INTRODUCTION
Celiac disease (CD) is an autoimmune disorder triggered by gluten in genetically predisposed individuals, affecting the small intestine. Initially recognized primarily in children, CD was long considered a childhood ailment, characterized by malnutrition, growth delays, persistent diarrhea, and significant mortality rates in pediatric cases[1]. However, our understanding has evolved, revealing that celiac disease can manifest at any age. Epidemiology and clinical presentations have shifted dramatically, with a notable rise in adult diagnoses, challenging previous assumptions about its prevalence[2]. The symptomatic spectrum has also broadened from classic malabsorption to encompass subtle presentations like anemia, osteoporosis, and even asymptomatic cases identified through screening of high-risk populations[3, 4].
This review aims to explore the key differences in celiac disease across different age groups, emphasizing the unique aspects of adult-onset CD and its diagnostic and management considerations. Understanding the nuances related to the Age Of Celiac Diagnosis is critical for effective clinical practice.
EPIDEMIOLOGY
Celiac disease is now recognized as a more common condition than previously thought, with prevalence rates varying from 1 in 100 to 1 in 500, depending on the population and diagnostic methods used. Females are diagnosed more frequently [2]. The accuracy of prevalence estimations has improved significantly with highly sensitive serological tests for anti-endomysial, anti-tissue transglutaminase, and anti-deamidated gliadin antibodies. Large-scale screenings using these tests have indicated a CD prevalence as high as 1% in general populations across Europe and North America [5]. However, many of these initial studies focused predominantly on pediatric populations. A study in Catalonia, utilizing serology, found a five times higher CD prevalence in children (1/71) compared to adults (1/357), suggesting a potential latency in adult presentations, which could account for lower adult prevalence figures [6]. Similar trends, with higher pediatric frequencies, have been observed in studies from Brazil and India [7, 8].
Contrasting these findings are prevalence studies in adult populations in Europe, such as the United Kingdom (1.2%) [9] and Finland, where a biopsy-confirmed prevalence of 2.4% was found in adults over fifty [10]. These studies, while not examining pediatric populations, show that prevalence in older adults can be similar to or even exceed rates found in children. A comprehensive review of global CD frequency, largely based on European data, revealed variable but often comparable prevalence and incidence rates between children and adults in recent years [2].
While the latency hypothesis in adults could explain lower prevalence, more research into the natural progression of CD is needed. Adult-onset celiac disease is clearly becoming more common, sometimes reaching or surpassing pediatric levels. Screening methods and population selection influence prevalence rates, with high-risk adult groups and first-degree relatives showing rates exceeding 15% [3]. Furthermore, serological screening alone may not fully capture adult prevalence, as antibody levels can be low or negative in adults, even with histological damage and symptoms consistent with CD [11]. This highlights the complexity of determining the true age of celiac diagnosis prevalence and the need for diverse diagnostic approaches across age groups.
SYMPTOMATOLOGY
A primary distinction between celiac disease in children and adults lies in the clinical presentation at diagnosis. Classical malabsorption is frequently observed in children at diagnosis, whereas it is present in less than 25% of adult cases [12]. Table 1 summarizes the varied clinical manifestations of CD based on the age of diagnosis.
Table 1. Age-Related Major Clinical Findings at Celiac Disease Diagnosis
| Children | Children > 2 yr | Adults |
|---|---|---|
| Diarrhea | Loose stools | Dyspepsia/Irritable Bowel Syndrome |
| Malnutrition | Iron deficiency | Iron deficiency |
| Bloating | Abdominal pain | Constipation |
| Vomiting | Dyspepsia | Osteoporosis |
| Irritability | Growth delay | Arthritis |
| Muscular atrophy | Headache | Hypertransaminasemia |
| Anemia | Pubertal delay | Extraintestinal symptoms |
As individuals age, there is a trend toward less pronounced clinical manifestations [4]. Older children and adults often present with limited symptoms, such as increased stool volume or gas due to lactose malabsorption or bacterial overgrowth. Notably, constipation can be the sole symptom in some adults with celiac disease.
Extraintestinal symptoms are more common in adults with CD and can occur alongside digestive issues or as the primary presentation. These include fatigue, oral ulcers, osteoporosis, and skin lesions. While children can also experience non-digestive symptoms, they are less frequent and typically secondary to digestive symptoms [13]. The shift in symptomatology with age of celiac diagnosis is a critical factor in recognizing and diagnosing CD across the lifespan.
Comorbid diseases are common in both children and adults with CD. However, adults appear to have a higher prevalence of associated pathologies, many of which are autoimmune in nature, mirroring CD’s autoimmune origin. Autoimmune thyroiditis, type 1 diabetes, Sjögren’s syndrome, and dermatitis herpetiformis are well-documented comorbidities of CD [14, 15].
Although malnutrition is a known feature of CD, particularly in classic presentations, it is important to note that overweight and obesity can also be present at diagnosis, especially in adults. Studies indicate that over half of adults diagnosed with CD are obese, while only a small percentage are underweight [16, 17]. This is particularly relevant in regions with high obesity prevalence like Europe and North America. Excess weight, even in adults, should not diminish suspicion for CD.
Functional dyspepsia and irritable bowel syndrome (IBS) are common functional gastrointestinal disorders in adults, but also seen in children. CD prevalence in adults with functional dyspepsia or IBS can be elevated, exceeding 10% in some studies. Current clinical guidelines recommend ruling out underlying CD, through serology or endoscopy, before diagnosing functional dyspepsia or IBS in adults [18, 19]. The varying clinical landscape based on the age of celiac diagnosis underscores the need for tailored diagnostic approaches.
DIAGNOSIS
Diagnosis of celiac disease relies on serological testing for antibodies against deamidated antigliadin peptides, endomysium, or tissue transglutaminase. Histological assessment of small-bowel mucosa, looking for intraepithelial lymphocytosis, villous atrophy, and crypt hyperplasia, is also crucial. Clinical improvement following gluten withdrawal further supports the diagnosis. Interestingly, anti-tissue transglutaminase antibody (tTGA) titers and the severity of histological lesions show an inverse correlation with the age of celiac diagnosis [12]. As diagnostic age increases, antibody levels tend to be lower, and histological damage may be less pronounced. Adults frequently present with inflammatory changes in duodenal biopsies without villous atrophy, such as lymphocytic enteritis (Marsh I) or with crypt hyperplasia (Marsh II), as illustrated in Figure 1.
Figure 1. Relationship Among Age, Antibodies, and Villous Atrophy.
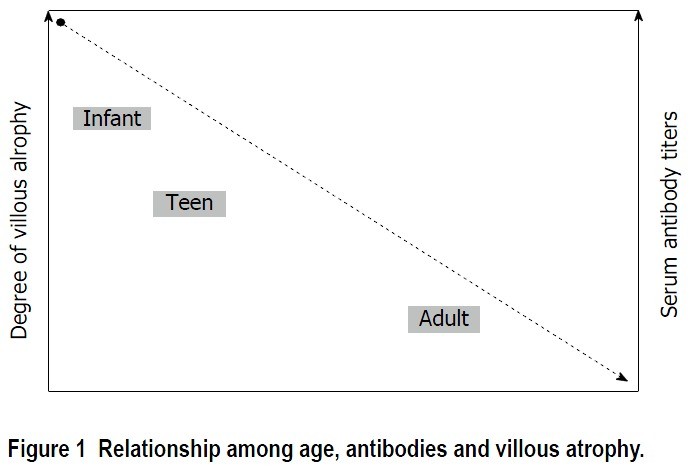 Figure 1
Figure 1
This reduced clinical, serological, and histological expressiveness in adult-onset CD makes diagnosis more challenging compared to pediatric cases. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria, updated in 2012, allow for CD diagnosis in children based on high tTGA titers alone, without requiring duodenal biopsy in certain cases [20]. This is predicated on the strong predictive value of high antibody titers for villous atrophy in children, thus avoiding invasive biopsies. Using pediatric criteria, up to 75% of duodenal biopsies can be avoided in children. However, high antibody titers (> 10 times the upper limit of normal) that permit biopsy omission are less common in adults, occurring in fewer than half of adult cases [21]. Furthermore, upper endoscopy with duodenal biopsies is a routine procedure for adults presenting with dyspeptic symptoms, irrespective of CD serology results [18]. Therefore, relying solely on serology, especially in adults, can be insufficient for accurate diagnosis, highlighting the importance of considering the age of celiac diagnosis in diagnostic protocols.
Correct diagnosis based only on high tTGA titers is infrequent in adults. For lower titers or when clinical suspicion remains, duodenal biopsies are necessary to confirm histological damage before initiating a gluten-free diet. Adults with symptoms and suggestive serology can present with two types of enteropathies on biopsy: (1) Mild villous atrophy and increased intraepithelial lymphocytes (Marsh 3A). If HLA-DQ2 or DQ8 genotypes are present, a gluten-free diet is recommended, with subsequent clinical and histological evaluation. This scenario fulfills 4 out of 5 diagnostic criteria for gluten-sensitive enteropathy proposed by Catassi and Fasano [22] (Table 2). (2) No villous atrophy but marked intraepithelial lymphocytosis (> 25 IELs/100 enterocytes), indicative of lymphocytic enteritis (Marsh I), which can have various non-CD etiologies like Helicobacter pylori infection, NSAID use, infections, or Crohn’s disease. If these are ruled out and a CD-compatible HLA genotype is present, further biopsy analysis can include: IgA antitransglutaminase subepithelial deposit assessment [23] and flow cytometry or immunohistochemistry to evaluate gamma-delta receptor expression on T lymphocytes, which can suggest CD [24].
Table 2. Diagnostic Criteria for Celiac Disease Proposed by Catassi and Fasano [22]
| The presence of signs and symptoms compatible with celiac disease |
|---|
| Positivity of serum celiac disease IgA class autoantibodies at high titer |
| Presence of the predisposing genes HLA-DQ2 and/or -DQ8 |
| Celiac enteropathy at the small intestinal biopsy¹ |
| Resolution of the symptoms and normalization of serology test following the implementation of a gluten-free diet² |
¹Including Marsh-Oberhuber 3 lesions, Marsh-Oberhuber 1-2 lesions associated with positive celiac antibodies positive at low/high titer, or Marsh-Oberhuber 1-3 lesion associated with IgA subepithelial deposits; ²Histological normalization in patients with sero-negative celiac disease or associated IgA deficiency. At least 4 of 5 (or 3 of 4 if the HLA genotype is not performed). HLA: Human leukocyte antigen.
While these advanced techniques can point towards CD in seronegative cases, confirming a diagnosis according to Catassi’s criteria requires evaluating the lymphocytic enteritis response to a gluten-free diet, including a follow-up biopsy after clinical improvement. The long-term implications of lymphocytic enteritis associated with adult CD and its relation to complications remain unclear. Therefore, lymphocytic enteritis in seronegative adults warrants careful CD diagnostic consideration and subsequent monitoring. The diagnostic pathway needs to be carefully considered based on the age of celiac diagnosis and the clinical context.
POSSIBLE PATHOGENIC DIFFERENCES
Intestinal microbiota is increasingly recognized as a factor in celiac disease pathogenesis [25]. Early life colonization and microbiota-immune system interactions may play a significant role in CD development.
Studies comparing intestinal microbiota in duodenal biopsies and stool samples from celiac children and adults to non-celiac controls have shown alterations in celiac patients [26]. Age-related variations in duodenal microbiota composition have also been observed between celiac and non-celiac individuals [27, 28]. Generally, intestinal microbiota richness increases with age. Furthermore, differences exist in the types of bacterial communities found in children versus adults. While these findings are preliminary, they suggest that microbiota-immune system interactions may differ based on age-related microbial composition, potentially contributing to the varying clinical, serological, and histological presentations seen across different age of celiac diagnosis groups.
Intraepithelial lymphocytes (IELs) are key players in the immune response in CD pathogenesis. A hallmark of CD, regardless of age, is an increase in CD3+ lymphocytes expressing the γδ receptor. The precise function of γδ IELs is still under investigation, but they may have a regulatory role in the immune response [29], potentially explaining their correlation with villous atrophy severity in both children and adults [30]. Another IEL population, CD3-, shows an inverse relationship with age, being twice as frequent in children under three compared to adults [31]. These age-related differences in IEL populations may contribute to the varying clinical presentations of CD depending on the age of celiac diagnosis. Further research into IELs and CD pathogenesis is essential for a deeper understanding.
EVOLUTION AND PROGNOSIS
The primary treatment for celiac disease is a strict, lifelong gluten-free diet (GFD). Clinical improvement is generally observed in most patients after starting a GFD. However, in contrast to children, up to 30% of adults may continue to experience symptoms despite gluten withdrawal. This necessitates investigating other potential causes for persistent symptoms in adult celiac patients, such as lactose intolerance, bacterial overgrowth, pancreatic insufficiency, microscopic colitis, or refractory CD. The impact of age of celiac diagnosis on treatment response is a significant consideration.
Beyond symptom persistence, histological recovery also differs between adults and children. Studies indicate that over 50% of adults do not achieve villous atrophy resolution even after two years on a strict GFD [32]. Conversely, children show duodenal mucosa recovery in the vast majority (95%) within the first two years post-diagnosis, although data is less extensive [33]. The main factor contributing to poor mucosal recovery in adults may be unintentional ingestion of small amounts of gluten, which is possibly more common in adults due to less closely monitored diets compared to children.
Strict adherence to a GFD and duodenal mucosa normalization are critical goals in adult CD management. Dietary non-compliance and persistent histological damage are linked to an increased risk of lymphoproliferative disorders in adults, the most severe complication associated with CD [34]. A Swedish population study found that celiac patients with persistent villous atrophy during follow-up had twice the risk of developing malignant lymphoproliferative disease, particularly T-cell lymphoma [35]. These findings underscore the importance of a follow-up biopsy around the second year post-diagnosis to assess villous atrophy recovery. This allows for the identification of individuals at higher risk of complications and enables closer monitoring of dietary adherence. Prognosis and follow-up strategies need to be tailored based on the age of celiac diagnosis and individual risk factors.
Celiac disease patients are at risk for other complications requiring monitoring. Reduced bone mineral density, likely due to vitamin D deficiency, is common. However, the fracture risk in CD patients remains unclear, and bone densitometry alone may not reliably identify those at high fracture risk. Bone densitometry is recommended for high-risk adult CD patients, including post-menopausal women, men over 55, and those with pre-existing osteopenia diagnosed before CD [36]. Further research is needed to determine the effectiveness and cost-effectiveness of routine bone densitometry in all adult CD patients at diagnosis and to optimize follow-up frequency [37]. Children may also have reduced bone mass at diagnosis but are more likely to fully recover bone mass within 6-12 months of a GFD, compared to adults. Routine bone densitometry is generally not needed in newly diagnosed pediatric patients with uncomplicated CD; however, monitoring growth and development is essential [38].
Hyposplenism can affect over one-third of adult CD patients but is not typically a complication in children. The incidence of hyposplenism correlates with the duration of gluten exposure before diagnosis and is higher in those with concurrent autoimmune disorders or pre-malignant conditions [39]. Splenic function assessment may be considered in select adult CD patients, such as older individuals at diagnosis, those with autoimmune or premalignant conditions, and those with a history of major infections or thromboembolism. Pitted red cell counting is a reliable, quantitative, and inexpensive diagnostic tool for hyposplenism [40]. Protein-conjugate vaccines should be recommended for patients with significant hyposplenism, defined by a pitted red cell value above 10% and/or low IgM memory B cell frequency. The prevalence and management of complications differ significantly based on the age of celiac diagnosis.
Pediatric CD follow-up can be similar to adults, but bone densitometry and follow-up biopsies are more selectively indicated. Children with good GFD adherence and normalized antibody levels can be followed annually rather than every two years, primarily for early detection of associated conditions and to ensure normal growth and development. Malignant complications and refractory CD are almost exclusively adult phenomena, necessitating different follow-up strategies compared to pediatric patients. The age of celiac diagnosis fundamentally shapes the long-term management and monitoring of celiac disease.
CONCLUSION
Celiac disease exhibits significant differences between children and adults. In adults, the disease presentation is often less pronounced than in childhood. Clinicians need to be aware of the subtle clinical profiles in adults, including atypical or minor symptoms, lower antibody titers, and milder mucosal lesions (Table 3). Pediatric diagnostic criteria (ESPGHAN) are not always applicable to adults. Duodenal biopsy is usually necessary for adult CD diagnosis and follow-up to assess mucosal recovery and detect complications. Understanding these age of celiac diagnosis related nuances is crucial for timely and accurate diagnosis and effective long-term management of celiac disease across all age groups.
Table 3. Summary of Key Features Associated with the Presentation of Celiac Disease in Adults
| High prevalence, even in advanced age |
|---|
| Oligosymptomatic presentation |
| Serology may have a low diagnostic yield |
| Duodenal biopsy usually shows mild atrophy or lymphocytic enteritis |
| Lymphocytic enteritis is a common presentation in adult celiac |
| The study of duodenal biopsy by an expert pathologist and the use of advanced techniques like flow cytometry may be useful for the diagnosis |
| Monitoring of the strict dietary compliance and the recovery of villous atrophy |
| The presence of associated complications should be identified at an early stage |
Footnotes
Conflict-of-interest statement: The authors declare that there are no conflicts of interest regarding the publication of this paper.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 3, 2015
First decision: August 4, 2015
Article in press: October 13, 2015
P- Reviewer: Bener A S- Editor: Ji FF
L- Editor: A E- Editor: Li D
References
[1] Anderson RP, Henry MJ, Taylor R, Middleton-Woods J, Mcgahan T, Antalis TM, et al. Tissue transglutaminase autoantibody ELISA: high sensitivity and specificity in celiac disease. Clin Chim Acta. 1998;275(1):23-34. [PMID: 9711737 DOI: 10.1016/s0009-8981(98)00075-3]
[2] Catassi C, Kryszak D, Bhatti B, Sturgeon C, Helzlsouer KJ, Clipp SL, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42(7):530-8. [PMID: 20923253 DOI: 10.3109/07853890.2010.512183]
[3] Volta U, De Giorgio R, Petrolini N, Diana G, Berti I, Caio G, et al. The changing clinical spectrum of coeliac disease: a 15-year experience of an Italian referral centre. Aliment Pharmacol Ther. 2001;15(8):1131-8. [PMID: 11472214 DOI: 10.1046/j.1365-2036.2001.01031.x]
[4] Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120(3):636-51. [PMID: 11223148 DOI: 10.1053/gast.2001.22123]
[5] Rewers M. Epidemiology of celiac disease: what are the prevalence, incidence, and risk factors? Gastroenterology. 2005;128(4 Suppl 1):S47-51. [PMID: 15824133 DOI: 10.1053/j.gastro.2005.02.031]
[6] Ribes-Koninckx C, Domínguez-Ortega G, Morán M, Catalán P, Rica I, Polanco I, et al. Celiac disease prevalence in the first decade of life in Spain. J Pediatr Gastroenterol Nutr. 2008;46(2):152-6. [PMID: 18223280 DOI: 10.1097/mpg.0b013e31815a2c61]
[7] Gandolfi L, Pratesi R, Tauil PL, Amorim AL, Hummel M, Rizzo G, et al. Celiac disease in Brazil: prevalence among blood donors and comparison of diagnostic criteria. Dig Dis Sci. 2000;45(12):2417-23. [PMID: 11152132 DOI: 10.1023/a:1005644919123]
[8] Makharia GK, Verma AK, Amarcha R, Bhatnagar S, Das P, Sachdev V, et al. Prevalence of celiac disease in India: a community-based study in children. Indian Pediatr. 2011;48(4):301-4. [PMID: 21525646 DOI: 10.1007/s13312-011-0055-1]
[9] Johnston SD, Watson RG, McMillan SA, Sloan J, Love AH. Coeliac disease: is adult presentation increasing? Postgrad Med J. 1990;66(775):370-2. [PMID: 2382425 DOI: 10.1136/pgmj.66.775.370]
[10] Collin P, Reunala T, Rasmussen M, Vilppula A, Keyriläinen O, Koskinen O, et al. Prevalence of celiac disease in elderly people. J Am Geriatr Soc. 2004;52(12):2083-6. [PMID: 15571540 DOI: 10.1111/j.1532-5415.2004.52601.x]
[11] Rodrigo L, Riestra S, Fuentes D, González S, López-Vázquez A, Alvarez N, et al. Diverse presentations of celiac disease in a large adult cohort in a tertiary care center. World J Gastroenterol. 2011;17(39):4503-7. [PMID: 22072853 DOI: 10.3748/wjg.v17.i39.4503]
[12] Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Age at diagnosis and clinical presentation of celiac disease: analysis of 600 adult patients. Am J Gastroenterol. 1998;93(3):413-7. [PMID: 9517847 DOI: 10.1111/j.1572-0241.1998.00413.x]
[13] Murch SH, Jenkins HR. Coeliac disease. Eur J Gastroenterol Hepatol. 1996;8(7):614-25. [PMID: 8842501]
[14] Sategna-Guidetti C, Volta U, Bianchi FB, Cesari P,ческому P, дивноне J, et al. Prevalence of thyroid disorders in adult patients with coeliac disease. Am J Gastroenterol. 2001;96(3):751-5. [PMID: 11261538 DOI: 10.1111/j.1572-0241.2001.03649.x]
[15] Elfström P, Granath F, Ekström-Smedby K, Ludvigsson JF. Increased risk of lymphoma in individuals with celiac disease. Gastroenterology. 2011;140(3):705-12. [PMID: 21145879 DOI: 10.1053/j.gastro.2010.11.040]
[16] Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical features, and effect of gluten-free diet. Am J Gastroenterol. 2006;101(10):2356-9. [PMID: 17032154 DOI: 10.1111/j.1572-0241.2006.00778.x]
[17] Hallert C, Grütznerström E, Larsson E, Tholstrup J, Hällström T. Increased body mass index in celiac disease is reversed after 12 months on a gluten-free diet. Scand J Gastroenterol. 2001;36(1):22-9. [PMID: 11204023 DOI: 10.1080/003655201750003413]
[18] Shahbazkhani B, Farsiani MA, Mehrabani D, Jedi S, Eshraghian A, Rostami-Nejad M, et al. Yield of routine duodenal biopsies in patients undergoing upper endoscopy for various indications. World J Gastroenterol. 2009;15(27):3375-8. [PMID: 19588493 DOI: 10.3748/wjg.15.3375]
[19] Sanders DS, Patel D, Stephenson Z, Gleeson M, Badreldin R, Wicks AC, et al. Coeliac disease is underdiagnosed in irritable bowel syndrome: a prospective study. Clin Gastroenterol Hepatol. 2003;1(5):371-8. [PMID: 15010827 DOI: 10.1053/cgh.2003.50064]
[20] Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for diagnosing coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136-60. [PMID: 22197656 DOI: 10.1097/mpg.0b013e31821acec2]
[21] Sugai E, Moreno ML, Hwang HJ, Núñez C, Garrote JA, Smecuol E, et al. Celiac disease serology in adult patients: is biopsy avoidable? World J Gastroenterol. 2010;16(33):4133-9. [PMID: 20738219 DOI: 10.3748/wjg.v16.i33.4133]
[22] Catassi C, Fasano A. Celiac disease. Curr Opin Gastroenterol. 2008;24(6):687-91. [PMID: 18849755 DOI: 10.1097/mog.0b013e328311a848]
[23] Dieterich W, Schuppan D, Junker E, Barwick T, Kupka R, Kagnoff MF, et al. Subepithelial deposits of transglutaminase 2-IgA antibodies in small bowel biopsies of patients with suspected celiac disease. Am J Gastroenterol. 2002;97(7):1696-701. [PMID: 12135041 DOI: 10.1111/j.1572-0241.2002.05846.x]
[24] Malamut G, Verkarre V, MacIntyre E, Terris B, Cerf-Bensussan N, Heyman M. Intraepithelial lymphocytes expressing gammadelta T cell receptor in children with celiac disease. J Pediatr Gastroenterol Nutr. 1997;25(3):318-24. [PMID: 9322443 DOI: 10.1097/00005176-199709000-00014]
[25] Olivares M, Laparra JM, Leeuwenkamp O, González N, Schipper R, Sanz Y. The ACE-inhibitory peptide-producing strain Lactobacillus casei subsp. paracasei CECT 5061 modulates the intestinal immune response and gut microbiota in healthy adults. Eur J Nutr. 2014;53(2):449-58. [PMID: 23736714 DOI: 10.1007/s00394-013-0552-1]
[26] Nistal E, Caminero A, Vivas S, Vaquero L, Herrán AR, Santander R, et al. Differences in faecal bacteria populations and faecal metabolites associated with celiac disease. Biochim Biophys Acta. 2012;1822(2):183-90. [PMID: 22057494 DOI: 10.1016/j.bbadis.2011.10.016]
[27] Cenit MC, Olivares M, Codoñer-Franch P, Sanz Y. Intestinal microbiota and celiac disease: an immunomodulatory relationship. BioMed Res Int. 2015;2015:382142. [PMID: 25866835 DOI: 10.1155/2015/382142]
[28] Collado MC, Sanz Y. Bacteriophages and probiotics in human health. FEMS Microbiol Rev. 2007;31(4):479-87. [PMID: 17588270 DOI: 10.1111/j.1574-6976.2007.00075.x]
[29] Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IFN-beta and TNF-alpha of MICA and chemokines and NKG2D receptor triggering promotes cytotoxic lymphocyte infiltration and destruction of epithelial cells in celiac disease. Immunity. 2004;21(3):357-66. [PMID: 15357784 DOI: 10.1016/j.immuni.2004.06.016]
[30] Ferrer-Ferrer M, Ruiz de Morales JG, Tagarro I, Menchén L, Polanco I, Garrote JA. Intraepithelial lymphocytes subsets in childhood celiac disease: γδ+T cells increase with Marsh lesion severity. Dig Dis Sci. 2012;57(1):106-13. [PMID: 21826484 DOI: 10.1007/s10620-011-1858-6]
[31] Nilsen EM, Jahnsen FL, Lundin KE, Cottier E, Scott H, Sollid LM, et al. Gluten induces an influx of CD3+ CD4- CD8- gamma delta + cells in celiac disease. Gastroenterology. 1995;108(4):917-28. [PMID: 7702285 DOI: 10.1016/0016-5085(95)90020-5]
[32] Kurppa K, Collin P, Vilppula A, Laurikka P, Mäki M, Kaukinen K. Postdiagnosis delay in mucosal healing increases lymphoma risk in celiac disease. Gastroenterology. 2012;142(7):1504-1511.e1. [PMID: 22425541 DOI: 10.1053/j.gastro.2012.03.006]
[33] Shamir R, Lerner A, Kerem E, Lebenthal E. Early mucosal recovery in children with celiac disease: is it really the rule? J Pediatr Gastroenterol Nutr. 2000;31(1):21-5. [PMID: 10914811 DOI: 10.1097/00005176-200007000-00006]
[34] Catassi C, Fabiani E, Rätsch IM, Gfeller R, Sergio F, Coppa GV, et al. Risk of lymphoma and other neoplasms in celiac disease. Gastroenterology. 1994;106(3):716-24. [PMID: 8119548 DOI: 10.1016/0016-5085(94)90838-2]
[35] Sjöberg V, Ekström-Smedby K, Stephansson O, Fored CM, Ludvigsson JF. Increased risk of non-Hodgkin lymphoma in patients with celiac disease. Clin Gastroenterol Hepatol. 2015;13(2):300-6.e1-2. [PMID: 25107549 DOI: 10.1016/j.cgh.2014.07.036]
[36] Meyer D, Stavropolous SN, Diamond B, Shane E. Osteoporosis in celiac disease: effects of gluten-free diet and calcium supplementation. Am J Gastroenterol. 2001;96(1):230-7. [PMID: 11197280 DOI: 10.1111/j.1572-0241.2001.03487.x]
[37] Valdimarsson T, Löfman O, Toss G, Stenlund H, Hallert C. Bone mineral density in coeliac disease: effect of gluten-free diet and calcium supplementation. Gut. 2000;46(2):181-7. [PMID: 10644305 DOI: 10.1136/gut.46.2.181]
[38] Mora S, Weber G, Barera G, Bellini C, Pasolli P, Sơncin C, et al. Effect of gluten-free diet on bone mineral content in children with celiac disease. Am J Clin Nutr. 1993;57(5):634-8. [PMID: 8475868 DOI: 10.1093/ajcn/57.5.634]
[39] McCarthy DM, Fraser ID, Evans KT, Toghill PJ, Hoffbrand AV. Splenic function in coeliac disease. Gut. 1978;19(2):121-4. [PMID: 637418 DOI: 10.1136/gut.19.2.121]
[40] Corazza GR, Quaglino F, Vaira D, Gasbarrini G. Pitted erythrocytes in coeliac disease. Lancet. 1990;335(8687):436-7.