Introduction
Acute pulmonary embolism (PE) stands as a critical, life-threatening condition arising from the obstruction of pulmonary arteries by blood clots, typically originating as deep vein thrombosis (DVT) in the lower extremities. Together, PE and DVT are recognized as venous thromboembolism (VTE), a spectrum of disease with substantial global impact on morbidity and mortality. While PE most often stems from thrombi, it can also result from embolization of air, fat, or tumor cells into the pulmonary circulation. Understanding the complexities of Acute Pulmonary Embolism Diagnosis is paramount due to its often nonspecific symptoms, such as dyspnea, chest pain, cough, and syncope, which can mimic various cardiopulmonary disorders. In severe instances, PE can manifest as hemodynamic instability and signs of right ventricular strain, underscoring the urgency for rapid and accurate diagnosis.
The cornerstone of acute pulmonary embolism diagnosis involves integrating clinical probability assessments, such as the Wells and Geneva criteria, with essential diagnostic tests. These tests range from D-dimer assays and CT pulmonary angiography (CTPA) to ultrasound, each playing a crucial role in confirming or excluding PE. For patients presenting with hemodynamic instability, immediate bedside imaging may be necessary to expedite diagnosis and intervention. The challenge in acute pulmonary embolism diagnosis lies in its varied presentation and the need to differentiate it from other conditions, making a systematic approach essential for clinicians. This article aims to enhance healthcare professionals’ proficiency in recognizing the clinical features of PE, selecting appropriate diagnostic modalities, and implementing effective interprofessional management strategies to improve patient outcomes in acute pulmonary embolism diagnosis.
Etiology and Risk Factors of Pulmonary Embolism
Understanding the Roots of Pulmonary Embolism
The vast majority of pulmonary emboli are thrombi that originate from deep vein thrombosis (DVT), most commonly in the lower extremities. Consequently, the risk factors for PE mirror those of DVT. The Virchow triad – hypercoagulability, venous stasis, and endothelial injury – provides a framework for understanding these risk factors in acute pulmonary embolism diagnosis.
Risk factors for pulmonary embolism are broadly categorized into genetic and acquired factors. Genetic predispositions include thrombophilias, such as factor V Leiden mutation, prothrombin gene mutation, protein C and S deficiencies, and hyperhomocysteinemia. Acquired risk factors are numerous and include prolonged immobilization (e.g., bed rest exceeding 3 days, travel longer than 4 hours), recent surgical procedures (especially orthopedic), active malignancy, the presence of indwelling venous catheters, obesity, pregnancy, cigarette smoking, and the use of oral contraceptives. Smoking is implicated in all forms of pulmonary infarction, including those associated with PE. Interestingly, younger age (peaking around 40 years) and increased height have been linked to a higher risk of PE complicated by pulmonary infarction, while obesity paradoxically shows a reduced likelihood in this specific context.
Additional Predisposing Factors for Venous Thromboembolism (VTE)
Beyond the common risk factors, several other conditions elevate the risk of VTE and, consequently, PE:
- Lower limb fractures
- Hospitalization for heart failure or atrial fibrillation/flutter within the preceding 3 months
- Hip or knee replacement surgery
- Major trauma
- Prior history of venous thromboembolism
- Central venous lines
- Chemotherapy regimens
- Congestive heart failure or respiratory failure
- Hormone replacement therapy
- Oral contraceptive therapy
- Postpartum period
- Infections, notably pneumonia, urinary tract infections, and HIV
- Cancer, with metastatic disease carrying the highest risk
- Thrombophilia
- Bed rest longer than 3 days
- Obesity
- Pregnancy
Cancer is a particularly potent risk factor for thrombus formation and subsequent PE, with pancreatic, hematological, lung, gastric, and brain cancers exhibiting the highest VTE risk. Infections throughout the body are also recognized triggers for VTE. Moreover, conditions like myocardial infarction and congestive heart failure increase PE risk. It’s also important to note that patients with VTE are at an elevated risk of future stroke and myocardial infarction, highlighting the systemic implications of this condition.
Categorization of Pulmonary Embolism
For effective acute pulmonary embolism diagnosis and management, PE is categorized based on hemodynamic stability. Hemodynamically unstable PE, previously termed massive or high-risk PE, is defined by the presence of hypotension (systolic blood pressure
Hemodynamically stable PE encompasses a spectrum from small, mildly symptomatic, or asymptomatic cases (low-risk PE or small PE) to those causing mild hypotension that stabilizes with fluid therapy or presenting with right ventricular dysfunction (submassive or intermediate-risk PE) while maintaining hemodynamic stability.
Epidemiology of Acute Pulmonary Embolism
The annual incidence of acute PE ranges from 39 to 115 per 100,000 individuals, while DVT incidence ranges from 53 to 162 per 100,000. Ranking as the third most frequent cardiovascular disease after coronary artery disease and stroke, acute PE represents a significant public health concern. Notably, men exhibit a higher incidence of acute PE compared to women. Pulmonary embolism complicated by pulmonary infarction occurs in a substantial proportion of cases, estimated between 16% and 31%.
Despite advancements in treatment and acute pulmonary embolism diagnosis, PE-related mortality remains high. In the United States alone, PE contributes to approximately 100,000 deaths annually. Accurately estimating mortality rates is challenging, as PE is suspected in many cases of sudden cardiac death. However, case-fatality rates for PE have shown a decline, likely attributable to improvements in diagnostic methodologies, earlier intervention strategies, and therapeutic advancements.
Pathophysiology of Pulmonary Embolism
Pulmonary embolism pathophysiology begins when a blood clot enters the pulmonary circulation, commonly involving multiple emboli distributed more frequently in the lower lung lobes and bilaterally. Larger emboli tend to obstruct the main pulmonary artery, potentially leading to saddle embolus and severe cardiovascular consequences. Conversely, smaller emboli typically lodge in peripheral arteries, possibly resulting in pulmonary infarction characterized by intra-alveolar hemorrhage.
PE triggers impaired gas exchange due to physical obstruction of the pulmonary vasculature, leading to ventilation-perfusion mismatch. Alveolar ventilation remains constant, but pulmonary capillary blood flow decreases, creating dead space ventilation and hypoxemia. The release of mediators like serotonin exacerbates the situation by causing vasospasm and further reducing pulmonary blood flow in unaffected lung regions. Local inflammatory mediators alter lung surfactant and stimulate respiratory drive, resulting in hypocapnia and respiratory alkalosis.
Image: Electrocardiogram findings in pulmonary embolism can include tachycardia, non-specific ST-segment and T-wave changes, and right ventricular strain patterns.
In acute pulmonary embolism diagnosis, understanding the hemodynamic consequences is crucial. Pulmonary vascular resistance increases due to thrombus-induced mechanical obstruction and hypoxic vasoconstriction. Pulmonary artery pressure rises when thromboembolic occlusion exceeds 30% to 50% of the pulmonary arterial bed’s cross-sectional area. This increased resistance elevates right ventricular afterload, impeding right ventricular outflow and causing dilation and interventricular septum bowing. Right bundle branch block may further desynchronize ventricles. Reduced right ventricular outflow and dilation compromise left ventricular filling, reducing cardiac output.
This cascade leads to decreased left ventricular filling during early diastole, diminishing cardiac output and causing systemic hypotension and hemodynamic instability. Right ventricular failure from acute pressure overload is the primary cause of death in severe PE. Clinical signs of right ventricular failure and hemodynamic instability are critical indicators of high early mortality risk.
Historically, underlying cardiac disease was considered a major risk factor for pulmonary infarction following PE, attributed to poor collateral circulation. However, recent studies suggest the opposite, indicating younger patients without cardiopulmonary disease are more susceptible to pulmonary infarction secondary to PE. This may be due to better bronchial vascular collateralization in those with chronic cardiopulmonary conditions, offering protection against infarction. The lung parenchyma receives oxygen from pulmonary arteries, bronchial circulation, and direct alveolar diffusion. Impedance in any of these sources can lead to infarction and necrosis. Inflammatory mediators from ischemic parenchyma can further impair gas exchange via vasoconstriction and bronchoconstriction. If ischemia is not promptly reversed, infarction occurs. Unilateral infarcts, predominantly in the right lower lobe, are common. Lower lobes are more prone to infarction than upper lobes, possibly due to gravity’s influence on alveolar, pulmonary, and bronchial arterial pressure relationships.
History and Physical Examination in Pulmonary Embolism
Clinical History: Recognizing the Signs
Prompt acute pulmonary embolism diagnosis is vital due to the high mortality and morbidity if left untreated. Untreated PE has a mortality rate of approximately 30%, which dramatically decreases to around 8% with timely intervention. However, diagnosing PE is often challenging due to the wide range of nonspecific clinical signs and symptoms. Common symptoms include dyspnea, pleuritic chest pain, cough, hemoptysis, presyncope, and syncope. Dyspnea severity can range from acute and severe in central PE to mild and transient in peripheral PE. In patients with pre-existing heart or lung conditions, worsening dyspnea may be the sole indicator. Chest pain is frequently caused by pleural irritation from distal emboli causing pulmonary infarction. Central PE chest pain may stem from right ventricular ischemia and needs differentiation from acute coronary syndrome or aortic dissection.
Less frequent presentations include arrhythmias (e.g., atrial fibrillation), syncope, and hemodynamic collapse. Hemodynamic instability, though rare, is a critical presentation indicating central or extensive PE with severely compromised hemodynamic reserve. Syncope can occur and may correlate with a higher incidence of hemodynamic instability and right ventricular dysfunction. It’s crucial to recognize that large PEs can sometimes be asymptomatic or present with mild symptoms. PE can be incidentally discovered during evaluations for other conditions. Thus, in addition to symptoms, clinicians must assess VTE risk factors to determine PE clinical probability in acute pulmonary embolism diagnosis.
Physical Examination Findings: What to Look For
Physical examination findings in PE patients can include tachypnea and tachycardia, common but nonspecific signs. Other findings may include calf swelling, tenderness, erythema, palpable cords indicative of DVT, pedal edema, rales, decreased breath sounds, and signs of pulmonary hypertension such as elevated jugular venous pressure, a loud P2 component of the second heart sound, a right-sided gallop, and a right ventricular parasternal lift.
PE is a recognized cause of sudden cardiac arrest (approximately 8% of cases). Massive PE can lead to acute right ventricular failure, presenting with jugular venous distension, parasternal lift, third heart sound, cyanosis, and shock. In PE patients with tachycardia at presentation who suddenly develop bradycardia or new broad complex tachycardia (with right bundle branch block), right ventricular strain and impending shock should be considered. PE should be suspected in hypotensive patients with jugular venous distension when acute myocardial infarction, pericardial tamponade, or tension pneumothorax are ruled out. These clinical assessments are crucial for guiding acute pulmonary embolism diagnosis.
Evaluation and Diagnostic Modalities for Acute Pulmonary Embolism
Diagnostic Evaluation: A Multi-faceted Approach
The acute pulmonary embolism diagnosis process integrates various laboratory and imaging studies with clinical probability scoring systems like the Wells and Geneva criteria.
Arterial Blood Gas (ABG) Analysis
Unexplained hypoxemia with a normal chest radiograph should raise suspicion for PE. Common ABG findings in PE include a widened alveolar-arterial oxygen gradient, respiratory alkalosis, and hypocapnia, reflecting the pathophysiological response to PE. Respiratory or lactic acidosis is less common but can occur in massive PE with obstructive shock and respiratory arrest.
Brain Natriuretic Peptide (BNP)
Elevated BNP levels have limited diagnostic utility in suspected PE. Right ventricular pressure overload from acute PE causes myocardial stretch, leading to BNP and N-terminal-proBNP release. Thus, natriuretic peptide levels reflect right ventricular dysfunction severity in acute PE, but are not specific for acute pulmonary embolism diagnosis.
Troponin
Serum troponin I is prognostically valuable but not primarily diagnostic in PE. As a marker of right ventricular dysfunction, troponin levels are elevated in 30% to 50% of patients with moderate to large PE and are associated with clinical deterioration and mortality post-PE.
D-dimer Testing
D-dimer levels are elevated in plasma during acute thrombotic processes due to simultaneous activation of coagulation and fibrinolysis. D-dimer testing has high negative predictive value; a normal D-dimer level makes acute PE or DVT unlikely, making it a useful rule-out test in acute pulmonary embolism diagnosis. However, its low positive predictive value means it cannot confirm PE.
Different D-dimer assays exist; clinicians should be familiar with their test’s performance. Quantitative enzyme-linked immunosorbent assay (ELISA) has a diagnostic sensitivity of at least 95% and can exclude PE in low- or intermediate-probability patients. A negative ELISA D-dimer and low clinical probability can rule out PE in about 30% of suspected cases without further testing.
D-dimer specificity decreases with age, reaching approximately 10% in those over 80. Age-adjusted cut-offs for patients over 50 can improve test performance. Using the age-adjusted cut-off (age in years x 10 ng/mL for patients >50) increases the number of patients where PE can be ruled out without increasing false negatives. For example, for a 75-year-old, the age-adjusted D-dimer cut-off is 750 ng/mL.
Electrocardiography (ECG)
ECG abnormalities in suspected PE are nonspecific. Common ECG findings include tachycardia and nonspecific ST-segment and T-wave changes. Less common findings are S1Q3T3 pattern, right ventricular strain, and new incomplete right bundle branch block.
Chest Radiograph (CXR)
CXR in PE is often normal or shows nonspecific abnormalities like atelectasis or effusion. It helps exclude alternative diagnoses in acute dyspnea. CXR may provide clues to pulmonary infarction. “Hampton hump” (wedge-shaped consolidation), Westermark sign (radiographic oligemia), and Fleischer sign (prominent pulmonary artery) are specific but lack sensitivity. Hampton hump has a sensitivity of 22% and specificity of 82%. Westermark sign, a sharp cut-off of pulmonary vessels with distal hypoperfusion, is rare but specific for acute PE, seen in up to 2% of cases.
Image: Chest X-ray showing a wedge-shaped opacity in the left lower lung field, indicative of a pulmonary infarction.
Computed Tomographic Pulmonary Angiography (CTPA)
Multidetector CTPA is the preferred diagnostic modality for suspected PE in acute pulmonary embolism diagnosis. It provides detailed visualization of pulmonary arteries down to subsegmental levels. The PIOPED II study reported CTPA sensitivity of 83% and specificity of 96% for PE diagnosis. CTPA is also commonly used to diagnose pulmonary infarction, with CT findings including a feeding vessel or “vessel sign,” central lucency, and semicircular shape. Air bronchograms make pulmonary infarction less likely. A vessel sign with central lucency and no air bronchogram has 99% specificity for pulmonary infarction.
PIOPED II highlighted pretest clinical probability influence on CTPA predictive value. A normal CTPA has high negative predictive value for PE in low (96%) and intermediate (89%) probability patients but only 60% in high-probability patients. Conversely, positive CTPA has high positive predictive value in intermediate (92% to 96%) and high probability patients but lower (58%) in low-probability patients. Clinicians should consider further testing if clinical judgment and CTPA results are discordant. Current data suggest negative CTPA is adequate to exclude PE in low- or intermediate-probability patients. Further investigation for negative CTPA in high clinical probability patients is debated.
CTPA may be relatively contraindicated in moderate to severe iodinated contrast allergy or renal insufficiency (eGFR 2). Risks must be weighed against clinical necessity and availability of alternatives like ventilation/perfusion (V/Q) scan. If feasible, CTPA can be postponed for allergy premedication or IV hydration for renal insufficiency.
CTPA can also detect right ventricular enlargement, a predictor of adverse outcomes. Right ventricular enlargement (right ventricular/left ventricular ratio ≥0.9) is a strong independent predictor of severe in-hospital outcomes in both overall and hemodynamically stable PE patients.
Lung Scintigraphy (V/Q Scan)
Planar V/Q scan is an established diagnostic test for suspected PE, especially when CTPA is contraindicated, inconclusive, or additional testing is needed. Normal CXR is usually required before V/Q scanning. V/Q scans in abnormal CXR patients are prone to false positives or low PE probability.
V/Q scanning is preferred for diagnosing PE in pregnancy and for patients with contrast allergy or severe renal failure. V/Q scan results are typically classified as normal (excluding PE), high-probability (diagnostic of PE in most cases), and nondiagnostic. Normal perfusion scans safely withhold anticoagulation.
PIOPED II analysis suggests high-probability V/Q scan can confirm PE, but its positive predictive value is insufficient in low clinical probability patients. High frequency of nondiagnostic scans is a limitation, often necessitating further testing for acute pulmonary embolism diagnosis.
Pulmonary Angiography
Pulmonary angiography, involving contrast injection via catheter into the right heart under fluoroscopy, was formerly the gold standard for PE diagnosis. PE diagnosis is based on thrombus evidence, such as pulmonary arterial branch amputation or filling defect.
With CTPA prevalence, pulmonary angiography is rarely used, reserved for patients with high clinical probability PE when CTPA or V/Q scan is nondiagnostic. Pulmonary angiography is operator-dependent and highly variable, potentially inferior to CTPA. Catheter-based pulmonary angiography is used in patients needing therapeutic intervention, aiding both diagnosis and clot lysis.
Magnetic Resonance Angiography (MRA)
MRA for suspected PE has been evaluated, but large studies do not recommend it as a first-line test due to low sensitivity, limited emergency availability, and high inconclusive scan rates. However, MRA can be considered when CTPA and V/Q scans are not feasible.
MRA advantages include no radiation exposure. MR pulmonary angiography showed sensitivity of 78% and specificity of 99% in 75% of patients with technically adequate images. Combining MR pulmonary angiography and venography showed sensitivity of 92% and specificity of 96% in 48% of patients with technically adequate images.
Echocardiography
Transthoracic echocardiography rarely definitively diagnoses PE unless thrombus is visualized in proximal pulmonary arteries. Echocardiography supports PE diagnosis by identifying right heart clot or new right heart strain, especially in hemodynamically unstable patients. Echocardiogram can justify emergency thrombolytic therapy.
Image: Chest X-ray demonstrating Hampton hump, a wedge-shaped pleural-based density often associated with pulmonary embolism and infarction.
Echocardiography for PE diagnosis has limitations. Right ventricle shape complexity means no single parameter accurately assesses size or function. Echocardiographic criteria for PE vary across studies. Negative predictive value is 40% to 50%, so negative result does not exclude PE. Right ventricular overload or dysfunction signs can occur without acute PE due to other cardiorespiratory diseases.
Right ventricular dilation is seen in ≥25% of PE patients on echo and aids risk stratification. More specific echocardiography findings, such as pulmonary ejection acceleration time (in right ventricular outflow tract) combined with right ventricular/left ventricular diameter ratio ≥1 and tricuspid annular plane systolic excursion (TAPSE), have high positive predictive value for PE, especially with pre-existing cardiorespiratory illness.
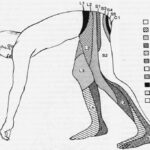
Compression Ultrasonography
PE often originates from lower-limb DVT, rarely from upper-limb DVT (mostly post-venous catheterization). DVT is found in about 70% of proven PE cases. Compression ultrasound has >90% sensitivity and about 95% specificity for proximal symptomatic DVT. Proximal DVT finding in suspected PE is sufficient for anticoagulant treatment without further testing, streamlining acute pulmonary embolism diagnosis. However, low sensitivity limits compression ultrasonography to cases where definitive imaging (CTPA, V/Q scan) is contraindicated or indeterminate.
Acute Pulmonary Embolus Diagnostic Criteria: Clinical Prediction Rules
Wells criteria and Geneva score are common scoring systems for estimating pretest PE probability. These systems classify patients into clinical probability categories, guiding diagnostic test selection and interpretation in acute pulmonary embolism diagnosis.
Revised Geneva Clinical Prediction Rule
The Geneva scoring system (original/simplified versions) uses the following points:
- Previous PE or DVT: 3/1
- Heart rate: 75-94 bpm (3/1), ≥95 bpm (5/2)
- Surgery or fracture (past month): 2/1
- Hemoptysis: 2/1
- Active cancer: 2/1
- Unilateral lower-limb pain: 3/1
- Pain on lower-limb deep palpation and unilateral edema: 4/1
- Age >65 years: 1/1
Clinical probability using Geneva criteria:
- Three-level score: Low (0-3/0-1), Intermediate (4-10/2-4), High (≥11/≥5)
- Two-level score: PE unlikely (0-5/0-2), PE likely (≥6/≥3)
Wells Criteria and Modified Wells Criteria
The Wells scoring system includes:
- Clinical DVT symptoms: 3
- Other diagnoses less likely than PE: 3
- Heart rate >100 bpm: 1.5
- Immobilization ≥3 days or surgery (past 4 weeks): 1.5
- Previous DVT or PE: 1.5
- Hemoptysis: 1
- Malignancy: 1
Clinical probability using traditional or modified Wells criteria: