Celiac disease (CD), a chronic autoimmune disorder triggered by gluten in genetically predisposed individuals, is increasingly recognized as a condition affecting individuals across all age groups. While historically considered a pediatric illness, the landscape of celiac disease has shifted, with a notable rise in diagnoses among adults. This review aims to explore the nuances of celiac disease diagnosis with a specific focus on the average age of diagnosis, highlighting the differences between adult and pediatric presentations and the implications for diagnosis and management.
EPIDEMIOLOGY AND AGE OF ONSET IN CELIAC DISEASE
Celiac disease is a global health concern, with prevalence rates varying between 1 in 100 to 1 in 500 individuals, depending on the population and diagnostic methods employed. Notably, females are diagnosed more frequently than males. Serological advancements have refined prevalence estimates, suggesting that approximately 1% of the population in both Europe and North America may be affected. However, epidemiological studies reveal a disparity in diagnosis rates across age groups.
Early studies suggested a higher prevalence in children. For instance, a study in Catalonia indicated a fivefold higher prevalence in children (1/71) compared to adults (1/357), proposing a potential latency period in adults. Similar trends were observed in Brazil and India, with children exhibiting twice the frequency of celiac disease.
Image alt text: Graph illustrating celiac disease prevalence in different age groups, highlighting the variations between pediatric and adult populations in epidemiological studies.
However, contrasting data emerge from European adult populations. Studies in the United Kingdom and Finland reported prevalence rates of 1.2% and 2.4% respectively in adults, even in older age groups, mirroring or exceeding pediatric prevalence. A comprehensive review of global celiac disease frequency suggests variable prevalence and incidence ratios, often similar between children and adults, challenging the latency hypothesis.
The increasing recognition of adult-onset celiac disease may be attributed to enhanced screening in high-risk adult groups, such as first-degree relatives of diagnosed individuals, where prevalence can exceed 15%. Furthermore, serological screening alone may underestimate adult prevalence, as antibody titers can be lower or even negative in adults despite histological damage and symptoms indicative of celiac disease. This highlights the complexity of diagnosing celiac disease across different age demographics and emphasizes the need for nuanced diagnostic approaches beyond serology, especially in adults.
SYMPTOMS AND CLINICAL PRESENTATION ACROSS AGES
A significant distinction between pediatric and adult celiac disease lies in the clinical presentation at diagnosis. Children often exhibit classic malabsorption symptoms, while adults frequently present with milder or atypical symptoms. Classical malabsorption is observed more frequently in children, whereas less than 25% of adult cases manifest with these textbook symptoms.
Table 1: Age-Related Clinical Manifestations of Celiac Disease at Diagnosis
| Children | Children > 2 years | Adults |
|---|---|---|
| Diarrhea | Loose stools | Dyspepsia/Irritable Bowel Syndrome (IBS)-like symptoms |
| Malnutrition | Iron deficiency anemia | Iron deficiency anemia |
| Bloating | Abdominal pain | Constipation |
| Vomiting | Dyspepsia | Osteoporosis |
| Irritability | Growth delay | Arthritis |
| Muscle wasting | Headache | Elevated liver enzymes (Hypertransaminasemia) |
| Anemia | Delayed puberty | Extraintestinal symptoms |
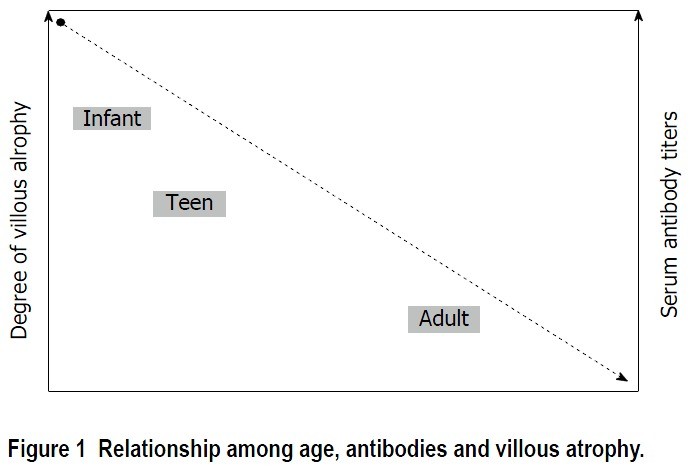

Table alt text: Table comparing and contrasting the major clinical findings at celiac disease diagnosis across different age groups: children, older children, and adults, emphasizing the shift from primarily digestive issues in children to more varied and often extraintestinal symptoms in adults.
As age increases, there is a tendency towards less pronounced clinical manifestations. Older children and adults may experience subtle symptoms like increased stool volume or gas, often attributed to lactose intolerance or bacterial overgrowth. Paradoxically, constipation can be the primary or even sole symptom in adult celiac disease.
Extraintestinal manifestations are more common in adults and may present alongside or independently of digestive symptoms. These include fatigue, mouth ulcers, osteoporosis, and skin lesions. While children can also experience non-digestive issues, they are less frequent and typically overshadowed by digestive symptoms.
Comorbid autoimmune disorders are also more frequently observed in adults with celiac disease, including autoimmune thyroiditis, type 1 diabetes, Sjögren’s syndrome, and dermatitis herpetiformis. The higher prevalence of associated autoimmune conditions in adults may reflect the longer duration of undiagnosed celiac disease and its cumulative impact on the immune system over time.
Interestingly, malnutrition, although a known feature of celiac disease, is not always present. Obesity or overweight is observed in a substantial proportion of adults at diagnosis, with some studies reporting over half of adult celiac patients as obese and only a small percentage underweight. This is particularly relevant in regions with high obesity prevalence. Therefore, clinicians should not dismiss celiac disease suspicion in overweight or obese adults.
Functional gastrointestinal disorders like dyspepsia and irritable bowel syndrome are common in adults, and celiac disease prevalence can be significantly elevated within these populations, reaching over 10% in some studies. Consequently, current guidelines recommend ruling out celiac disease through serology and/or endoscopy before diagnosing functional dyspepsia or IBS in adults. This is crucial because symptoms can overlap, and misdiagnosis can delay appropriate celiac disease management.
DIAGNOSTIC APPROACHES AND AGE-RELATED CONSIDERATIONS
Celiac disease diagnosis relies on a combination of serological testing, histological assessment of duodenal biopsies, and clinical response to a gluten-free diet. Serum antibody tests, including anti-deamidated gliadin peptide (DGP), anti-endomysial (EMA), and anti-tissue transglutaminase (tTGA) antibodies, are pivotal. However, the diagnostic accuracy and interpretation of these tests can vary with age.
Notably, tTGA titers and the severity of histological lesions tend to inversely correlate with age. As the age at diagnosis increases, antibody levels may be lower, and histological damage may be less pronounced. It is not uncommon to find adults with inflammatory changes in duodenal biopsies (lymphocytic enteritis – Marsh I or Marsh II) without significant villous atrophy.
Image alt text: Microscopic image illustrating duodenal biopsy Marsh classification, emphasizing the spectrum of histological changes in celiac disease from mild lymphocytic infiltration (Marsh I) to severe villous atrophy (Marsh III), and the relevance of these stages in diagnosing celiac disease across different ages.
This reduced clinical, serological, and histological expressiveness in adults complicates diagnosis compared to pediatric cases. Pediatric diagnostic criteria, such as those from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), allow for celiac disease diagnosis in children based on high tTGA titers alone, without mandatory biopsy. This is because high antibody titers in children strongly predict villous atrophy, thus potentially avoiding biopsies in up to 75% of pediatric cases. However, these criteria are less applicable to adults, as high antibody titers (greater than 10 times the upper limit of normal) are less frequent, occurring in less than half of adult celiac disease cases.
In adults presenting with dyspeptic symptoms, upper endoscopy with duodenal biopsies is often routinely performed irrespective of celiac serology, making histological assessment a cornerstone of adult diagnosis. Therefore, while high tTGA titers in adults can facilitate diagnosis, lower titers necessitate duodenal biopsies to confirm histological damage and guide dietary intervention.
In cases with low antibody titers or negative serology but persistent symptoms, duodenal biopsies are crucial. Biopsy findings in symptomatic, seropositive adults may reveal:
- Mild Villous Atrophy (Marsh 3A): In conjunction with HLA-DQ2 or DQ8 genotype, a gluten-free diet is warranted, and clinical and histological response should be monitored. This scenario fulfills most of the diagnostic criteria for gluten-sensitive enteropathy.
- Lymphocytic Enteritis (Marsh I): Characterized by increased intraepithelial lymphocytes without villous atrophy. This can have various etiologies beyond celiac disease, including Helicobacter pylori infection, NSAID use, infections, or Crohn’s disease. If these are excluded and HLA compatibility exists, further investigations like IgA anti-transglutaminase subepithelial deposit analysis and duodenal lymphocyte flow cytometry (gamma-delta T-cell receptor expression) may support celiac disease diagnosis.
In lymphocytic enteritis cases, response to a gluten-free diet must be evaluated, including follow-up biopsies to confirm diagnosis according to Catassi and Fasano criteria. The long-term implications of lymphocytic enteritis associated with adult celiac disease and its relationship to complications are still under investigation, requiring careful diagnosis and follow-up.
Table 2: Diagnostic Criteria for Celiac Disease (Catassi and Fasano)
| Diagnostic Criterion | Description |
|---|---|
| Compatible Signs and Symptoms | Clinical presentation consistent with celiac disease |
| Positive Serology | High titer of IgA anti-celiac autoantibodies |
| Genetic Predisposition | Presence of HLA-DQ2 and/or -DQ8 genes |
| Celiac Enteropathy | Small intestinal biopsy showing Marsh-Oberhuber grade 3 lesions, Marsh 1-2 lesions with positive celiac antibodies, or Marsh 1-3 lesions with IgA subepithelial deposits |
| Response to Gluten-Free Diet | Resolution of symptoms and serological normalization following gluten withdrawal |
Table alt text: Table outlining the diagnostic criteria for celiac disease as proposed by Catassi and Fasano, listing the five key criteria including symptoms, serology, genetics, enteropathy, and response to a gluten-free diet, emphasizing the comprehensive approach needed for accurate diagnosis, especially in cases with atypical presentations.
PATHOGENIC MECHANISMS AND AGE-RELATED VARIATIONS
The intestinal microbiota’s role in celiac disease pathogenesis is increasingly recognized. Early life colonization and microbiota-immune system interactions may significantly influence disease development. Studies show altered intestinal microbiota composition in both children and adults with celiac disease compared to controls. Age-related differences in duodenal microbiota are also observed between celiac and non-celiac individuals.
Generally, intestinal microbiota richness increases with age, and compositional differences exist between children and adults. It is hypothesized that age-varying microbial compositions could lead to differing microbiota-immune system interactions, potentially contributing to the clinical, serological, and histological variations seen across age groups in celiac disease.
Intraepithelial lymphocytes (IELs) are crucial in celiac disease’s immune response, irrespective of age. Increased CD3+ lymphocytes expressing the γδ receptor are a hallmark of celiac disease. While their exact function remains unclear, γδ IELs may have a regulatory role in the immune response and correlate with villous atrophy severity in both children and adults. Another IEL population, CD3-, is inversely related to age, being twice as frequent in children under three years compared to adults. These age-related IEL population changes may contribute to the varying clinical presentations of celiac disease across the lifespan.
EVOLUTION, PROGNOSIS, AND AGE AT DIAGNOSIS
Celiac disease management centers on a strict, lifelong gluten-free diet (GFD). While most patients experience clinical improvement on a GFD, up to 30% of adults may continue to have symptoms despite gluten withdrawal. In adults with persistent symptoms on a GFD, other conditions like lactose intolerance, bacterial overgrowth, pancreatic insufficiency, microscopic colitis, or refractory celiac disease should be investigated.
Furthermore, histological recovery rates differ between adults and children. Over 50% of adults may not achieve mucosal healing despite two years on a GFD, whereas duodenal mucosa recovery is seen in the vast majority of children (95%) within the first two years post-diagnosis. Poorer dietary adherence in adults, leading to inadvertent gluten ingestion, is a likely factor in this disparity, as children’s diets are often more closely managed.
Strict adherence to a GFD and duodenal mucosal normalization are critical goals in adult celiac disease management. Dietary non-compliance and persistent histological damage are associated with increased risk of lymphoproliferative disorders, the most severe celiac disease complication, particularly in adults. Studies indicate that adults with persistent villous atrophy have twice the risk of developing malignant lymphoproliferative diseases, especially T-cell lymphoma. Therefore, a follow-up biopsy within two years of diagnosis is recommended in adults to assess mucosal recovery and identify individuals at higher risk for complications, warranting closer monitoring and dietary compliance reinforcement.
Osteoporosis and reduced bone mineral density, often linked to vitamin D deficiency, are also concerns in celiac disease. While fracture risk is less clear, bone densitometry is recommended in high-risk adult celiac patients, such as post-menopausal women, men over 55, and those with pre-existing osteopenia. The cost-effectiveness and optimal frequency of bone densitometry in all adult celiac patients require further study. Children may have reduced bone mass at diagnosis but typically achieve full recovery within 6-12 months on a GFD. Routine bone densitometry is not generally needed in uncomplicated pediatric celiac disease, but monitoring growth and development is essential.
Hyposplenism is another complication affecting over one-third of adult celiac patients but is rare in children. Its incidence correlates with pre-diagnosis gluten exposure duration and is higher in those with autoimmune or premalignant conditions. Splenic function assessment may be considered in older adults at diagnosis, those with comorbidities, or a history of infections or thromboembolism. Pitted red cell counting is a cost-effective diagnostic tool for hyposplenism. Protein-conjugate vaccines are recommended for patients with significant hyposplenism.
Pediatric celiac disease follow-up can be less intensive than in adults. Bone densitometry and follow-up biopsies are selectively indicated in children. Annual follow-up for children with good GFD adherence and normalized antibody levels may be appropriate to ensure early detection of associated conditions and normal growth and development. Malignant complications and refractory celiac disease are essentially adult-specific concerns, necessitating a more rigorous follow-up strategy in adults compared to children.
CONCLUSION: AGE AT DIAGNOSIS AND CLINICAL PRACTICE
Celiac disease exhibits significant age-related differences in presentation, diagnosis, and management. In adults, the clinical presentation is often subtle, with atypical or minor symptoms, lower antibody titers, and milder mucosal lesions compared to children. Pediatric diagnostic criteria are not always applicable in adults, necessitating duodenal biopsies in most adult diagnoses and follow-up to assess mucosal recovery and detect complications. Clinicians must be aware of these age-related nuances to ensure timely and accurate celiac disease diagnosis and management across all age groups.
Table 3: Key Features of Celiac Disease Presentation in Adults
| Feature | Description |
|---|---|
| High Prevalence Across Age Range | Significant prevalence even in older adults |
| Oligosymptomatic Presentation | Often presents with subtle or atypical symptoms |
| Serology Limitations | Lower diagnostic yield of serology compared to children |
| Mild Histopathology | Duodenal biopsy frequently shows mild atrophy or lymphocytic enteritis |
| Lymphocytic Enteritis Relevance | Lymphocytic enteritis is a common presentation in adults |
| Advanced Diagnostic Techniques | Expert pathology and flow cytometry may be useful for diagnosis |
| Monitoring Key Outcomes | Strict dietary compliance and villous atrophy recovery are crucial |
| Complication Vigilance | Early identification of associated complications is essential |
Table alt text: Summary table of key features associated with celiac disease presentation in adults, emphasizing the challenges in diagnosis due to subtle symptoms and the importance of comprehensive diagnostic and monitoring strategies.
REFERENCES
References from the original article should be listed here, maintaining the original numbering if possible or re-numbering sequentially. Please refer to the original article for the full list of references.