Age-related macular degeneration (AMD) stands as a principal cause of irreversible vision impairment in individuals aged 65 and above within industrialized nations. Globally, it’s estimated that AMD contributes to approximately 9% of all blindness cases. In Germany, a significant portion, about half of all instances of blindness and severe visual impairment, are attributed to AMD. This review aims to provide a detailed overview of AMD, encompassing its primary risk factors, clinical presentations, and current treatment modalities, with a focus on AMD diagnosis and early intervention strategies.
Prevalence and Impact of AMD
By 2020, AMD was estimated to affect around 200 million individuals worldwide, underscoring its widespread impact. Alarmingly, the prevalence of AMD has shown a notable increase in recent years. In Germany alone, the number of individuals with early, predominantly asymptomatic AMD, surged from 5.7 million in 2002 to approximately 7 million by 2017 – a substantial 23% increase within just 15 years. Concurrently, the more symptomatic and vision-threatening later stages of AMD also became more prevalent in Germany during the same period, rising from about 360,000 to roughly 490,000 individuals, marking a 36% increase. It’s estimated that late-stage AMD is responsible for half of all cases of blindness and severe visual impairment in Germany. Among patients with late-stage AMD, neovascular AMD is observed to be 1.4 times more frequent than geographic atrophy, the advanced stage of dry AMD. The escalating prevalence might be attributed not only to the aging global population but also to enhanced detection rates through improved diagnostic methodologies, highlighting the importance of effective AMD diagnosis techniques. The demographic trend’s significant influence on AMD prevalence is evident in the age-adjusted prevalence rates, escalating from 24% in individuals aged 65 to 74 to over 44% in those aged 70 to 95.
Risk Factors for AMD
Age is unequivocally the primary risk factor for AMD. Consequently, its prevalence in industrialized countries is steadily climbing in tandem with population aging.
Figures for Germany
Currently, approximately 7 million individuals in Germany are living with AMD.
The profound effect of age on AMD development is further illustrated by the observation that individuals under 50 typically exhibit minimal to no typical AMD-related changes. Conversely, the Gutenberg Health Study conducted in Mainz, Germany, revealed that 24% of individuals aged 65 to 74 already present with characteristic AMD changes, although many are still asymptomatic. The most frequently observed early AMD-related changes include funduscopically visible deposits of metabolic byproducts beneath or above the retinal pigment epithelium of the macula, specifically drusen and pseudodrusen. The prevalence of such changes increases with the age of the patient cohort studied. For instance, the AugUR cohort study in Regensburg, Germany, identified intermediate-stage AMD in 44% and late AMD in 19% of subjects aged 70 to 95. Other studies have estimated AMD prevalence in individuals over 85 to be as high as 30%.
Stages of AMD
AMD is clinically categorized into early, intermediate, and late stages. Late AMD is further divided into two primary forms: dry (atrophic) and neovascular (wet, exudative). It’s not uncommon to find a combination of both late AMD types in the same eye. Accurate staging is crucial for appropriate management and relies heavily on precise AMD diagnosis.
Learning Objectives
Upon reading this review, you will be able to:
- Understand the prevalence and risk factors associated with AMD.
- Recognize the typical clinical manifestations of AMD and know the appropriate diagnostic evaluation process.
- Comprehend the treatment strategies for each stage of AMD and be familiar with potential treatment complications.
Methodology
This review is based on a thorough analysis of relevant publications identified through a focused PubMed search for original research articles and reviews. It also incorporates current guidelines and position statements from pertinent specialist societies in the field of ophthalmology, ensuring the information is grounded in evidence-based medicine and reflects current best practices in AMD diagnosis and management.
Progression of AMD
A significant proportion, 64.5%, of patients with AMD exhibit the same disease stage in both eyes. For those with asymmetric involvement, monitoring disease progression in the better-seeing eye is paramount. Population-based studies indicate that in patients initially diagnosed with AMD in only one eye, the second eye becomes affected within 5 years in 19% to 28% of cases.
Late AMD poses a greater threat to vision than early AMD, which is often asymptomatic, or intermediate AMD, which is typically oligosymptomatic. The progression rate from intermediate to late AMD in the natural course of the disease is generally reported as 28% within five years. Initial symptoms often manifest as distorted vision or central visual field defects, frequently described as stationary, centrally located gray spots. Late AMD presents in two main forms: dry (atrophic) and wet (exudative or neovascular). Atrophic AMD is characterized by the gradual loss of retinal pigment epithelium, photoreceptors, and choroidal capillaries in the macula, the region responsible for sharp central vision. This form typically progresses slowly over years and can lead to complete central vision loss, resulting in a central scotoma in advanced stages. Natural history studies of AMD have shown that atrophic areas develop in 19% of eyes with intermediate AMD within 5 years. Importantly, peripheral and orienting vision are usually preserved even in late AMD because the degenerative process primarily affects the macular region, sparing the rest of the retina. Given that the macula is the central retinal area with the highest spatial resolution, patients with AMD often experience increasing difficulty with reading and facial recognition, while spatial orientation remains intact due to preserved peripheral vision. Effective AMD diagnosis is crucial to differentiate between these stages and forms, guiding appropriate intervention strategies.
Functional Impairment
AMD primarily affects the macula, thus impairing central vision and consequently limiting the patient’s ability to perform tasks such as reading, driving, and recognizing faces.
The exudative form of late AMD is typically associated with a more rapid and severe loss of vision compared to the atrophic form. Reading ability can deteriorate within days. Untreated patients with exudative AMD may lose an average of three lines (15 letters) of visual acuity in two years. The visual loss in exudative AMD is attributed to the development of choroidal neovascularization (CNV) in the macular area. These newly formed vessels are prone to acute rupture, leading to hemorrhage into the macula and subsequent scarring. Pathological neovascularization is thought to be a misguided attempt by damaged retinal areas to repair themselves, complicated by exudation or rupture of abnormal vessels and/or the retinal pigment epithelium, resulting in rapid vision decline. Thus, while both dry and exudative AMD originate from retinal pigment epithelium degeneration, they follow distinct clinical courses. Transitional or mixed forms between these two main types are also observed. Accurate and timely AMD diagnosis is essential for differentiating between these forms and initiating appropriate treatment, especially for the exudative form where timely intervention can significantly impact visual outcomes.
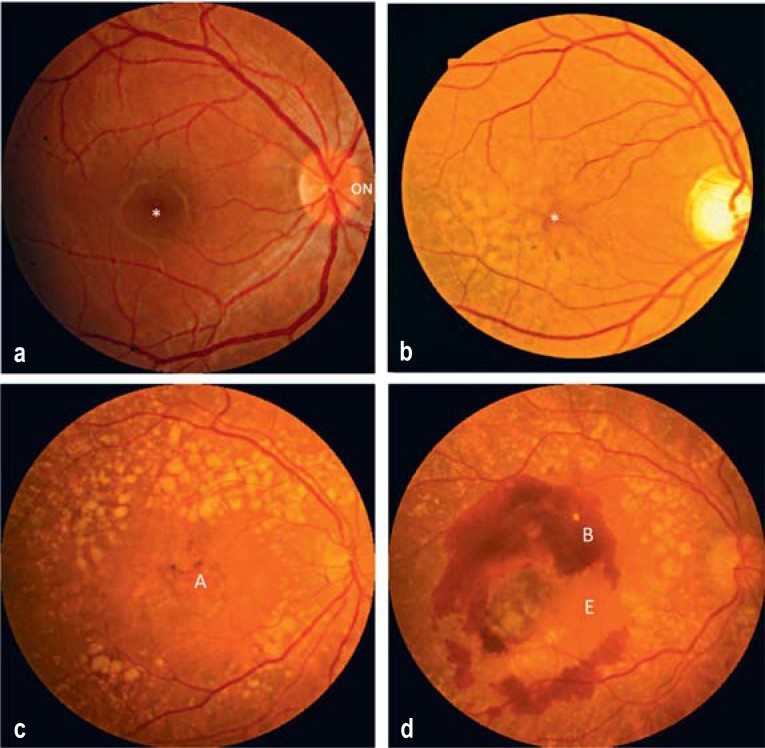
Figure 1: Stages of Age-Related Macular Degeneration (AMD). This image illustrates the progression of AMD, from a normal macula to early/intermediate AMD with drusen, and then to late-stage dry (atrophic) and wet (exudative) forms, highlighting key morphological changes for effective AMD diagnosis.
Figure 2: Clinical Manifestations of Age-Related Macular Degeneration (AMD). This figure demonstrates the Amsler grid test, showing normal perception versus distorted lines and a central scotoma typical of late AMD, crucial for self-monitoring and early AMD diagnosis.
Risk Factors of AMD
The primary risk factor for AMD is advancing age. Pathogenetically, it’s hypothesized that the macula’s high metabolic activity places a significant cumulative burden on the retinal pigment epithelium throughout life to process and eliminate metabolic waste. AMD develops when retinal pigment epithelium cells can no longer effectively manage this demand in older age. However, AMD doesn’t necessarily correlate with other age-related conditions like osteoporosis. Instead, factors beyond age modulate both AMD onset and progression. Smoking is a major modifiable risk factor; smokers have a 2.6 to 4.8 times higher odds ratio for developing AMD compared to non-smokers. Even former smokers have an elevated odds ratio of 1.7 for AMD development.
Exudative Late AMD
Exudative late AMD exhibits the most aggressive disease course among AMD subtypes. It often leads to rapid central visual function deterioration due to pathological blood vessel growth, accompanied by exudation, hemorrhage, fibrosis, and sometimes retinal pigment epithelium tears.
Besides smoking, numerous genetic risk alleles for AMD have been identified. The most significant are polymorphisms in CFH (complement factor H) and ARMS2 (age-related maculopathy susceptibility 2). Together, these alleles may account for up to 45% of AMD risk. Furthermore, studies have linked AMD with body-mass index, cardiovascular disease, and arterial hypertension. Dyslipidemias and metabolic dysfunction have also been associated with AMD in some studies, though definitive causal relationships remain unclear. Drusen deposits in AMD resemble atherosclerotic deposits in blood vessels, commonly seen in cardiovascular high-risk patients, but research on a potential link between AMD and atherosclerosis has yielded inconsistent results. Recognizing these risk factors is crucial for targeted screening and early AMD diagnosis, enabling timely interventions and lifestyle modifications to potentially slow disease progression.
Diagnostic Evaluation of AMD
Patient history can provide valuable clues for AMD diagnosis. Patients often report acute or gradual vision worsening in one or both eyes, frequently more noticeable in low light conditions. Inquiry about distorted vision (metamorphopsia) is essential, as its presence strongly suggests macular disease. Metamorphopsia can manifest when viewing straight lines like road stripes, windowpanes, or tiles. Some patients also report facial distortion or size discrepancies between images seen by each eye. However, patients in early AMD stages or when the fovea is not yet involved may remain asymptomatic for extended periods. Therefore, a comprehensive AMD diagnosis requires a thorough ophthalmological examination, including best-corrected visual acuity measurement, dilated funduscopic evaluation, macular layer imaging with optical coherence tomography (OCT), and, in certain cases, fluorescein angiography, particularly when considering treatment for exudative AMD.
Genetic Risk Alleles
Apart from age and smoking, genetic risk alleles in complement system genes and the ARMS2 HTRA locus are major risk factors for AMD development and progression. Genetic testing may play an increasingly important role in risk stratification and personalized AMD diagnosis in the future.
OCT has become a cornerstone in AMD diagnosis. Its clinical utility is underscored by its assignment of a reimbursable procedure code in Germany in 2019. OCT is non-invasive and easily performed in almost all patients. However, maintaining minimal image quality standards is crucial to avoid missing subtle but clinically significant changes. It’s important to note that neither OCT alone nor OCT angiography can entirely replace conventional fluorescein angiography in differentiating between dry and exudative late AMD. Fluorescein angiography remains the gold standard for directly visualizing active exudation from pathological blood vessels into the retinal parenchyma, providing critical information for accurate AMD diagnosis, particularly in exudative AMD.
eFigure 1: Normal Findings and Retinal Pigment Epithelium Tear in Exudative Late AMD. This image contrasts normal funduscopy and OCT findings with those in exudative AMD, illustrating hemorrhage and retinal pigment epithelium tear, key indicators for AMD diagnosis and monitoring treatment response.
eFigure 2: Fluorescein Angiography for the Diagnosis of Exudative AMD. This figure showcases fluorescein angiography findings in a healthy macula versus exudative AMD with a classic CNV membrane, demonstrating leakage in the late phase, essential for confirming AMD diagnosis and guiding treatment.
Treatment
Appropriate AMD treatment is stage-dependent. In all stages, mitigating risk factors is paramount, especially smoking cessation. Numerous prospective studies have shown that smokers have a higher risk of AMD progression, even post-diagnosis. A Korean study further indicated that visual acuity gains from anti-VEGF treatment are less in smokers with exudative AMD than non-smokers. Early AMD diagnosis can thus motivate patients to adopt healthier lifestyle habits to curb disease progression.
Symptoms Indicative of AMD
Typical symptoms that raise suspicion for AMD diagnosis include distorted vision (metamorphopsia), reduced visual acuity, and central scotoma.
Dietary supplements for AMD are extensively discussed. The AREDS trials provide the most robust clinical trial data. AREDS-1 and AREDS-2 were long-term randomized controlled trials evaluating dietary supplements’ impact on AMD progression. AREDS-1 (2001) found that high-dose vitamin C and E, beta-carotene, and zinc supplementation had benefits for intermediate AMD. However, other studies linked high-dose beta-carotene and vitamin supplementation in smokers (current or former) with increased cancer risk. A vitamin E and beta-carotene combination reportedly increased lung cancer risk by 18% (95% CI [3; 36%], p = 0.01), while vitamin A and beta-carotene supplementation was associated with a relative risk of 1.28 ([95% CI 1.04; 1.57]; p = 0.02). Consequently, AREDS-2 replaced beta-carotene with lutein/zeaxanthin and omega-3 fatty acids. AREDS-2 showed efficacy only in intermediate or late AMD, with smaller effect sizes than smoking cessation. AREDS-1’s odds ratio for AMD progression was 0.72 (99% CI [0.52; 0.98]). AREDS-2’s hazard ratio was 0.89 (98.7% CI: [0.75; 1.06]) for lutein + zeaxanthin + omega-3 fatty acids (DHA + EPA). Supplementation had a limited effect on intermediate AMD and no effect on early or late stages. Thus, routine dietary supplements for primary prevention (before intermediate AMD signs) are not generally recommended. German ophthalmological societies advise a balanced diet for primary prophylaxis, aligning with the German Nutrition Society’s guidelines.
Nanosecond laser therapy for drusen in intermediate AMD (large drusen without atrophy or exudation) has also been explored. The LEAD trial assessed if laser treatment of drusen could slow AMD progression in intermediate AMD. The primary endpoint was not met; no protective effect was found. In patients with reticular pseudodrusen, disease progression was actually faster. Thus, retinal laser therapy is not recommended for dry AMD outside controlled clinical trials.
Dry (Atrophic) Late AMD
Key Clinical Evaluation Techniques
Essential techniques for AMD diagnosis and monitoring include visual acuity testing, dilated bilateral fundoscopy, optical coherence tomography (OCT), and, in some cases, fluorescein angiography. Patients can use the Amsler grid for early self-detection of metamorphopsia.
Currently, there is no proven effective treatment for atrophic late AMD. Clinical trials, including recent ones focusing on complement system modulators, have yielded negative results. Common reasons cited for these failures, similar to those in other central nervous system degenerative diseases, include initiating treatment too late in a disease cascade that has reached a point of no return. At a certain stage, neural tissue, specifically retinal photoreceptors in this case, is irreversibly lost. No method has yet been found to prevent further photoreceptor loss at the periphery of existing macular atrophy. Current research in atrophic late AMD focuses on better understanding disease progression pathogenesis to identify optimal therapeutic targets and timing for future interventions. Early and accurate AMD diagnosis of the dry form remains crucial for patient education, prognosis, and potential enrollment in clinical trials.
Wet (Exudative) Late AMD
The aging population, as mentioned earlier, is a major factor in rising AMD prevalence. Logically, one might expect a parallel increase in blindness or severe visual impairment cases. However, statistics from Germany and other countries show a stagnation or even decrease in blindness and severe visual impairment rates, despite increasing AMD prevalence. This is largely attributed to the 2005 introduction of effective treatments for exudative late AMD, the most aggressive form. In 2006, anti-VEGF therapy for exudative macular degeneration was recognized by Science as a top ten scientific breakthrough after successful phase 3 clinical trials. This treatment involves intravitreal injection of anti-VEGF drugs. Four are currently available: bevacizumab (off-label, since 2005) and three approved in Europe: ranibizumab (2007), aflibercept (2012), and brolucizumab (2020). Approved drugs cost around 1000 euros per injection, while bevacizumab costs approximately 40 euros. Bevacizumab is not expected to gain intraocular use approval. Numerous biosimilar drugs are anticipated in the coming years.
Lifestyle Modifications
Smoking cessation is recommended for all AMD stages to prevent onset or progression. Dietary supplements have limited effects and are only effective in certain AMD stages. Early AMD diagnosis and lifestyle counseling are important components of patient care.
Though anti-VEGF drugs vary chemically, in binding affinity, and specificity, they share a mechanism: blocking vascular endothelial growth factor (VEGF). VEGF is pro-angiogenic, promoting pathological blood vessel growth in exudative AMD, and a permeability factor, facilitating blood plasma component extravasation into the retina. Sub- and/or intraretinal fluid from hyperpermeable choroidal vessels is a primary cause of vision worsening in exudative AMD. VEGF inhibitors’ efficacy against wet macular degeneration largely stems from reducing vessel permeability rather than inhibiting angiogenesis.
Unfortunately, anti-VEGF therapy usually requires long-term, repeated administrations, especially in the initial years. Patients must understand this and the associated time and logistical demands to ensure compliance. AMD is chronic; even with effective treatments for certain stages, the underlying pathogenic cascade cannot be halted early on. Long-term, consistent, intensive treatment is necessary to positively impact its course. Early and accurate AMD diagnosis is crucial to initiate timely and appropriate anti-VEGF therapy in exudative AMD.
Various established anti-VEGF therapy strategies exist for exudative AMD. Initial clinical trials tested regular monthly VEGF inhibitor administrations. Each intravitreal injection is an outpatient surgical procedure with a per-injection risk of severe intraocular infection (endophthalmitis) around 0.029% (roughly 1:3500). Endophthalmitis is typically treated with vitrectomy and intraocular antibiotics, and visual outcomes are variable and often poor, depending on the pathogen’s aggressiveness and other factors. Other rare but clinically relevant intravitreal therapy risks include sterile inflammatory reactions (0.09–2.9%) and, rarely, retinal detachment (0.013%).
Intravitreal Anti-VEGF Therapy
Four anti-VEGF drugs are available: bevacizumab (off-label, since 2005) and three approved in Europe: ranibizumab (2007), aflibercept (2012), and brolucizumab (2020). Prompt AMD diagnosis of the wet form enables access to these vision-saving treatments.
To personalize treatment, “pro re nata” (PRN) and “treat and extend” strategies have been developed and are preferred over regular monthly injections at most AMD treatment centers. These strategies aim to provide patients with the optimal number of injections based on individual needs. Clinical trials show most patients need about 7–8 injections in the first treatment year to effectively control exudative AMD, with fewer injections generally needed in subsequent years. Real-world German studies indicate patients are more likely to receive too few than too many injections. In practice, treatment intervals are often suboptimal due to comorbidities, transportation difficulties, etc. Numerous studies have linked undertreatment phases with irreversible vision loss. Ensuring uninterrupted treatment is a critical therapeutic goal. Proper management, depending on disease activity, involves intravitreal injection and/or follow-up with visual acuity measurement, funduscopy, and OCT.
In summary, current exudative late AMD treatment, while not addressing the underlying etiology, effectively preserves visual acuity in many patients. Visual acuity stabilizes in over 70% of treated eyes, with nearly 20% showing marked improvement after initial treatments. However, anti-VEGF therapy is ineffective in early, intermediate, and atrophic late AMD. Therefore, accurate AMD subtype diagnosis is vital to initiate timely treatment for exudative late AMD, ideally before irreversible vision loss occurs.
Atrophic Late AMD
No evidence-based treatment exists for atrophic late AMD, but clinical trials are exploring several approaches. Continued research and improved AMD diagnosis methods are crucial for future breakthroughs.
Exudative Late AMD Treatment
Exudative late AMD is treated with intravitreal anti-VEGF drugs. Most patients require multiple injections, typically 7-8, in the first year. Timely AMD diagnosis and initiation of treatment are key to maximizing visual outcomes.
Further Information on CME.
- CME credit for this unit can be obtained online until July 19, 2021.
- New CME units are available for 12 months. Answers are available online four weeks post-publication. Please note submission deadlines on the website.
- This article is certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed with the uniform CME number.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 19 July 2021. Only one answer is possible per question. Please select the answer that is most appropriate.
(Questions 1-10 are identical to the original article and are omitted for brevity, as per instructions.)
► Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Acknowledgement
I am grateful to Prof. Hansjürgen Agostini and Prof. Daniel Pauleikhoff for critically reviewing and commenting on this article, and to Prof. Clemens Lange and Dr. Bastian Grundel for making relevant images available.
Footnotes
Conflict of interest statement
Prof. Stahl has served as a paid consultant for Novartis and Bayer. He has received reimbursement of scientific meeting participation fees and of travel and accommodation expenses, as well as payment for the preparation of continuing medical education sessions, from Allergan, Bayer, and Novartis.
References
(References are identical to the original article and are omitted for brevity, as per instructions.)