mortality but also placing a considerable burden on healthcare infrastructure. In the UK alone, the annual expenditure for managing HF is estimated at £980 million [2], with global economic costs reaching approximately $108 billion per year according to World Bank estimates [3]. Prevalence rates of HF range around 1–2% in the general population but escalate to over 10% in individuals aged 70 years and older [4]. This might even underestimate the actual disease burden, considering that asymptomatic left ventricular (LV) systolic dysfunction is estimated to affect 5.5% of those over 65 years [5]. Lifetime risk assessments suggest a 33% risk for men and 28% for women to develop HF [6].
Clinical guidelines [1••] commonly use the term HF in the context of chronic heart failure (CHF), where symptom severity is often graded using the New York Heart Association (NYHA) functional classification. Over the past few decades, significant advancements have been made in understanding CHF pathophysiology and in developing disease-modifying therapies, driven by extensive clinical research. However, the acute presentation and management of acute heart failure (AHF) remain less clear. AHF is characterized by a rapid onset, often in patients with pre-existing cardiomyopathy. Hospital admission for AHF signals a grim prognosis, marked by high risks of readmission and mortality post-discharge. UK National Heart Failure Audit data indicates approximately 10% mortality during initial hospitalization, with post-discharge 30-day and 1-year mortality rates of 6.5% and 30%, respectively [7].
This article aims to provide a comprehensive introduction to AHF, emphasizing its clinical relevance. By examining epidemiological and demographic data, we will highlight the complexities in Acute Heart Failure Diagnosis and management, and the crucial role of clinical trials in advancing our understanding and treatment of this challenging condition.
De Novo Acute Heart Failure: Initial Presentation and Diagnosis
Acute heart failure is broadly defined as a rapid onset or worsening of heart failure signs and symptoms [8]. It is a potentially life-threatening condition frequently necessitating hospitalization. Initial emergency treatment primarily aims to address fluid overload and haemodynamic instability. This broad category includes patients presenting with heart failure symptoms for the first time (de novo AHF) and those experiencing exacerbation of pre-existing cardiomyopathy (acute decompensated heart failure). Accurate acute heart failure diagnosis is critical for guiding immediate and long-term management strategies.
De novo AHF arises from a sudden surge in intracardiac filling pressures and/or acute myocardial dysfunction, potentially leading to reduced peripheral perfusion and pulmonary edema. A leading cause is cardiac ischemia, where coronary artery occlusion impairs contractility in the affected myocardial region. Management in such cases involves addressing haemodynamic compromise and myocardial reperfusion to restore contractile function.
Non-ischemic myocardial insults represent less common triggers for AHF. These include inflammatory conditions like viral cardiomyopathy, toxic exposures such as drug-induced cardiomyopathy, and idiopathic conditions like peripartum cardiomyopathy. Hospitalization for AHF can be the initial step towards a CHF diagnosis, as these acute insults may have lasting effects on myocardial function. Conversely, AHF can occur with reversible myocardial dysfunction seen in tachycardia-induced cardiomyopathy, stress-induced (Takotsubo) cardiomyopathy, or endocrine-related conditions like thyroid storm. In these scenarios, management focuses on stabilizing haemodynamics during hospitalization and identifying and correcting the underlying cause to improve acute heart failure diagnosis and treatment.
Acute valvular incompetence can also precipitate AHF, particularly in ischemic contexts leading to acute mitral regurgitation due to sub-valvular apparatus damage. It can also occur non-ischemically, as seen in infective and non-bacterial thrombotic endocarditis. Extra-cardiac pathologies like pulmonary embolism or pericardial effusion causing tamponade can also induce AHF by reducing LV output and peripheral perfusion.
Therefore, de novo AHF can stem from various causes beyond pump failure, typically presenting with reduced perfusion pressures and pulmonary edema. Management strategies are geared towards haemodynamic support, pharmacologically or mechanically, and addressing the root cause to improve acute heart failure diagnosis and patient outcomes.
Acute Decompensated Heart Failure: Diagnosis in the Context of Chronic Conditions
The majority of patients diagnosed with acute heart failure experience it as an exacerbation of pre-existing cardiomyopathy, termed acute decompensated heart failure (ADHF). This patient group differs significantly from those with de novo AHF, influencing haemodynamic assessment and management approaches. Accurate acute heart failure diagnosis in ADHF requires consideration of the patient’s chronic condition and typical presentation patterns.
Unlike de novo AHF, ADHF patients often present with congestion and fluid retention signs and symptoms (weight gain, exertional dyspnoea, orthopnoea, dependent edema), rather than the acute pulmonary edema or cardiogenic shock seen in acute LV systolic dysfunction. This is due to chronic neuro-humoral compensatory mechanisms attempting to maintain haemodynamic stability despite worsening LV function. Decompensation occurs when these mechanisms are overwhelmed, leading to fluid overload. Data from the IMPACT-HF registry indicates that ADHF follows a more gradual course, with patients presenting after days or weeks of worsening congestion symptoms prior to hospitalization [9].
Similar to de novo AHF, ADHF exacerbations can occur in various clinical settings. Demographic studies show high comorbidity prevalence in ADHF patients, including atrial fibrillation/flutter (30–46%), valvular heart disease (44%), and dilated cardiomyopathy (25%) [10]. These pre-existing conditions are delicately balanced in patients with myocardial dysfunction, and events like rapid atrial fibrillation can trigger decompensation and AHF hospitalization. Studies such as OPTIMIZE-HF have identified uncontrolled hypertension, new or worsening ischemia, and arrhythmias (primarily atrial) as common comorbidities precipitating ADHF admissions [11].
Chronic comorbidities are integral to acute heart failure diagnosis and management in ADHF patients. Renal dysfunction and diabetes mellitus are highly prevalent non-cardiac comorbidities in decompensated heart failure, negatively impacting outcomes. Observational studies show that 20–30% of ADHF patients have renal dysfunction, and 40% have diabetes mellitus [10]. Polypharmacy and medication side effects, such as NSAIDs and thiazolidinediones, can also precipitate ADHF. Conversely, medication non-compliance, particularly with diuretics or CHF prognostic medications, is a significant precipitating factor. Furthermore, multiple comorbidities increase susceptibility to infections like cellulitis or COPD exacerbations, placing haemodynamic strain that can trigger ADHF in patients with limited cardiac reserve. These complex, interconnected factors often make it challenging to pinpoint a clear precipitant in 40–50% of ADHF admissions [12].
ADHF patients typically present with a more gradual onset, complicated by numerous comorbidities and predominantly with congestion. Management aims to address acute precipitants and reinforce adherence to disease-modifying therapies. Accurate acute heart failure diagnosis in ADHF necessitates a holistic assessment considering both acute presentation and chronic health context.
Epidemiology of Acute Heart Failure: Diagnostic and Prognostic Implications
Our understanding of AHF epidemiology is largely derived from extensive registries in the USA (ADHERE [13••], OPTIMIZE-HF [14]), Europe (EHFS I [15], EHFS II [16], ESC-HF Pilot Registry [17]), and internationally (ALARM-HF [18]). These registries provide comprehensive insights into patients presenting with AHF, highlighting key demographic features relevant to acute heart failure diagnosis and prognosis. Firstly, AHF patients are predominantly male, with a mean age over 70 years, consistent with the epidemiology of ischemic heart disease and CHF. Secondly, most patients (66–75%) have a prior HF history, presenting with decompensation rather than de novo AHF. Finally, these patients exhibit a significant burden of comorbidities, including diabetes mellitus (up to 40% in some registries) and chronic obstructive pulmonary disease (approximately 20%) [7].
In-hospital mortality in AHF registries like ADHERE [19], OPTIMIZE [20], and EHFS [10] ranges from 4 to 7%, with a median hospital stay of 4–11 days. ALARM-HF registry data suggests a higher in-hospital mortality of around 11%, possibly due to a greater proportion of patients with cardiogenic shock. The UK National Heart Failure Audit (2016) provides a clearer picture, reporting an overall in-patient mortality rate of 9.6% from 56,915 admissions between April 2014 and March 2015. Mortality rates were significantly higher in older patients, reaching 12% in those over 75 years. Notably, in-hospital mortality was lower in specialist cardiology settings (7.1%) compared to general medical wards (9.6%). These epidemiological data underscore the severity of AHF and the importance of timely and appropriate acute heart failure diagnosis and management.
Post-discharge mortality remains high and has not significantly improved over the last decade. Prior studies reported 1-year mortality for AHF hospitalization around 20% [21]. The ADHERE registry showed a 1-year mortality as high as 36% [13••], possibly influenced by a higher proportion of cardiogenic shock patients. The National Heart Failure Audit reported an overall 1-year mortality of 29.6% for HF hospitalization in the UK. Mortality was influenced by the care setting (cardiology vs. general medicine) and follow-up received. Patients prescribed comprehensive disease-modifying therapy (ACE inhibitor/ARB, beta-blocker, MRA) and enrolled in HF disease management programs had improved 1-year mortality compared to those without these interventions [7]. These observations strongly advocate for specialist care and evidence-based pharmacotherapy in long-term AHF management. Epidemiological findings emphasize the need for improved acute heart failure diagnosis strategies to facilitate timely interventions and better long-term outcomes.
Interestingly, 40–55% of HF admissions in registries and 30% in the National Heart Failure Audit involved patients with normal or near-normal LV systolic function. These HF patients with preserved ejection fraction (HFpEF) tend to be older, female, and more likely to have hypertension as a comorbidity [22]. HFpEF is a significant component in AHF clinical trials. In RELAX-AHF, assessing serelaxin, only 55% of patients had an ejection fraction (EF) below 40%. Compared to patients with heart failure with reduced ejection fraction (HFrEF), hospitalizations and deaths in HFpEF are more often due to non-cardiovascular causes [23], reflecting this population’s demographics and comorbidities. However, the difference in cardiovascular death and HF hospitalization between HFpEF and HFrEF is modest, despite no proven effective mortality or morbidity treatment for HFpEF. The EVEREST trial, investigating oral tolvaptan, enrolled patients with a mean EF of 27.5% in the placebo arm and showed a 1-month cardiovascular mortality and/or HF hospitalization of 9–10% [24]. In contrast, RELAX-AHF’s placebo arm, with a higher mean EF of 38.6%, had comparable 1-month cardiovascular death or HF readmission (8–9%) [25]. These discrepancies may result from the interplay between the worse haemodynamic profile in HFrEF (potentially offsetting therapy benefits) and the poorer pre-morbid condition in HFpEF (negatively impacting cardiovascular outcomes). Furthermore, variations in trial endpoints and patient populations may contribute to these differences. It may also indicate that current acute heart failure diagnosis and classification criteria do not fully capture the variability in LV systolic function and underlying pathophysiological entities encompassed within AHF.
Classification of Acute Heart Failure: Enhancing Diagnostic Precision
The definition of AHF is broad, leading to numerous stratification attempts [26]. A primary challenge in classifying AHF as a single entity is patient population heterogeneity. Patients admitted with HF present across a disease spectrum, from severe LV systolic dysfunction and low cardiac output to severe hypertension with normal LV function. Most AHF patients fall between these extremes, exhibiting diverse underlying pathologies and precipitants culminating in fluid overload. Refining acute heart failure diagnosis through improved classification systems is crucial for targeted management.
Older European Society of Cardiology guidelines [27] categorized patients into six groups (I–VI) based on clinical and haemodynamic characteristics. ADHF (I), hypertensive AHF (II), and AHF with pulmonary edema (III) constitute over 90% of hospital presentations. ADHF typically presents with mild-to-moderate symptoms, whereas AHF with pulmonary edema (III) is dominated by respiratory distress and hypoxemia, ranging in severity from low-output states (IVa) to cardiogenic shock (IVb). High-output failure (V), uncommon in AHF, is associated with conditions like anemia, thyrotoxicosis, and Paget’s disease, generally presenting with warm extremities and pulmonary congestion, and hypotension in sepsis. Right-sided heart failure (VI) is categorized, mainly including patients with pre-existing lung disease and cor pulmonale, though acute myocardial ischemia/infarction affecting the right ventricle is also included.
This classification system directs management towards the underlying cause of AHF. However, patient comorbidities often obscure the primary decompensation reason, or multiple factors may contribute. From a practical standpoint, stratifying AHF patients by initial clinical presentation may be more useful. This approach helps identify high-risk patients needing immediate interventions like inotropic agents and/or mechanical circulatory support. Clinical presentation-based classification is vital for effective acute heart failure diagnosis and early intervention.
Systolic blood pressure (SBP) on admission is one stratification marker. Most AHF patients present with preserved (90–140 mmHg) or elevated (>140 mmHg) SBP, with higher SBP generally indicating a better prognosis. This may be due to the feasibility of vasodilator therapy or reflect preserved LV function. Less than 10% of patients present with systolic hypotension (SBP 28].
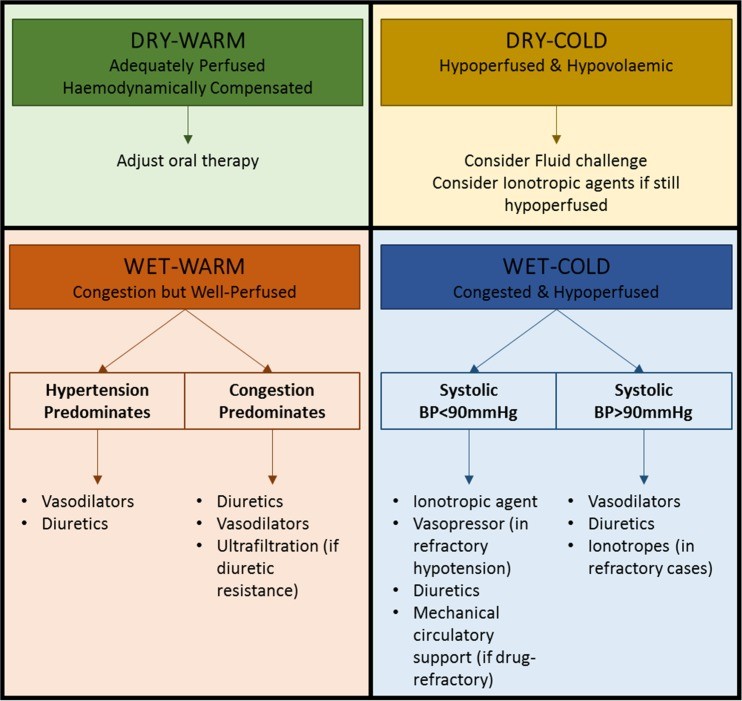
A more comprehensive AHF classification method, developed by Stevenson et al. [29] and endorsed by current ESC guidelines [1••], focuses on initial clinical assessment based on congestion signs/symptoms (orthopnoea, edema, elevated JVP) and peripheral perfusion (cold extremities, oliguria, narrow pulse pressure). Patients are categorized as ‘wet’ or ‘dry’ (fluid status) and ‘cold’ or ‘warm’ (perfusion status). This assessment yields four groups (warm and wet, warm and dry, cold and dry, cold and wet) for initial stratification, guiding therapy (Fig. 1) and providing prognostic information [1••]. Warm and dry patients have a 6-month mortality of 11%, compared to 40% for the cold and wet profile [29]. This clinical presentation-based stratification is a practical step in AHF management and improves acute heart failure diagnosis and risk assessment.
Fig. 1.
Stratification of patients admitted with AHF based on initial clinical presentation. Patients may be classified, irrespective of underlying aetiology, according to both their perfusion status (COLD vs WARM) and degree of fluid congestion (WET vs DRY). Based on the initial clinical assessment, prognosis can be determined and an appropriate management strategy put in place. It is important to note that 95% of patients presenting with AHF to the hospital have clinical features of congestion (WET). Adapted from the 2016 ESC guidelines [1••]
Challenges Posed by Classification and Identification in Acute Heart Failure Diagnosis
Before 1990, patients with stable chronic heart failure (CHF) faced a 60–70% mortality rate [30], now halved to 30–40% 1-year mortality [31]. This improvement is mainly due to therapies proven beneficial in randomized controlled trials, based on improved understanding of CHF pathophysiology. However, similar improvements in outcomes for AHF patients have not occurred. UK National Heart Failure Audit data shows a 30-day mortality of approximately 15%, unchanged over the past 6 years [7]. Other studies indicate poor prognosis for AHF hospitalization, with death or recurrent hospitalization nearing 50% at 6 months [32]. These statistics highlight significant challenges in acute heart failure diagnosis and management.
A paradox exists: AHF signs and symptoms are often successfully treated during hospitalization, yet mortality outcomes remain poor. This may reflect current AHF pathophysiology understanding, where haemodynamic abnormalities cause early congestion, while end-organ damage contributes to morbidity and mortality [33]. AHF pharmacological treatments (loop diuretics, vasodilators, inotropes) have remained largely unchanged since the 1970s [34], primarily targeting haemodynamic compromise and fluid overload. Furthermore, randomized placebo-controlled trials in AHF have been scarce, with the first only in 2002 [35]. Trials to date have not shown convincing mortality impact from interventions in AHF patients.
Several factors contribute to this, including how AHF is defined and patient recruitment criteria for trials. AHF trials often compare treatment to placebo plus standard medical therapy. However, ‘standard therapy’ is rarely explicitly defined and varies widely among patients due to AHF’s nature. Variations in diuretic, inotrope, vasodilator, and non-invasive ventilation use, influenced by clinical condition during hospitalization, can significantly affect long-term outcomes. Furthermore, initiation, up-titration, and cessation of oral neurohumoral antagonists (proven beneficial in CHF) vary in AHF patients, influenced by factors like SBP and renal function. Given these drugs’ proven benefits, treatment variations during hospitalization likely impact long-term outcomes. Evaluating new therapies, especially considering ‘hard’ endpoints like cardiovascular mortality or readmission, can be obscured by variable ‘standard’ therapy. Standardizing acute heart failure diagnosis criteria and treatment protocols in trials is crucial.
Diagnostic criteria variability for AHF further complicates standardized study inclusion. AHF diagnosis is clinical, based on fluid overload symptoms/signs, with or without hypoperfusion evidence, supported by chest X-ray (pulmonary congestion) and biomarkers (BNP or NT-proBNP). Similar to NYHA classification for CHF symptoms, clinical assessment of fluid overload or LV function does not always correlate with symptom severity. Patients with similar congestion levels can be classified as ‘stable’ CHF or ‘acutely decompensated’ HF based on semi-subjective factors like symptom severity, functional status, and outpatient care infrastructure. This inherent heterogeneity in patient populations recruited into AHF trials using clinical criteria may dilute the intervention’s effect. Improving the consistency and objectivity of acute heart failure diagnosis is essential for clinical research.
Timing is another critical issue. Patients with similar fluid overload signs may present at different disease stages. Limited data exists on a therapeutic window in AHF treatment for long-term outcome improvement. Patients presenting at different cardiac function decompensation stages add further heterogeneity to clinical trials. Objective criteria like plasma natriuretic peptide concentrations may partially mitigate this. ADHERE registry data suggests earlier natriuretic peptide measurement and therapy implementation may improve long-term outcomes [36]. However, patient heterogeneity and uncertainties about admission and intervention timing remain significant challenges in AHF trials. Addressing these challenges in acute heart failure diagnosis is vital for advancing treatment strategies.
Finally, a lack of consensus on appropriate phase III study endpoints in AHF exists [37]. Endpoints should be consistent, reproducible, sensitive, and clinically meaningful for progress. No AHF trials to date have shown convincing ‘hard’ outcome improvements (cardiovascular mortality, readmission) from hospitalization interventions. Recent trials have focused on shorter-term symptom relief (RELAX-HF, Likert dyspnoea scale [25]) or shorter-term outcomes like worsening heart failure (ASCEND-HF [38]). This shift reflects the lack of ‘hard’ endpoint improvement evidence and the greater achievability and clinical relevance of ‘softer’ endpoints in AHF management. Trial designs also reflect a fundamental question: can acute hospitalization interventions improve post-discharge outcomes? While shown in acute myocardial infarction (reperfusion therapy) or hyperacute stroke (thrombolytic therapy), no short-term AHF therapy has convincingly improved long-term mortality. Recent trials aim to address this. RELAX-AHF2, for example, a phase III trial of serelaxin in AHF, with primary endpoints of cardiovascular death and time to worsening heart failure [39•], did not show benefit for serelaxin on these endpoints. Nevertheless, using hard clinical endpoints in trial design is a positive step in assessing novel disease-modifying therapies and refining acute heart failure diagnosis and management approaches.
Conclusions
Acute heart failure is a clinical syndrome characterized by fluid overload signs and symptoms requiring hospitalization. Patients may present with AHF as their first heart disease manifestation or, more commonly, as decompensation of pre-existing cardiomyopathy. In the latter, hospitalization marks a significant prognostic event, associated with increased mortality and morbidity. Classification and treatment strategies have focused on managing initial haemodynamic disturbances in patients often with multiple comorbidities. However, unlike chronic stable heart failure, few therapies have improved long-term survival post-AHF admission. Future clinical trials must be designed and conducted with a more comprehensive understanding of AHF pathophysiology and better patient population definition. Improving acute heart failure diagnosis, classification, and trial design are crucial steps towards enhancing patient outcomes.
Compliance with Ethical Standards
Conflict of Interest
Sameer Kurmani and Iain Squire report personal fees from NOVARTIS.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Decompensated Heart Failure
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
[1••]
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
[2]
2. Heart failure: the hidden costs of heart failure. 2nd ed. London: British Heart Foundation; 2006.
[3]
3. World Economic Forum. Global economic burden of non-communicable diseases. Geneva: World Economic Forum; 2011.
[4]
4. Cowie MR, Mosterd A, Wood DA, Deckers JW, Simoons ML, Loeve AJ, et al. Epidemiology of heart failure characterising the population at risk. Heart. 1997;78(5):421–6.
[5]
5. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Erwa JA, Bailey KR, et al. Prevalence and predictors of preclinical diastolic dysfunction (stage A heart failure) in the community a population-based study. Circ Heart Fail. 2009;2(3):191–8.
[6]
6. Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, et al. Lifetime risk for developing heart failure the Framingham Heart Study. Circulation. 2002;106(24):3068–72.
[7]
7. National Institute for Cardiovascular Outcomes Research (NICOR). National Heart Failure Audit 2014–2015. London: NICOR; 2016.
[8]
8. Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(4):384–416.
[9]
9. Hauptman PJ, Good JK, Chatterjee K, O’Connor CM, Gheorghiade M. Decompensated heart failure in the elderly. Am J Cardiol. 1999;83(3):472–5.
[10]
10. Tavazzi L, Swedberg K, Komajda M, Weidinger F, Bohm M, Borer JS, et al. Clinical profiles and outcomes in patients hospitalised with acute heart failure: data from the European Heart Failure Survey II (EHFS II). Eur J Heart Fail. 2006;8(4):414–21.
[11]
11. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Stevenson LW, Westfall RE, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–16.
[12]
12. O’Connor CM, Gattis WA, Adams KF Jr, Hasselblad V, Gheorghiade M, for the ADHERE Investigators. Predictors of 90-day mortality after hospitalization for heart failure: the ADHERE registry. Am Heart J. 2001;141(4):S73–9.
[13••]
13. Fonarow GC, Adams KF Jr, Gheorghiade M, O’Connor CM, Yancy CW, Young JB, et al. Outcomes following hospitalization for heart failure: high 30-day readmission rates and death across all payer groups. J Am Coll Cardiol. 2007;50(5):428–35.
[14]
14. Gheorghiade M, Fonarow GC, Young JB, Sopko G, Yancy CW, Komajda M, et al. Design and rationale of the OPTIMIZE-HF registry: a prospective, randomized, outcomes trial and registry of patients hospitalized with heart failure. Am Heart J. 2004;148(1):9–16.
[15]
15. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, et al. EuroHeart Failure Survey II: baseline characteristics of patients hospitalized for acute heart failure in 48 centres in Europe. Eur Heart J. 2006;27(22):2715–24.
[16]
16. Komajda M, Anker SD, Cowie MR, Cleland JG, Gelder IC, Hall AS, et al. EuroHeart Failure Survey II (EHFS II): a survey on acute heart failure in 110 centres in Europe. Eur J Heart Fail. 2006;8(2):111–8.
[17]
17. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Early evaluation of patients with acute heart failure: the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) registry. Eur Heart J. 2011;32(12):1479–87.
[18]
18. Peacock WF, Young J, Salmon M, Maisel AS, люк A, McCord J, et al. ALARM-HF Investigators. Association of baseline characteristics with outcomes in patients with acute heart failure: an ALARM-HF analysis. Acad Emerg Med. 2009;16 Suppl 1:S9–16.
[19]
19. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Acute heart failure syndromes: current state and future directions. Eur Heart J Suppl. 2006;8(Suppl A):A23–9.
[20]
20. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297(1):61–70.
[21]
21. Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark DM, Tu JV. Predicting mortality after hospitalization for heart failure in the community: derivation and validation of a risk-adjustment model. JAMA. 2003;290(19):2581–7.
[22]
22. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9.
[23]
23. Shah MR, Hernandez AF, Swaminathan M, McNulty SE, Miklos Z, Patel CB, et al. Patients hospitalized for heart failure with preserved ejection fraction baseline characteristics and outcomes. Am Heart J. 2008;156(6):1169–77.
[24]
24. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure. JAMA. 2007;297(12):1313–21.
[25]
25. Teerlink JR, Metra M, Felker GM, Ponikowski P, Serpytis P, Silva Moreira L, et al. Relaxin in acute heart failure (RELAX-AHF) rationale and design. J Card Fail. 2009;15(4):336–44.
[26]
26. Felker GM, Hasselblad V, Gheorghiade M. The categorization of acute heart failure syndromes: application to clinical trial design. Eur Heart J. 2007;28(15):1777–84.
[27]
27. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J. 2012;33(14):1787–847.
[28]
28. Zannad F, Mebazaa A, Juilliere Y, Cohen-Solal A, Guize L, Alla F, et al. Clinical profile, contemporary management and one-year prognosis of patients with acute heart failure syndromes: the EFICA study. Eur J Heart Fail. 2006;8(7):697–705.
[29]
29. Stevenson LW. Tailored therapy before hemodynamic monitoring for pulmonary edema complicating heart failure. Chest. 1993;104(4):1273–6.
[30]
30. The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316(24):1429–35.
[31]
31. Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJ, et al. Hospitalization for heart failure in the UK 1995–2003: temporal trends and geographical variation. Heart. 2006;92(1):94–100.
[32]
32. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Koenig W, গুরুতর P, et al. দীর্ঘস্থায়ী G, for the ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients hospitalized with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol. 2006;47(1):76–84.
[33]
33. Metra M, Ponikowski P, Dickstein K, McMurray JJV, Gavazzi A, Berghmans T, et al. Heart failure with preserved left ventricular systolic function: pathophysiology, clinical profile, and perspectives. Eur Heart J. 2003;24(4):351–8.
[34]
34. Cuffe MS, Califf RM, Adams KF Jr, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287(12):1541–7.
[35]
35. Publication Committee for the VMAC Trial Investigators. Intravenous nesiritide vs placebo for short-term management of acutely decompensated heart failure: results of the VMAC trial. JAMA. 2002;287(12):1531–40.
[36]
36. Yancy CW, জামার G, Albert NM, Butler J, কুত্তাপ্পান P, ডিজাই P, et al. প্রশ্ন J, for the ADHERE Investigators. Natriuretic peptide-guided therapy for acute decompensated heart failure: the ASCEND-HF trial. J Am Coll Cardiol. 2011;58(1):25–34.
[37]
37. Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Voors AA. Endpoints for clinical trials in acute heart failure: a консенсус statement from the Heart Failure Association of the European Society of Cardiology Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2016;37(7):536–44.
[38]
38. O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, গড় R, et al. ASCEND-HF Investigators. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43.
[39•]
39. Metra M, Teerlink JR, Felker GM, Ponikowski P, Davison BA, Givertz MM, et al. স্ট্রাকচার, Rationale, and design of the Relaxin for Acute Heart Failure 2 (RELAX-AHF-2) trial. Eur J Heart Fail. 2017;19(3):412–9.