Introduction
Soil-transmitted helminths (STHs) pose a significant global health burden, infecting over 1.45 billion individuals worldwide. Among these, Ascaris lumbricoides is a prevalent species, affecting an estimated 819 million people. Ascariasis, the infection caused by Ascaris, can lead to a spectrum of health issues, ranging from mild discomfort to severe and even life-threatening conditions. These infections are particularly common in areas with inadequate sanitation and hygiene, but global travel and migration patterns have also increased their incidence in non-endemic regions.
Recognizing the widespread impact of STHs, the World Health Assembly has set ambitious goals for morbidity control, emphasizing mass drug administration (MDA) with albendazole or mebendazole to treat at least 75% of school-age children and high-risk groups in endemic areas by 2020. Achieving these goals and advancing towards elimination necessitates sensitive, specific, user-friendly, and cost-effective Ascariasis Diagnosis Tests. These tests are crucial not only for individual patient management but also for the effective planning, monitoring, and evaluation of public health interventions, especially in measuring reduced infection intensities and drug efficacy. As national STH control programs expand, research into improved Ascaris diagnostics becomes increasingly vital.
This article reviews current literature on diagnostic techniques for A. lumbricoides infection, exploring both established methods and promising novel assays. We aim to provide a comprehensive overview of diagnostic approaches applicable in various settings, from clinical diagnosis to large-scale control programs, highlighting the importance of integrated diagnostic strategies for multi-species infections.
Methods
A thorough literature search was conducted across PubMed, Google Scholar, Web of Science, and EMBASE databases to gather relevant publications on Ascaris diagnostic techniques. Search terms included combinations of “Ascaris,” “Ascariasis,” “A. lumbricoides,” “soil-transmitted helminths,” “STH,” “helminth,” and “diagnostics,” “diagnosis,” “sensitivity,” “specificity,” “Kato-Katz,” “FLOTAC,” “ethyl,” “midi,” “ether,” “antigen,” “immunology,” “immunoglobulins,” “LAMP,” “loop,” “polymerase chain reaction,” “PCR,” “FECPACK,” and date ranges from 2010 to 2015. Additionally, specific publications were searched by title and authors when needed. Initial screening based on titles yielded 368 papers, followed by abstract review of 146 articles, with final reference selection guided by publisher guidelines.
Clinical Presentation of Ascariasis
A. lumbricoides infection manifests in two primary forms of pathology: immune reactions to larval migration and complications arising from adult worms in the gastrointestinal tract, such as nutrient depletion or intestinal obstruction. Often, ascariasis is asymptomatic and can co-occur with other conditions, making it a differential diagnosis for a broad spectrum of illnesses, as detailed in Table 1.
Fig. 1. Ascaris lumbricoides Life Cycle and Diagnostic Markers
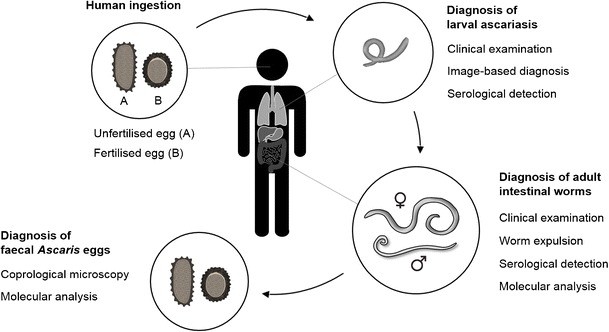 Fig. 1
Fig. 1
Life cycle of Ascaris lumbricoides, highlighting diagnostic stages and transmission routes. Infective eggs are ingested, larvae migrate through the body, mature in the intestine, and eggs are excreted in stool.
Table 1. Differential Diagnoses of Ascariasis Morbidity
| Findings | Differential diagnoses |
|---|---|
| Larval ascariasis | |
| Urticarial rash, other rashes | Allergy, drug reactions, infections (including other parasites), environmental factors, dermatological conditions |
| Tender hepatomegaly | Infections (including intestinal ascariasis, malaria, amoebiasis, echinococcosis, bacterial, viral, fungal), Tumors, Vascular causes, Toxicity, Metabolic disorders |
| Cough, dyspnea | Pulmonary infections/inflammation, Tumors, Vascular causes, Mechanical causes |
| Eosinophilia | Other parasitic infections, allergy, drug reactions, rare congenital or malignant diseases |
| Increased IgE titers | Other parasitic infections, allergy, drug reactions, rare congenital or malignant diseases |
| Intestinal ascariasis | |
| Acute abdominal pain | Infection/inflammation, Vascular causes, Other conditions |
| Ileus | Bowel obstruction, Intestinal paralysis |
| Acute pancreatitis | Acute pancreatitis, other causes of acute abdominal pain |
| Acute cholecystitis | Acute cholecystitis, other causes of acute abdominal pain |
| Liver abscess, cholangitis | Infections, cholelithiasis, tumors |
Differential diagnoses for larval and intestinal ascariasis, outlining the broad range of conditions that ascariasis can mimic.
Individual diagnosis of ascariasis often necessitates a comprehensive evaluation, especially in non-endemic areas where travel history to endemic regions might be a key indicator. Clinical and laboratory examinations, potentially including serological, molecular, and imaging techniques, are essential. It is also increasingly important to consider Ascaris suum, a species primarily infecting pigs, as a potential cause of human ascariasis, even in the absence of travel to A. lumbricoides endemic areas.
Migrating Ascaris Larvae and Diagnosis
Löffler syndrome, or eosinophilic pneumonitis, is an immune-mediated response to migrating larvae in the lungs, typically seen during initial or intermittent infections. Symptoms include fever, cough, and dyspnea, usually appearing 4 to 16 days post-infection. Clinical signs may also involve urticaria, abnormal lung sounds, and hepatomegaly. Eosinophilia is a common laboratory finding, and chest X-rays may reveal pulmonary infiltrates. Serological tests can support diagnosis before egg excretion begins, although cross-reactivity with other parasites should be considered. While usually resolving within three weeks, severe cases can be fatal. Ectopic larval migration, though rare, can lead to complications associated with eosinophilia.
Adult Ascaris Worms and Diagnosis
Mild ascariasis infections are often asymptomatic. However, heavy infections frequently present with acute abdominal pain and ileus, caused by mechanical bowel obstruction, volvulus, or intussusception, particularly in children. In endemic regions, intestinal ascariasis is also a common cause of hepatobiliary and pancreatic diseases, such as acute pancreatitis and cholecystitis. Diagnostic imaging techniques like ultrasonography, abdominal X-ray, CT scans, and MRI can help identify the cause. Endoscopic retrograde cholangiopancreatography (ERCP) may be used for both diagnosis and treatment, and capsule endoscopy is an option even when conventional gastrointestinal endoscopy is negative. In endemic areas, chronic Ascaris infection is a significant contributor to malabsorption, undernutrition, micronutrient deficiencies, growth stunting, cognitive impairment, and immune dysregulation, increasing susceptibility to other infections.
Coprological Diagnosis for Ascariasis
Microscopy-based stool examination for eggs remains the cornerstone of ascariasis diagnosis, especially for large-scale assessments. These techniques are also widely used for detecting other intestinal parasites.
The Kato-Katz thick smear is the WHO-recommended method for STH detection in endemic areas. It provides a reliable measure of infection intensity, quantified as eggs per gram of stool (EPG), correlating well with worm burden. Kato-Katz is cost-effective, simple to perform, has a low false-positive rate, and can detect multiple co-endemic parasites. However, its sensitivity is limited, particularly at low infection intensities, due to high EPG variability from sample to sample, non-random egg distribution within stool, daily egg count fluctuations, and stool density variations affecting volume-to-weight ratios. Accurate Kato-Katz diagnosis also depends on well-trained technicians.
Increasing the number of Kato-Katz smears, especially from stools collected on consecutive days, significantly improves sensitivity and prevalence estimates. Using two slides per stool over three days can reduce false negatives for A. lumbricoides to ≤1% in moderate prevalence settings. However, multiple smears increase resource demands and may introduce bias due to age-related compliance issues.
FLOTAC® exhibits higher sensitivity than single or multiple Kato-Katz slides, possibly due to processing a larger stool volume (1g). This makes FLOTAC® valuable for mapping and monitoring control programs, especially in low-endemic areas. Despite requiring a centrifuge and not achieving 100% sensitivity, FLOTAC® generally provides more reliable egg counts compared to Kato-Katz.
Mini-FLOTAC, a simplified version not requiring specialized equipment, shows comparable or better sensitivity to Kato-Katz for STH infection intensity determination across various settings. Flotation solution (FS) choice impacts species-specific diagnosis; FS2 is recommended for hookworm, FS7 for Schistosoma mansoni and A. lumbricoides, and FS4 for all STHs. While Mini-FLOTAC may be more expensive upfront, it can be faster than Kato-Katz in low-intensity infection scenarios post-treatment. However, cost per detected case increases as prevalence decreases. Although FLOTAC® is generally more expensive than Kato-Katz, a single FLOTAC® test can be cheaper and more sensitive than triplicate Kato-Katz.
The McMaster egg counting technique offers accurate EPG estimates and is easy to use, providing reliable drug efficacy assessments. However, it is less sensitive than FLOTAC®. Other techniques like TF-Test®, Baermann-Moraes, Paratest, formalin-ethyl acetate sedimentation, SAF, Hoffman-Pons-Janer, and SSTT have shown promise for A. lumbricoides diagnosis, while Midi Parasep® has been less effective. Further research is needed to fully evaluate these tests for Ascaris and other intestinal parasites.
Diagnosis in Infants
Kato-Katz has reduced sensitivity for ascariasis diagnosis in breastfed infants due to their liquid stools and lower EPGs. Modified Wisconsin floatation and simple gravity sedimentation are more sensitive in infants than Kato-Katz, formal-ethyl acetate sedimentation, or modified formal-ethyl acetate sedimentation. Gravity sedimentation, although labor-intensive, can distinguish fertilized from unfertilized Ascaris eggs and is less affected by diarrheal stool consistency than the Wisconsin method.
Drug Efficacy Assessment
Statistical models suggest that McMaster and Kato-Katz provide reliable drug efficacy estimates for control program monitoring and evaluation. FLOTAC® has also demonstrated higher post-treatment sensitivity compared to Kato-Katz for detecting STHs, particularly with preserved samples. However, some studies report decreased helminth egg recovery with sample preservation time. Kato-Katz and McMaster sensitivity decreases post-treatment, while FLOTAC® remains high, making it a potentially valuable tool for drug efficacy studies.
Mobile Phone Technology in Diagnosis
Mobile devices adapted for Kato-Katz slide examination can accurately diagnose helminth eggs in moderate- to high-intensity infections, achieving 81% sensitivity for A. lumbricoides. Lens-free mobile devices combined with digital image analysis hold potential for improving point-of-care stool-based diagnosis, especially with continued technological and software advancements.
Serological Diagnosis of Ascariasis
Serological tests, detecting antibodies or antigens, could offer simpler and faster ascariasis diagnosis compared to stool microscopy. While point-of-care tests exist for other NTDs like lymphatic filariasis and schistosomiasis, none are currently available for STHs.
Humoral Immune Response in Ascariasis
A. lumbricoides infection triggers antibody production, varying with exposure and infection intensity, particularly in high-endemic areas. Factors like age, genetics, atopy, nutritional status, and co-infections can influence the humoral response. Total immunoglobulin (Ig) titers correlate with worm burden in endemic populations. Some studies indicate IgG4 isotype as a sensitive and specific marker for chronic A. lumbricoides infection, positively correlating with infection intensity. However, cross-reactivity of anti-Ascaris antibodies with other helminth epitopes is common. Standardization of Ascaris antigens, including recombinant antigens, allergens, and antigens from other ascarid species, is needed for improved research and diagnostic applications.
Antibodies as Markers of Active Infection
Limited research has evaluated serological ascariasis diagnosis at the community level. Studies show that anti-Ascaris IgG4 levels can decrease to baseline after successful treatment in individuals with high-intensity infections over several months. However, antibody titers may not always correlate with worm expulsion post-treatment. Anti-Ascaris antibodies are particularly associated with larval stage ascariasis and can remain elevated for months, even post-treatment, especially in re-infection-prone areas. Therefore, antibody detection is generally not considered ideal for active Ascaris infection diagnosis and might overestimate treatment needs in mass control programs. Commercial diagnostic tests for anti-A. lumbricoides IgG and IgM are available but often rely on somatic worm antigens, leading to cross-reactivity. Saliva-based IgG detection showed good performance in high-intensity T. trichiura infections but not as effectively in Ascaris infections.
Antigen Detection for Ascariasis
Antigen detection, unlike antibody detection, would specifically indicate current infections. While urine-based schistosome antigen tests are highly sensitive and commercially available, studies on antigen detection in blood or other specimens for A. lumbricoides were not found in this review. Given the intestinal location of adult STHs, coproantigen tests might be more sensitive than urine or blood tests. Research in this area is warranted.
Serological Diagnosis in Children
Antibodies may serve as valuable markers of infection in young children, especially in areas with high exposure to intestinal pathogens, as control programs aim for STH elimination.
Biomedical Markers for Ascariasis
Limited research exists on specific biomedical markers for A. lumbricoides infection. Urinary fatty acid products of Ascaris infection, detectable by gas-liquid chromatography, correlate with worm burden. However, these tests are not yet commercially available.
Molecular Diagnosis of Ascariasis
Molecular tools offer highly sensitive and specific ascariasis diagnosis. Rapid advancements are reducing costs and improving techniques like real-time quantitative PCR (qPCR) and multiplex assays.
DNA extraction and amplification of the nuclear first internal transcribed spacer region (ITS1) from single Ascaris eggs are optimized for population genetic analysis. Applied to stool samples, these techniques can provide highly sensitive Ascaris detection, particularly by amplifying DNA from single eggs. Methods like molecular paleoparasitological hybridization for ancient DNA detection may further enhance sensitivity for very low-intensity infections.
Multiplex PCR enables simultaneous detection of multiple parasite species, simplifying diagnostics by replacing multiple tests with one. High-throughput PCR assays and multiplex PCRs for A. lumbricoides, T. trichiura, and N. americanus have shown promising results.
qPCR, unlike conventional PCR, quantifies amplicon and infection intensity. It is more sensitive than Kato-Katz and flotation (FS7) for detecting A. lumbricoides and co-infections. Multiplex qPCR assays have successfully detected A. lumbricoides alongside multiple intestinal parasites, highlighting the prevalence of co-infections, even in young children. qPCR quantification strongly correlates with EPG, indicating its potential for measuring parasite reduction post-treatment.
Alternatively, amplicons for multiple STHs and protozoa can be hybridized to beads on a Luminex platform, providing high-throughput diagnosis with less equipment than qPCR. Reverse transcriptase PCR can identify specific schistosome stages and could be useful for differentiating new and treatment-resistant Ascaris infections.
However, the higher sensitivity of qPCR over microscopy seen with many parasites isn’t always observed for Ascaris due to high egg output and challenges in extracting parasite DNA from the resistant Ascaris eggshell. This may limit the utility of methods like USB DNA-chip technology for field ascariasis diagnosis. Multiplex assays are also limited to targeted species, and DNA from high-intensity infections can impede detection of lower intensity infections. Cost remains a significant barrier to molecular diagnostic techniques in endemic areas, potentially making other methods more cost-effective for now.
Parallels to Diagnostic Tools for Ascaris suum
Recent evidence suggests A. suum may be a more common cause of human infection than previously thought, even in non-A. lumbricoides endemic areas. This necessitates species-specific ascariasis diagnosis on a larger scale. Zoonotic potential of Ascaris spp., potentially exacerbated by drug resistance in pigs, could reshape public health strategies. While adult A. lumbricoides and A. suum differ structurally, their eggs are morphologically indistinguishable, complicating stool-based species diagnosis.
Serological Diagnosis for Ascaris suum
An A. suum antigen-based immunoblot assay has been developed to diagnose human visceral larva migrans (VLM) suspected to be caused by A. suum. An ELISA using A. suum hemoglobin antigen correlates well with EPG and worm load in pigs, showing higher sensitivity than microscopy and low cross-reactivity with Trichuris suis. This veterinary tool could be adapted for rapid, multi-species diagnosis of human Ascaris infection.
Molecular Diagnosis for Ascaris suum
While PCR can detect single Ascaris eggs, it may not differentiate between A. lumbricoides and A. suum. Further research using multi-locus genotype data in sympatric populations is needed to assess cross-transmission globally and determine appropriate diagnostics. Detailed genomic comparisons between A. lumbricoides and A. suum may reveal genes suitable for species differentiation. However, mitochondrial DNA variations are minimal, raising questions about whether they are truly distinct species.
Discussion
Despite global STH control efforts, only about 30% of children needing treatment receive preventive chemotherapy. Accurate ascariasis diagnosis tests and optimized survey protocols are crucial for effective program planning, monitoring, and surveillance. The choice of diagnostic technique and protocol depends on the specific research question. Development of ascariasis diagnostics has been hampered by insufficient investment and the lack of a true gold standard for test comparison. There is a need for studies comparing adult worm expulsion, repeated Kato-Katz and other EPG estimation tests, and PCR to standardize current and future diagnostic methods. Ideal correlations should be linear with low variance. This review has identified available diagnostic tests to support endemic countries in achieving global targets, with recommendations summarized in Table 2.
Table 2. Characteristics and Application of Diagnostic Techniques in Control Programs
| Strengths and limitations | Recommendations for use in control programmes | Integration with other NTDs |
|---|---|---|
| Spec | Sens | Field-based |
| Coprological | ||
| Kato-Katz | ✓✓✓ | ✓ |
| McMaster | ✓✓✓ | ✓ |
| FLOTAC | ✓✓✓ | ✓✓ |
| Mini-FLOTAC | ✓✓✓ | ✓ |
| Serological | ||
| Antibodies | ✓✓ | ✓✓ |
| Antigens | ? | ? |
| Molecular | ||
| PCR | ✓✓✓ | ✓✓✓ |
| qPCR | ✓✓✓ | ✓✓✓ |
Comparison of ascariasis diagnostic test characteristics, including specificity (Spec), sensitivity (Sens), field applicability, cost, sample type, and recommendations for use in mapping, monitoring & evaluation (M&E), drug efficacy studies, and surveillance, highlighting integration potential with other NTD and common intestinal pathogen diagnostics.
Geographical Mapping of Disease Distribution
Currently Available Tests
Disease mapping for MDA frequency relies on stool microscopy, primarily Kato-Katz, using A. lumbricoides EPG categories (light, moderate, heavy). These thresholds need refinement, and further research is needed to correlate EPG values across FLOTAC, McMaster, and Kato-Katz. Questionnaires are not sensitive enough for identifying communities needing STH treatment.
Ideal Tools
While current stool-based tests may suffice for mass treatment strategies, particularly in moderate- to high-endemic areas, more sensitive tests are needed as infection intensity decreases. Point-of-care ascariasis diagnosis tests, similar to those for other infectious diseases, are needed for STH control programs. Ideal tools would include antigen, host immunological marker, or parasite DNA detection in urine, blood, or oral fluid. However, coproantigen tests may prove more sensitive due to STH location in the intestines. Improved coordination of disease mapping, integrating specimens for other NTD surveys, could enhance inter-sectoral collaboration and funding sustainability.
Monitoring and Evaluating Impact of Anti-helminthic Treatment
Currently Available Tests
Control program impact is evaluated via sentinel site surveys. Repeated mapping is sometimes used, but comparing cross-sectional surveys with varying protocols is questionable. Stool microscopy, especially Kato-Katz, remains the main diagnostic tool for impact evaluation, measuring prevalence, cure rate (CR), EPG, and egg reduction rates (ERRs). While cost and ease of use have been prioritized, more sensitive tools are needed as control programs reduce prevalence and intensity.
Ideal Tools
As infection intensity declines, measuring disease transmission becomes more critical, requiring direct infection markers like antigens. For integrated control programs, more precise diagnostics can better assess the impact of complementary interventions like WASH. Coordinated disease impact surveys, leveraging albendazole use for both LF and STH, and convenient sample collection would enhance data validity and cost-effectiveness. Techniques like FLOTAC®, with sample preservation, and centralized stool processing could be incorporated. However, rapid on-site STH diagnostic tools are highly needed as LF surveillance scales down. While current STH stool test limitations can be mitigated by adjusted reporting metrics, a convenient point-of-care test is needed for M&E of STH control programs, ideally detecting other tropical diseases like malaria on a multi-array platform. Coproantigen tests may be the most sensitive for A. lumbricoides infection. In areas aiming for STH elimination, antigen, antibody, and multiplex qPCR assays may improve disease detection. However, PCR results need to be correlated with morbidity, and PCR remains expensive.
Measuring Drug Efficacy
Currently Available Tests
Drug resistance monitoring is not routine in STH control programs. Benzimidazole resistance, though rare, may arise from drug pressure. Few studies assess coprological method accuracy for drug efficacy (CR or ERR) estimation. ERR via Kato-Katz is recommended for drug efficacy measurement, but other stool techniques, including pooled samples, may be more sensitive and cost-effective.
Ideal Tools
Despite high costs, PCR and qPCR’s increased sensitivity and specificity could establish accurate baselines and drug efficacy measures. Better understanding of EPG and worm burden correlation with qPCR quantification is needed. PCR methods should be used with Kato-Katz, and ideally another stool microscopy method, for accurate drug efficacy measurement. Single-nucleotide polymorphisms linked to drug resistance in veterinary nematodes may serve as early resistance markers in human A. lumbricoides, but more research is needed. Drug resistance monitoring could be integrated with other NTD control programs.
Surveillance of Disease Elimination and Recrudescence
Currently Available Tests
Global STH control targets do not currently include elimination. However, coordinated NTD surveillance surveys are recommended. Stool collection for STH diagnosis remains a challenge compared to blood or urine for other NTDs. In elimination settings, antibody detection in young children may indicate active transmission, but appropriate Ig isotypes need identification.
Ideal Tools
Serology and PCR-based diagnostic tests are being developed for other NTDs, with Ascaris assays as potential add-ons. Research on antigen, antibody, and molecular-based tools for Ascaris must be prioritized for integrated STH diagnosis.
Conclusions
Novel, convenient diagnostics for ascariasis are lacking compared to other NTDs. Standardized protocols and validated diagnostics are needed for assessing epidemiology, disease burden, and drug efficacy. This review has presented current tools for clinical and field ascariasis diagnosis. Ascaris diagnostics may shift towards more sensitive techniques like FLOTAC®, serological tests, and qPCR as low-intensity infection areas become more common. As control programs scale up, the evolving STH epidemiology requires addressing, and quantitative rapid, point-of-care tests are crucial for successful control. Increased investment in Ascaris and other STH diagnostic research is urgently needed to develop simple, affordable tools to alleviate human suffering caused by these infections.
Acknowledgments
The authors thank Professors Patrick Lammie, Lisette van Leishout, Peter Geldof, and Drs Bruno Levecke and Govert van Dam for discussions on Ascaris diagnostic methods, and Professor Roy Anderson for manuscript comments.
Compliance with Ethics Guidelines
Conflict of Interest
Poppy H. L. Lamberton and Peter M. Jourdan declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article involves no animal studies. Cited articles with human participant involvement, where the authors were involved, obtained and maintained local Institutional Review Board approval for all procedures.
Footnotes
This article is part of the Topical Collection on Topics Exploring Loa-Loa, Onchocerciasis, Hookworm, Ascaris, Trichuris.
Contributor Information
Poppy H. L. Lamberton, Phone: +44(0)207 5943819, Email: [email protected]
Peter M. Jourdan, Phone: +44 (0)207 5943254, Email: [email protected]
References
Papers of particular interest, published recently, have been highlighted as:
- • Of importance
- •• Of major importance