Dementia poses a significant global health challenge, with accurate and early diagnosis being critical for effective management and patient care. Traditional diagnostic methods often face limitations in differentiating between the various etiologies of dementia, leading to delays in appropriate treatment strategies. However, the advent of Artificial Intelligence (AI) offers a transformative approach. This article delves into a groundbreaking study leveraging Ai-based Differential Diagnosis Of Dementia Etiologies On Multimodal Data, showcasing its potential to revolutionize the field.
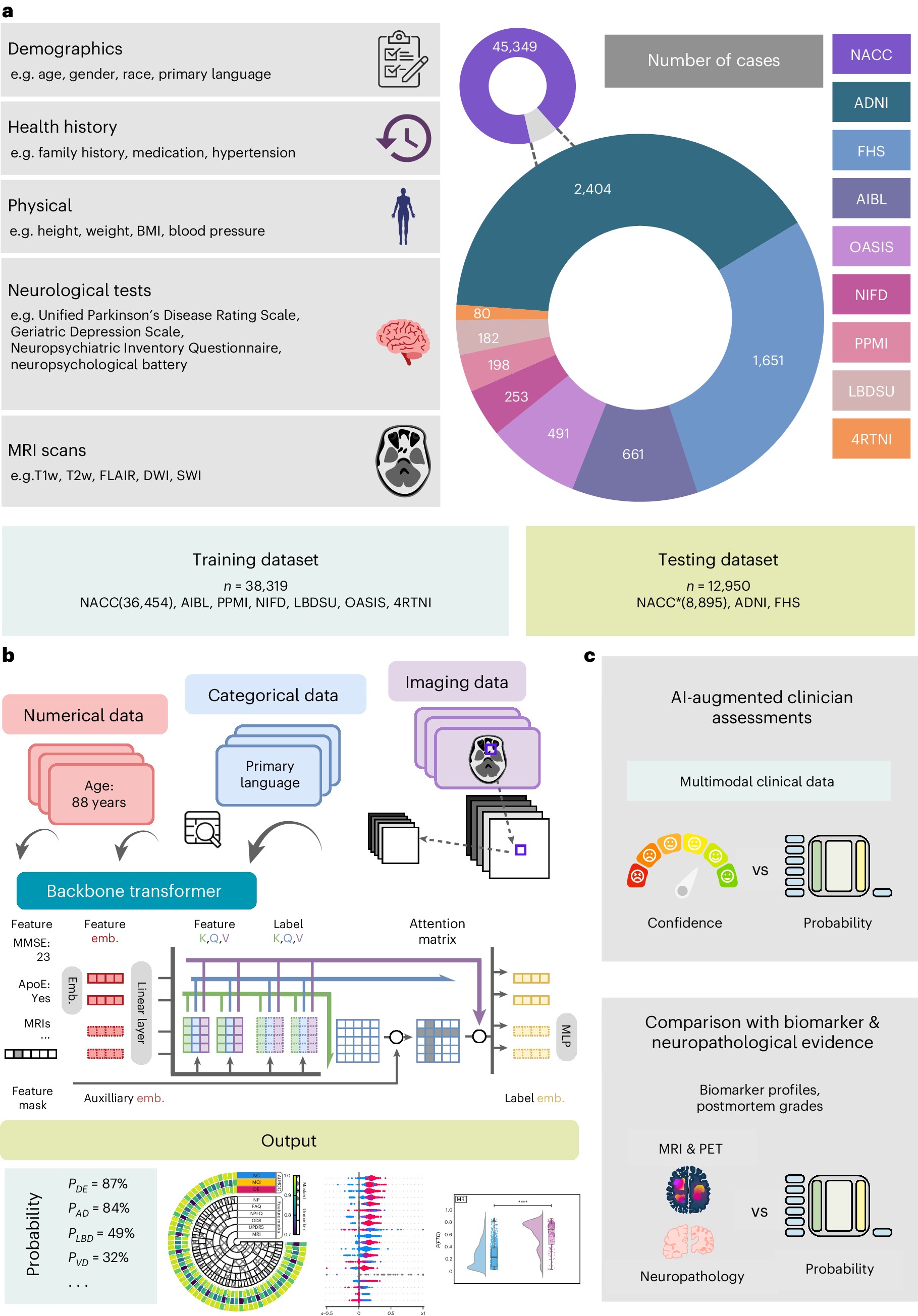
This research meticulously compiled and analyzed data from 51,269 participants across nine distinct cohorts, creating a vast and diverse dataset for training and validating an AI model. The study encompassed individuals with normal cognition (NC), mild cognitive impairment (MCI), and dementia, representing a comprehensive spectrum of cognitive health. Crucially, the dataset included detailed multimodal data, incorporating demographics, medical and family history, laboratory results, neurological examinations, medication records, neuropsychological assessments, functional evaluations, and multisequence magnetic resonance imaging (MRI) scans. This rich array of information provides a robust foundation for AI to learn complex patterns and nuances associated with different dementia types.
Building a Robust AI Model for Dementia Differentiation
The cornerstone of this study lies in the development and implementation of a sophisticated AI model designed for ai-based differential diagnosis of dementia etiologies on multimodal data. The researchers employed a transformer-based architecture, renowned for its ability to process and interpret complex, high-dimensional data. This architecture is particularly well-suited for integrating diverse data modalities, such as clinical information and neuroimaging, into a unified diagnostic framework.
Multimodal Data Integration and Processing
A key innovation in this AI approach is its capacity to effectively handle multimodal data. The model was trained to process numerical data (e.g., age, test scores), categorical data (e.g., gender, medical history), and imaging data (MRI scans) in a cohesive manner. Each data type underwent specific embedding techniques to transform them into a uniform vector representation that the transformer model could process. For MRI data, the team utilized Swin UNETR, a 3D transformer-based architecture, to extract meaningful features from various MRI sequences, including T1-weighted, T2-weighted, FLAIR, and SWI images. This ensured that the AI could leverage the rich spatial and textural information contained within brain scans.
Overcoming Data Challenges: Missing Features and Labels
Real-world medical datasets often suffer from incompleteness, with missing features and diagnostic labels being common challenges. To address this, the researchers incorporated innovative strategies into their model training. Random feature masking was employed to simulate missing data during training, forcing the model to learn robust representations even with incomplete information. Furthermore, to handle missing diagnostic labels across different cohorts, a multilabel classification approach with label masking was implemented. This allowed the model to learn from all available data, maximizing its utility and generalizability.
Training and Optimization for Accurate Differential Diagnosis
The AI model was trained using a carefully designed loss function that combined focal loss and ranking loss, alongside L2 regularization. Focal loss addressed class imbalance issues, common in dementia etiology distributions, by focusing on harder-to-classify cases. Ranking loss incorporated inter-class relationships, further refining the model’s ability to differentiate between closely related dementia types. This rigorous training process, utilizing a large and diverse dataset, resulted in a highly accurate and robust AI system for ai-based differential diagnosis of dementia etiologies on multimodal data.
Validating AI Performance: Benchmarking Against Traditional Methods and Expert Clinicians
To rigorously evaluate the AI model’s performance, the researchers conducted comprehensive validation studies, comparing it against traditional machine learning models and expert clinician assessments.
Superior Performance Compared to Traditional ML Models
The AI model’s classification accuracy for NC, MCI, and dementia was benchmarked against the CatBoost model, a traditional tree-based machine learning framework. The AI model demonstrated superior performance, highlighting the advantages of deep learning and transformer architectures in handling complex medical data for dementia diagnosis. This underscores the potential of ai-based differential diagnosis of dementia etiologies on multimodal data to surpass conventional approaches.
Biomarker and Neuropathological Validation
The study went beyond standard performance metrics by validating the AI model’s predictions against gold-standard biomarkers and neuropathological evaluations. The model’s predicted probabilities for Alzheimer’s disease (AD), frontotemporal dementia (FTD), and Lewy body dementia (LBD) showed strong correlations with established biomarkers for each respective etiology, including amyloid PET, tau PET, FDG PET, and DaTscan. Furthermore, neuropathological validation, using data from NACC, FHS, and ADNI cohorts, confirmed the AI model’s alignment with post-mortem diagnoses, reinforcing its accuracy in identifying different dementia pathologies.
AI Augments Clinician Diagnostic Accuracy
Perhaps the most compelling aspect of the validation was the assessment of AI-augmented clinician performance. A group of neurologists and neuroradiologists were tasked with diagnosing dementia etiologies in a subset of NACC cases, both with and without AI assistance. The results demonstrated that AI significantly enhanced clinician diagnostic accuracy. By averaging clinician confidence scores with the AI model’s predicted probabilities, the AI-augmented diagnoses showed improved AUROC and AUPR metrics compared to clinician-only diagnoses. This highlights the potential of ai-based differential diagnosis of dementia etiologies on multimodal data to serve as a valuable clinical decision support tool, empowering clinicians to make more accurate and confident diagnoses.
Interpretability and Clinical Implications
Beyond diagnostic accuracy, the study also explored the interpretability of the AI model using Shapley value analysis. This analysis provided insights into which features were most influential in the model’s predictions for different dementia etiologies. Understanding feature importance is crucial for building trust in AI systems and for gaining deeper clinical insights into the factors driving dementia diagnosis.
The findings of this research have significant clinical implications. The demonstrated effectiveness of ai-based differential diagnosis of dementia etiologies on multimodal data opens new avenues for improving early and accurate dementia diagnosis. This technology has the potential to:
- Enhance diagnostic accuracy: AI can process complex multimodal data to identify subtle patterns indicative of specific dementia etiologies, improving diagnostic precision.
- Accelerate diagnosis: AI-powered tools can streamline the diagnostic process, reducing delays in diagnosis and enabling earlier intervention.
- Improve clinical decision-making: AI can serve as a valuable decision support tool for clinicians, augmenting their expertise and confidence in dementia diagnosis.
- Personalize treatment strategies: Accurate differential diagnosis facilitated by AI can pave the way for personalized treatment plans tailored to the specific etiology of dementia.
Conclusion: AI as a Catalyst for Transforming Dementia Care
This study provides compelling evidence for the transformative potential of ai-based differential diagnosis of dementia etiologies on multimodal data. By leveraging advanced AI techniques and integrating diverse clinical and imaging data, this research has developed a robust and accurate diagnostic tool. The validation results, demonstrating superior performance compared to traditional methods and significant augmentation of clinician accuracy, underscore the clinical utility of this AI approach. As AI technology continues to evolve, its integration into dementia care pathways promises to revolutionize diagnosis, treatment, and ultimately, improve the lives of individuals affected by these devastating conditions.