I. Introduction
Pancreatic cysts (PCs), increasingly detected through routine abdominal imaging, present a significant clinical challenge in primary care. Differentiating between benign and malignant cysts is crucial for appropriate patient management. While traditional diagnostic methods such as imaging and cyst fluid analysis have limitations, advanced health assessment & clinical diagnosis in primary care are being significantly improved by molecular techniques. This study explores the transformative potential of preoperative DNA-based testing, specifically next-generation sequencing (NGS), of pancreatic cyst fluid (PCF) in enhancing diagnostic accuracy and risk stratification for patients with PCs. This innovative approach promises to refine clinical pathways, moving beyond traditional diagnostic boundaries to provide more precise and personalized care strategies in primary and specialized settings.
II. Significance of DNA-Based Pancreatic Cyst Fluid Testing
What Was Previously Understood?
Prior to advancements in molecular diagnostics, the evaluation of pancreatic cysts relied heavily on imaging modalities like CT scans, MRI, and endoscopic ultrasound (EUS), combined with cyst fluid analysis for cytology and tumor markers like Carcinoembryonic Antigen (CEA). DNA-based testing emerged as a promising adjunct, offering the potential to analyze the genetic material shed from the cyst lining into the PCF. This was particularly relevant because PCF aspirates often yielded suboptimal cellular content and fluid volume, limiting the effectiveness of conventional ancillary studies. Previous studies, while insightful, were often retrospective, used postoperative specimens, had limited sample sizes, lacked long-term follow-up, or employed less sensitive detection methods like Sanger sequencing. These limitations underscored the need for prospective, large-scale evaluations of preoperative DNA-based testing.
Novel Findings from Prospective Molecular Analysis
This prospective study on a large cohort of patients undergoing preoperative PCF DNA testing using NGS revealed several key findings:
- Enhanced Sensitivity and Specificity of NGS for Mucinous Cysts: Mutations in KRAS and/or GNAS, detected by NGS, demonstrated high sensitivity and specificity for intraductal papillary mucinous neoplasms (IPMNs), a type of mucinous PC. However, the sensitivity for mucinous cystic neoplasms (MCNs) was lower, suggesting the need for additional markers for comprehensive MCN detection.
- Superiority of NGS over Sanger Sequencing: NGS exhibited superior sensitivity for detecting KRAS/GNAS mutations in preoperative PCF compared to Sanger sequencing. This highlights the importance of utilizing highly sensitive molecular techniques for accurate PC characterization.
- Identification of Advanced Neoplasia Markers: The preoperative detection of mutations or deletions in TP53, PIK3CA, and/or PTEN, with mutant allele frequencies (MAFs) comparable to KRAS and/or GNAS mutations, was strongly indicative of advanced neoplasia (high-grade dysplasia and invasive adenocarcinoma) within IPMNs.
- Low-Level Mutations as Potential Risk Indicators: The study identified low-level mutations in TP53, PIK3CA, and/or PTEN in IPMNs with low-grade dysplasia. This suggests that these mutations might serve as early indicators of malignant transformation risk in a subset of IPMNs, warranting closer surveillance.
- Correlation of GNAS MAF with High-Grade Dysplasia: Elevated MAFs for GNAS mutations (>55%) were found to correlate with IPMNs exhibiting high-grade dysplasia, further refining the molecular risk stratification of these lesions.
Impact on Clinical Practice
These findings strongly advocate for the integration of preoperative NGS of PCF into routine clinical practice. NGS-based molecular testing offers a significant advancement in accurately classifying PCs and identifying IPMNs harboring advanced neoplasia. This improved diagnostic precision can guide clinical decision-making, potentially leading to more targeted surveillance strategies, optimized surgical interventions, and ultimately improved patient outcomes.
III. Introduction to Pancreatic Cyst Challenges and Molecular Solutions
The escalating incidental detection of pancreatic cysts (PCs) through advanced abdominal imaging has created a complex diagnostic and management dilemma in contemporary healthcare. PCs represent a heterogeneous group of lesions, ranging from benign entities like pseudocysts and serous cystadenomas (SCAs) to mucinous cysts such as intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs), which carry a risk of progression to invasive pancreatic ductal adenocarcinoma. While pseudocysts and SCAs are generally considered benign and manageable with clinical monitoring, mucinous PCs pose a greater challenge due to their malignant potential.
Distinguishing between these cyst types based on standard clinical findings, imaging characteristics, and traditional cyst fluid studies is often challenging. Furthermore, predicting the progression rate of mucinous PCs to malignancy remains a significant clinical hurdle. Balancing the risk of cancer development with the inherent risks of surgical intervention has led to the development of both consensus-based and evidence-based guidelines to aid in the appropriate surveillance and treatment of PCs. However, multiple studies have demonstrated the limitations of these guidelines, underscoring the need for more precise diagnostic tools to personalize PC management.
In recent years, DNA-based testing has emerged as a powerful adjunct in the assessment of PCs. Despite the frequent limitations in cellular content and fluid volume of PC aspirates for conventional ancillary studies like cytopathology and CEA quantification, the DNA derived from lysed or exfoliated cyst epithelial cells within the PCF offers a valuable resource for genetic analysis. Sequencing studies have elucidated distinct mutational profiles associated with different PC types and their progression to invasive adenocarcinoma. For instance, KRAS mutations are commonly found in IPMNs and MCNs, while GNAS mutations are highly specific to IPMNs. Conversely, VHL mutations or deletions are characteristic of SCAs, and CTNNB1 mutations, in the absence of other genetic alterations, are observed in solid-pseudopapillary neoplasms. Moreover, IPMNs with advanced neoplasia are frequently associated with mutations in TP53, PIK3CA, PTEN, and/or AKT1.
Although numerous studies have explored DNA testing of PCs, many have been limited by retrospective designs, reliance on postoperative specimens, small sample sizes, inadequate follow-up, or insensitive detection strategies. Consequently, the definitive diagnostic utility of PCF DNA analysis in routine clinical practice remained uncertain.
This study aimed to address these limitations by prospectively evaluating DNA-based molecular testing on a large, consecutive cohort of patients. A highly sensitive, targeted next-generation sequencing (NGS) assay was developed, focusing on genes known to be frequently mutated or deleted in PCs and those with advanced neoplasia (KRAS, GNAS, NRAS, HRAS, BRAF, CTNNB1, TP53, PIK3CA, PTEN, and AKT1). While VHL was not included in the NGS panel due to technical constraints, it was assessed using Sanger sequencing, acknowledging its lower sensitivity compared to NGS. This molecular testing was implemented within a CLIA-certified and CAP-accredited clinical laboratory, utilizing PCF obtained through endoscopic ultrasound-fine needle aspiration (EUS-FNA) as part of routine PC assessment. The primary objectives were to: (1) determine the prevalence and distribution of genetic alterations in PCs, (2) evaluate the diagnostic accuracy of molecular analysis using both NGS and Sanger sequencing, and (3) compare these molecular findings with established diagnostic modalities in the preoperative evaluation of PCs, based on surgical pathology follow-up.
IV. Materials and Methods: Prospective Study Design and Molecular Analysis
Study Cohort and Sample Collection
This prospective study received ethical approval from the University of Pittsburgh Institutional Review Board (IRB# PRO13020493). Between January 2014 and July 2017, a total of 673 PCF specimens obtained via EUS-FNA were prospectively submitted to the Molecular & Genomic Pathology Laboratory at the University of Pittsburgh Medical Center (UPMC) for molecular testing. In all cases, the clinical indication for PCF molecular testing was a clinical suspicion of a mucinous PC. Patient demographics, clinical presentation, EUS findings, fluid viscosity (as documented by the endoscopist), CEA analysis, and cytopathological diagnoses were meticulously recorded through medical record review. Main duct dilatation on endoscopy was defined as a diameter ≥5 mm, consistent with established guidelines. The presence of a mural nodule was defined as a uniform echogenic nodule of any size lacking a lucent center or hyperechoic rim, according to established criteria. A PCF CEA value >192 ng/mL was used as the threshold for elevated CEA. Cytopathology specimens were assessed for adequacy using a three-tiered system: satisfactory, less than optimal, and unsatisfactory. Satisfactory specimens contained sufficient epithelial cells and/or mucin representative of the target cyst. Less than optimal specimens had scant epithelium without mucin but with at least a few histiocytes. Unsatisfactory specimens were virtually acellular and lacked mucin. Malignant cytopathology was defined as a diagnosis of at least suspicious for adenocarcinoma or positive for adenocarcinoma. Pathology slides from all surgical specimens were reviewed, and diagnoses for all PCs were established based on standard histomorphological criteria. Advanced neoplasia was defined as mucinous PCs (IPMN and MCN) diagnosed with high-grade dysplasia or invasive adenocarcinoma, consistent with established definitions. Pathological staging was performed according to the American Joint Committee on Cancer Cancer Staging Manual (eighth edition).
Molecular Testing Procedures
Molecular testing was conducted prospectively as part of routine clinical care, with a turnaround time of 14 days (mean, 10 days) within the CLIA-certified and CAP-accredited Molecular and Genomic Pathology Laboratory at UPMC, at a cost of $750 per PCF specimen. Genomic DNA was extracted from EUS-FNA-obtained PCF using the MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche, Indianapolis, Indiana, USA) on a Compact MagNA Pure instrument (Roche, Indianapolis, Indiana, USA). DNA concentration was quantified using the Qubit V.2.0 Fluorometer with the dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, Maryland, USA). Amplification-based targeted NGS (PancreaSeq) was performed using primers designed for genomic regions of interest, including KRAS, GNAS, NRAS, HRAS, BRAF, CTNNB1, TP53, PIK3CA, PTEN, and AKT1, with primer sequences and performance characteristics previously described. Amplicons were barcoded, purified, and ligated with specific adapters. DNA quantity and quality were assessed using the 2200 TapeStation (Agilent Technologies, Santa Clara, California, USA). Template preparation and enrichment were performed using the Ion One Touch 2 and One Touch ES, enabling testing on Ion Sphere Particles on a semiconductor chip. Massively parallel sequencing was carried out on an Ion Torrent Personal Genome Machine Sequencer or Ion Proton, following the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, Maryland, USA), and data were analyzed using Torrent Suite Software V.3.4.2. Bioinformatic data analysis details are available in the online supplementary material. The limit of detection was 5% mutant allele frequency (MAF) at 500× coverage or 3% MAF at 1000× coverage for each tested region, with a minimal depth of coverage of 500×. For each identified mutation, MAF was calculated as the ratio of mutant allele reads to total reads (mutant + wild-type) and reported as a percentage. Copy number assessment was performed as previously described. Biallelic inactivation was inferred from a decrease in sequencing coverage below established cut-offs, coupled with the presence of a sequence variant at high MAF.
Sanger sequencing was employed to analyze exons 1–3 of the VHL gene. DNA amplification was performed using primers flanking VHL exons 1, 2, and 3. The quality of PCR products was evaluated by agarose gel electrophoresis. Bidirectional Sanger sequencing was performed using the BigDye Terminator Kit on an ABI3730 instrument (Thermo Fisher Scientific, Waltham, Maryland, USA). Mutation detection was performed using Mutation Surveyor V.3.01 (SoftGenetics, State College, Pennsylvania), with a limit of detection of approximately 10%–20% mutant alleles in a background of normal DNA. The primers included exon–intron boundaries, enabling the detection of deletions and insertions within exons or complete exon loss through visual inspection of electropherograms. However, this method does not detect loss of the entire VHL gene.
Statistical Analysis
Statistical analyses were performed using SPSS Statistical software, V.23 (IBM, Armonk, New York, USA). Differences in mutational status for dichotomous variables were compared using Fisher’s exact test. Sensitivity and specificity were calculated using standard 2×2 contingency tables for cases with confirmed diagnostic pathology. Statistical significance was defined as a p-value of <0.05.
V. Results: Molecular Findings and Clinicopathological Correlations
Patient Cohort and Specimen Characteristics
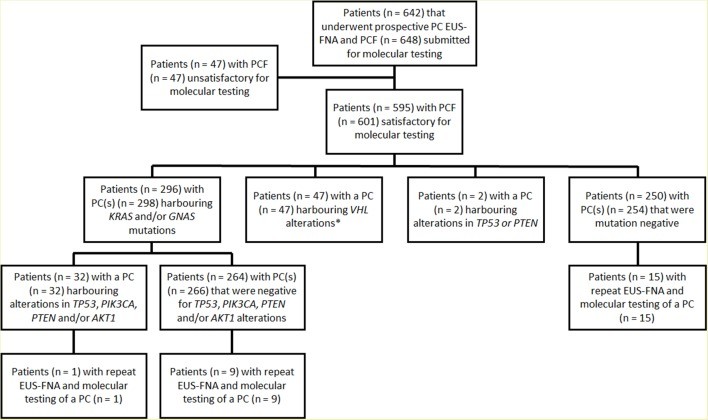
A total of 673 EUS-FNA-obtained PCF specimens from 642 patients were prospectively analyzed for genetic alterations over a 43-month period (Figure 1). Among these, 626 (93%) specimens from 595 patients were deemed satisfactory for molecular testing (Table 1 and online supplementary data). The remaining 47 cases were excluded due to insufficient DNA for analysis. Six of 595 (1%) patients submitted two separate specimens corresponding to distinct PCs. Additionally, 25 of 595 (4%) patients underwent repeat EUS-FNA and molecular testing of the same PC during the study period.
Figure 1: Study cohort illustrating patient and pancreatic cyst fluid (PCF) specimen flow through the prospective molecular testing process. EUS-FNA, endoscopic ultrasound–fine needle aspiration; PC, pancreatic cyst.
While sufficient for molecular studies, cyst fluid volume was insufficient for CEA analysis in 174 of 626 (28%) cases. Furthermore, 375 (60%) specimens were categorized as either less than optimal (n=297, 47%) or unsatisfactory (n=78, 13%) for cytopathological diagnosis, primarily due to absent to scant cellularity.
Prevalence and Distribution of Genetic Alterations
The DNA concentration in submitted EUS-FNA-obtained PCF specimens ranged from 0.01 to 248 ng/uL (mean, 6.93 ng/uL; median, 4.7 ng/uL). Overall, genetic alterations within the 11-gene panel were detected in 357 (57%) PCs. NGS analysis identified activating mutations in KRAS, GNAS, BRAF, and CTNNB1 in 264 (42%), 162 (26%), 5 (1%), and 4 (1%) cases, respectively. No mutations were detected in HRAS and NRAS. In total, KRAS and/or GNAS mutations were present in 308 (49%) PCs, with 119 (19%) cases harboring mutations in both genes (online supplementary table 2). Multiple KRAS mutations were observed in 10 specimens, involving various combinations of codon 12, 13, and 61 substitutions. MAFs for KRAS ranged from 3% to 55% (mean, 24%; median, 24%). Multiple GNAS mutations were detected in three specimens, consisting of substitutions in codons 201 and 227. GNAS MAFs ranged from 3% to 92% (mean, 28%; median, 26%). Two PCs exhibited GNAS MAFs >55%, specifically 88% and 92%. BRAF and CTNNB1 MAFs ranged from 24% to 46% and 6% to 46%, respectively. Mutations in BRAF and CTNNB1 were exclusively observed in the presence of a KRAS and/or GNAS mutation. Sanger sequencing of VHL exons 1–3 identified VHL mutations and/or deletions throughout the gene coding sequence in 47 of 626 (8%) PCs.
NGS analysis also assessed the status of TP53, PIK3CA, PTEN, and AKT1 (Table 2). Genetic alterations in TP53, PIK3CA, PTEN, and AKT1 were identified in 24 (4%), 11 (2%), 2 (1%), and 1 (1%) PCs, respectively. Overall, alterations in TP53, PIK3CA, PTEN, and AKT1 were present in 35 (6%) cases. For TP53 and PTEN, genetic alterations included mutations and/or deletions throughout the gene coding sequence. MAFs for TP53, PTEN, and AKT1 ranged from 4% to 43%, 11%, and 8%, respectively. Homozygous deletions in TP53 and PTEN were detected in 2 (1%) and 1 (1%) cases, respectively. Alterations in PIK3CA were activating point mutations in exon 9 (n=7) and/or exon 20 (n=5) with MAFs ranging from 3% to 50%.
PCs with alterations in TP53, PIK3CA, and/or PTEN were significantly associated with co-occurring mutations in KRAS and/or GNAS (p<0.001). Notably, only three cases with TP53 mutation (n=2) or PTEN deletion (n=1) were wild type for both KRAS and GNAS. Among the two TP53 mutant cases, one also harbored a VHL deletion, while the other was negative for other genetic alterations. No other genetic alterations were found in the single PC with a PTEN deletion.
Correlation with Clinicopathological Features
Table 1 presents the clinical and pathological characteristics of 595 patients with PCs, correlated with KRAS, GNAS, and VHL mutational status. Table 2 shows the correlation with TP53, PIK3CA, PTEN, and AKT1 status. KRAS/GNAS mutations were more frequent in PCs located in the body and tail of the pancreas (59%) compared to the head, neck, and uncinate (43%). PCs with KRAS/GNAS mutations were also associated with cyst multifocality (66% mutant vs. 34% wild type, p=0.002) and increased fluid viscosity (73% mutant vs. 27% wild type, p<0.001). Elevated CEA (>192 ng/mL) was more common in KRAS/GNAS mutant PCs (74% mutant vs. 26% wild type, p<0.001). In contrast, VHL mutations were associated with symptomatic presentation (p=0.036) and absence of cyst multifocality (p=0.002).
PCs with TP53, PIK3CA, PTEN, and/or AKT1 alterations were more frequently observed in men (9% mutant vs. 4% in women, p=0.014), in cysts ≥3 cm in size (9% mutant vs. 91% wild type, p=0.025), and associated with main duct dilatation (12% mutant vs. 88% wild type, p=0.008). Malignant cytopathology was strongly correlated with these alterations (70% mutant vs. 30% wild type).
Follow-up and Diagnostic Surgical Pathology
Follow-up data were available for 571 of 595 (96%) patients, ranging from 1 to 42 months (mean, 27 months; median, 26 months). Diagnostic pathology was obtained for 102 of 595 (17%) patients who underwent surgical resection within 1–16 months (mean, 4 months; median, 3 months) after initial EUS-FNA and molecular testing (online supplementary table 2). Surgical indications for most PCs, except for 2 SCAs, 8 cystic pancreatic neuroendocrine tumors (PanNETs), and 14 pseudocysts, were based on concerns for advanced neoplasia in mucinous PCs, guided by Fukuoka guidelines and molecular testing considerations.
Preoperative KRAS and/or GNAS mutations were detected in all 56 IPMNs within the resection cohort. KRAS mutations were also identified in two MCNs with high-grade dysplasia and one MCN with low-grade dysplasia. However, the remaining seven MCNs with low-grade dysplasia were KRAS/GNAS-negative. MAFs for KRAS and GNAS ranged from 3% to 47% and 3% to 92%, respectively. The two PCs with MAFs >55% both corresponded to IPMNs with high-grade dysplasia. No KRAS and/or GNAS mutations were found in non-mucinous PCs in the resection cohort.
VHL alterations were preoperatively identified in two of three SCAs. While Sanger sequencing failed to detect a VHL alteration in one SCA by EUS-FNA, repeat testing of the surgical resection specimen confirmed a VHL frameshift mutation. No VHL alterations were observed in other mucinous and non-mucinous PCs.
Genetic alterations in TP53, PIK3CA, and/or PTEN were found in all 13 IPMNs with adenocarcinoma, 2 IPMNs with high-grade dysplasia, 3 IPMNs with low-grade dysplasia, and 1 MCN with low-grade dysplasia (Table 3 and online supplementary table 3). MAFs for TP53, PIK3CA, and PTEN ranged from 8% to 43%, 3% to 50%, and 10%, respectively. Except for the MCN with low-grade dysplasia, co-mutations in KRAS and/or GNAS were detected in all PCs with TP53, PIK3CA, and/or PTEN alterations. In IPMNs with adenocarcinoma and high-grade dysplasia, MAFs for KRAS and/or GNAS were at least equal to MAFs for TP53, PIK3CA, and/or PTEN (Figure 2). The three IPMNs with low-grade dysplasia harbored activating PIK3CA mutations, but their MAFs were lower than KRAS MAFs. Two IPMNs with high-grade dysplasia lacked TP53, PIK3CA, and/or PTEN alterations, but exhibited GNAS MAFs >55%. The remaining MCNs with high-grade dysplasia, IPMNs and MCNs with low-grade dysplasia, and non-mucinous PCs were negative for TP53, PIK3CA, PTEN, and/or AKT1 alterations.
Figure 2: Illustrative case of DNA-based molecular testing guiding diagnosis in a pancreatic head cyst. (A, B) Imaging findings and (C) cytopathology results contrasted with (D) surgical pathology revealing adenocarcinoma arising in an IPMN, highlighting the clinical utility of molecular analysis. MAFs, mutant allele frequencies.
Among the 469 patients with follow-up data but without diagnostic surgical pathology, 230 (49%) had KRAS and/or GNAS mutant PCs. Fourteen of these 230 (6%) also had mutations in TP53, PIK3CA, and/or AKT1. However, MAFs for TP53, PIK3CA, and/or AKT1 (4%–9%) were lower than MAFs for KRAS and/or GNAS (28%–45%). Two patients with TP53 mutant PCs (MAFs 5%) were wild type for KRAS and GNAS. One of these TP53 mutant cases also had a VHL deletion. None of these PCs showed concerning features for advanced neoplasia by imaging or cytopathology, and all 16 patients remained alive and without pancreatic cancer on follow-up.
Repeat EUS-FNA and molecular testing in 25 patients showed consistent KRAS and GNAS genetic status compared to initial testing. However, in one case with a KRAS and TP53 mutation on initial testing, the TP53 mutation was absent on subsequent testing, with corresponding MAF reduction for KRAS. The remaining cases showed essentially unchanged MAFs for KRAS and/or GNAS between initial and repeat specimens.
Diagnostic Performance of Molecular Testing and Comparison with Other Modalities
Based on 102 cases with diagnostic pathology, preoperative NGS detection of KRAS and/or GNAS mutations exhibited 100% sensitivity and 96% specificity for IPMNs (Table 4). For both IPMNs and MCNs, KRAS and/or GNAS mutations had 89% sensitivity and 100% specificity. Increased fluid viscosity and elevated CEA showed lower sensitivities (77% and 57%, respectively) and specificities (89% and 80%, respectively).
In combination with KRAS and/or GNAS mutations, alterations in TP53, PIK3CA, and/or PTEN had 88% sensitivity and 97% specificity for IPMNs with advanced neoplasia. Sensitivity and specificity increased to 100% when selection criteria were modified to include cases with either GNAS MAFs >55% or TP53/PIK3CA/PTEN MAFs at least equivalent to KRAS/GNAS MAFs. In contrast, main pancreatic duct dilatation and mural nodules on EUS had sensitivities of 47% and 35%, and specificities of 74% and 94%, respectively. Preoperative cytopathological diagnosis of at least suspicious for adenocarcinoma showed 35% sensitivity and 97% specificity.
For mucinous PCs with advanced neoplasia (IPMNs and MCNs), the combination of KRAS and/or GNAS mutations with TP53, PIK3CA, and/or PTEN alterations yielded 79% sensitivity and 96% specificity. Modified selection criteria incorporating GNAS MAFs >55% or TP53/PIK3CA/PTEN MAFs equal to or greater than KRAS/GNAS MAFs improved sensitivity to 89% and maintained 100% specificity. Cytopathology diagnosis of at least suspicious for adenocarcinoma had 32% sensitivity and 98% specificity.
Prospective Sanger Sequencing Analysis of KRAS and GNAS
Prior to this study, prospective EUS-FNA PCF testing for KRAS and GNAS using Sanger sequencing was performed for 175 PCs from 169 patients over 12 months (online supplementary material and supplementary table 3). Of these, 159 (91%) PCs from 153 patients were satisfactory for molecular analysis. Sanger sequencing detected KRAS and/or GNAS mutations in 39% of PCs (62 of 159), compared to 49% by NGS in the current study. In the Sanger sequencing cohort, 34 cases had diagnostic pathology, including 5 IPMNs with adenocarcinoma, 1 IPMN with high-grade dysplasia, 12 IPMNs with low-grade dysplasia, and 2 MCNs with low-grade dysplasia. Sanger sequencing detected KRAS and/or GNAS mutations in 13 of 18 (72%) IPMNs and 0 of 2 (0%) MCNs. Overall, Sanger sequencing for KRAS and/or GNAS mutations had 72% sensitivity and 100% specificity for IPMNs, and 65% sensitivity and 100% specificity for both IPMNs and MCNs. Repeat NGS testing for KRAS and GNAS on 24 cases from the Sanger cohort (8 KRAS/GNAS mutant and 16 KRAS/GNAS wild type by Sanger) confirmed consistent KRAS and GNAS status by NGS. However, 3 of 16 KRAS/GNAS wild type PCs by Sanger sequencing were found to harbor KRAS (n=3) and/or GNAS (n=1) mutations by NGS, highlighting the increased sensitivity of NGS.
VI. Discussion: Clinical Implications of Preoperative NGS in Pancreatic Cyst Management
When evaluating a patient with a pancreatic cyst, critical questions guide clinical decision-making: What type of PC is present? Is it mucinous or non-mucinous, given the malignant potential of mucinous cysts? Does a mucinous PC harbor malignancy? If not malignant, what is its lifetime malignant potential?
This prospective study, mirroring findings from retrospective and postsurgical specimen studies, demonstrates that preoperative DNA-based PC testing, specifically NGS for KRAS and/or GNAS mutations, is highly effective for mucinous PC diagnosis, achieving 89% sensitivity and 100% specificity. For IPMNs specifically, KRAS and/or GNAS mutation detection reached 100% sensitivity, and GNAS mutations alone were 100% specific for IPMNs. However, KRAS mutations were detected in only 30% of MCNs. While KRAS mutations are common in MCNs, their prevalence increases with dysplasia severity. In this study, KRAS mutations were found in 100% of MCNs with high-grade dysplasia but only 13% of those with low-grade dysplasia. Given the typical recommendation for surgical resection of MCNs due to their malignant potential and frequent occurrence in younger patients and the pancreatic body/tail, KRAS assessment alone is insufficient for MCN detection. Additional markers are needed to enhance the sensitivity of DNA testing for MCNs. Notably, one MCN with low-grade dysplasia in this study harbored a PTEN deletion without a KRAS mutation, potentially serving as a marker for MCNs. Despite lower sensitivity for MCNs, DNA testing for mucinous PCs demonstrated higher sensitivity and specificity compared to surrogate markers like fluid viscosity and CEA.
Molecular markers are also valuable for excluding mimics of mucinous PCs. Oligocystic and unilocular SCA variants can be clinically and radiographically indistinguishable from branch duct IPMNs and MCNs. VHL alterations are highly specific for SCAs, although they may also occur in cystic PanNETs. In this study, VHL mutations and/or deletions by Sanger sequencing showed 100% specificity for SCAs. However, preoperative VHL alteration detection failed in one SCA, likely due to the limitations of Sanger sequencing. Repeat testing of the surgical specimen confirmed a VHL mutation. Prospective Sanger sequencing for KRAS and GNAS in a prior cohort showed lower sensitivity (72% for IPMNs) compared to NGS (100% in this study), attributable to Sanger sequencing’s higher detection limit (10%–20% mutant alleles) versus NGS (3%–5%). A significant proportion of KRAS (24%) and GNAS (22%) mutant cysts had MAFs below Sanger sequencing’s detection limit, highlighting NGS’s superiority for preoperative PCF molecular analysis. Therefore, Sanger sequencing is not recommended for preoperative PCF molecular evaluation due to its lower sensitivity. NGS is the preferred method for accurate and sensitive detection of clinically relevant mutations in PCF.
PC DNA testing is particularly valuable for distinguishing between mucinous PCs with low-grade dysplasia and those with advanced neoplasia. TP53 and mTOR pathway gene alterations are implicated in mucinous PC malignant transformation. Combining KRAS and/or GNAS mutations with TP53, PIK3CA, and/or PTEN alterations achieved 79% sensitivity and 96% specificity for mucinous PCs with advanced neoplasia. This study’s sensitivity is higher than reported in some prior studies, potentially due to deeper NGS coverage (minimum 500×, often >1000×) compared to others (e.g., median 200×). Deeper coverage enhances assay reliability and sensitivity.
Two key findings from this study further improved the detection of mucinous PCs with advanced neoplasia. First, GNAS MAFs >55% were associated with IPMNs with high-grade dysplasia. Activating KRAS and GNAS mutations are typically heterozygous with MAFs ≤50% due to non-neoplastic cell contamination. Mutant allele-specific imbalance (MASI), where MAF >50%, indicates increased mutant allele dosage via wild-type allele deletion or mutant allele copy number gain. KRAS MASI in PCF has been previously linked to advanced neoplasia. This study newly identifies GNAS MASI in PCF, associated with high-grade dysplasia in IPMNs.
Second, TP53/PIK3CA/PTEN MAFs at least equal to KRAS/GNAS MAFs correlated with advanced neoplasia in IPMNs. While KRAS/GNAS and TP53/PIK3CA/PTEN mutations were common in advanced neoplasia, mutations in KRAS/GNAS and PIK3CA were also found in IPMNs with low-grade dysplasia and clinically indolent PCs. However, in these cases, TP53/PIK3CA/PTEN MAFs were lower than KRAS/GNAS MAFs. Modifying selection criteria to include GNAS MAF >55% or TP53/PIK3CA/PTEN MAFs at least equivalent to KRAS/GNAS MAFs improved sensitivity and specificity for advanced neoplasia in mucinous PCs to 89% and 100%, respectively. This outperforms cytopathology, which had 32% sensitivity and 98% specificity for diagnosing at least suspicious for adenocarcinoma in mucinous PCs. NGS testing appears superior to other modalities for detecting high-grade dysplasia and invasive adenocarcinoma in mucinous PCs.
The presence of TP53, PIK3CA, and/or PTEN alterations in IPMNs with low-grade dysplasia and clinically low-risk IPMNs is notable. Traditionally, these mutations were thought to arise during progression from low- to high-grade dysplasia and invasive adenocarcinoma. However, recent studies, including this one, suggest early TP53 mutations may occur, potentially indicating higher malignant potential even in low-grade IPMNs. While the natural history of IPMNs with low-level TP53, PIK3CA, and/or PTEN alterations is still being elucidated, these findings suggest they might represent a higher-risk subgroup requiring closer monitoring. Given the high prevalence of PCs and IPMNs, risk-stratified surveillance strategies are needed. Detecting KRAS and/or GNAS mutations and low-level TP53, PIK3CA, and/or PTEN alterations in PCF may provide predictive markers for malignant potential in IPMNs.
Study limitations include surgical selection bias (diagnostic pathology only available for a subset) and testing selection bias (only specimens satisfactory for NGS/Sanger included). However, the 7% failure rate for molecular testing likely minimizes selection bias impact. The follow-up period is relatively short to fully assess the clinical impact of TP53, PIK3CA, PTEN, and/or AKT1 alterations. Longer-term follow-up is planned. The molecular panel, while comprehensive, excluded RNF43, CDKN2A, and SMAD4. RNF43 mutations might improve MCN detection sensitivity, but RNF43 alterations are relatively infrequent in MCNs. CDKN2A and SMAD4 deletions are associated with advanced neoplasia in IPMNs, but the optimized selection criteria in this study (incorporating MAFs) achieved 100% sensitivity and specificity for advanced neoplasia in IPMNs without these genes. Further studies are needed to define the minimal gene set for PC assessment. Finally, this study does not address optimal integration of DNA-based molecular testing into current PC surveillance protocols. While prior work proposed an algorithmic approach using molecular testing for patient stratification, further validation is needed before implementation. This study applied Fukuoka guidelines with consideration of molecular testing’s impact based on prior research.
In conclusion, this large prospective study of preoperative DNA-based molecular testing of EUS-FNA-obtained PCF supports NGS analysis in PCF due to its high sensitivity and specificity for PC classification, especially IPMNs, and for diagnosing advanced neoplasia in IPMNs. Limitations include MCN assessment using KRAS alone and the unsuitability of Sanger sequencing. Future research should focus on integrating DNA-based molecular testing into PC management guidelines.
VII. Acknowledgements
The authors express gratitude to the clinical coordinating staff at the UPMC Digestive Disorders Center, Robyn L Roche and Kate Smith for administrative support, and the Pancreatic Cancer Action Network, Pittsburgh Affiliate, and the National Pancreas Foundation, Western Pennsylvania Chapter for their invaluable support.
VIII. Footnotes
Contributors: ADS, KM, REB, AK, HJZ, AS, AIW and MNN: study concept and design. ADS, KG, REB, AK, HJZ, JSC, KEF, GIP, AS, DLB, AKD, MH, KKL, JWM, SEM, NPO, JFP, AT, AHZ, AIW and MNN: acquisition of data. ADS, AIW and MNN: analysis and interpretation of data. ADS, KM, HJZ and MNN: drafting of the manuscript.
Funding: This study was supported in part by grants from the Pancreatic Cancer Action Network (Translational Research Grant), National Pancreas Foundation, Western Pennsylvania Chapter and the University of Pittsburgh (to AD Singhi).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: University of Pittsburgh Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
IX. References
[List of References as in the original article]
X. Associated Data
Supplementary Materials
Supplementary file 1
gutjnl-2016-313586supp001.pdf (47.4KB, pdf)
Supplementary file 2
gutjnl-2016-313586supp002.pdf (84.4KB, pdf)
Supplementary file 3
gutjnl-2016-313586supp003.pdf (136.2KB, pdf)
Supplementary file 4
gutjnl-2016-313586supp004.pdf (121.5KB, pdf)