1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) stands as a major global health challenge, recognized as a leading cause of both illness and death worldwide. While COPD can manifest with varied symptoms, they typically begin to surface after the age of 40. The way COPD presents itself clinically is far from uniform. It differs significantly from the initial stages of the disease through its progression and in how it affects patients’ lives at different points in time. This complexity highlights the need to fully grasp the diverse elements that contribute to the overall impact of COPD on an individual’s health [1,2].
Current COPD treatments are largely shaped by findings from extensive clinical trials. These trials often involve participants who represent a typical COPD patient profile. However, they don’t always delve deeply into the role of co-existing health conditions that can worsen COPD. Furthermore, pivotal clinical trials that guide COPD treatment strategies often focus on a patient demographic with an average age of around 65 years old, plus or minus 8 years [3,4,5,6,7]. This age focus in research can limit how well these findings apply to a broader COPD population, particularly older individuals or those with unique health profiles who might need more tailored treatment plans.
To truly understand COPD, it’s crucial to recognize that a patient’s condition is frequently influenced by other health issues. These conditions may not always be directly linked to the degree of lung function impairment measured by spirometry but often worsen as patients age [8,9]. It’s common in COPD patients to observe varying levels of functional decline, different clinical signs, inconsistent exacerbation frequencies, and quality of life issues, even among those with similar forced expiratory volume in 1 second (FEV1) measurements. This variability may stem from the disease’s heterogeneous nature, possibly originating from different disease mechanisms and showing up as different clinical presentations. Research within the general population has increasingly shown that as COPD patients get older, the likelihood of having multiple chronic conditions increases. These comorbidities often speed up the health decline in COPD patients [10,11].
Besides the increasing number of comorbidities, aging itself is considered a factor that can affect COPD progression. While there are parallels between natural lung aging (sometimes called “senile lung”) and COPD, the normal changes in the lungs that come with age should not be seen as a disease requiring medical intervention. Currently, there isn’t enough evidence to say if lung aging alone is a major factor in COPD patients needing medical care or hospitalization for COPD flare-ups. Aging might only explain some related changes, mainly emphysema [12]. Regardless of aging’s role in COPD development, it’s clear that as COPD patients age, they develop distinct clinical traits that need identification as they can significantly impact how the disease progresses.
Recent research emphasizes the need to include time, or aging, when studying how genetics and environment contribute to COPD’s development. The age when genetic and environmental factors interact is significant, as are past exposures and even parental history. A comprehensive approach that includes genetics, environment, and time can offer deeper insights into lung function and explain the variety in COPD clinical presentations [13].
Our study proposes that the clinical picture of COPD changes with a patient’s age, leading to different disease burdens. This age factor is crucial when designing studies and treatment plans, enabling more personalized patient care focused on specific treatable aspects, potentially lessening the current impact of COPD.
The use of big data and artificial intelligence in healthcare allows us to handle and gain insights from large, complex datasets from electronic health records (EHR). This technology allows for the evaluation of key indicators in clinical processes, reducing biases beyond data availability. Big data is now essential, changing how we understand complex issues in fields like epidemiology. It’s especially useful in public health, enabling early disease detection, tracking disease patterns, and creating better prevention strategies.
In epidemiology, big data integrates information from EHRs, social media, and wearable devices. Big data analysis also enables tailored health interventions and policies to meet specific community needs. Combining big data with artificial intelligence (AI) further enhances health management. AI is vital for processing and analyzing large datasets, identifying real-time patterns and correlations for early disease detection and faster responses to health crises. AI’s machine learning improves predictive models over time with new data. This synergy between big data and AI is crucial for strengthening epidemiological resilience and improving global health today.
This study aims to identify, in routine clinical settings, how COPD patient profiles differ by age and how this impacts the overall disease burden. This approach will improve our understanding of gene-environment-time interactions, leading to better treatments and prevention for COPD.
2. Materials and Methods
This study was designed as an observational, retrospective, and non-interventional analysis, utilizing secondary data extracted from the unstructured text within electronic medical records (EMR). Conducted within the Castilla-La Mancha region of Spain, the study leveraged the healthcare administration’s (SESCAM) Savana Manager v.3.0 tool, which facilitated the analysis of data dating back to January 1, 2011. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [14]. The study population encompassed patients over 40 years of age diagnosed with COPD and treated between January 1, 2011, and January 24, 2021.
The methodology employed has been previously detailed in other publications [15,16,17,18]. Savana Manager is a data extraction system powered by artificial intelligence (natural language processing, NLP) and big data techniques. This technology is designed to extract unstructured clinical information (natural language or free text) from electronic health records (EHR) and transform it into organized, reusable data for research, all while ensuring patient anonymity. Utilizing computational linguistic techniques, Savana Manager accurately detects and validates comprehensive clinical content using the SNOMED CT [19] tool, drawing data from the SESCAM specialized care network (including hospitalization, emergency, outpatient consultations) and primary care services.
Data management and protection: Hospital IT services manage and anonymize data before it reaches Savana, ensuring no identifiable information is disclosed. An algorithm further protects patient data by randomly inserting misleading information and only recovering partial individual data. This process creates a fully anonymized patient database, with study reports containing only aggregated, non-identifiable data. In compliance with the European Data Protection Authority, anonymized clinical records are exempt from General Data Protection Regulation. The study received approval from the Research Ethics Committee (Comité de Ética de la Investigación, or CEIm) of the Guadalajara public healthcare area (ref 1/2023, approved January 17, 2023).
Information extraction assessment: For this study, COPD identification in free text was achieved using a named-entity recognition approach, enhanced with negation and temporality detection layers. The negation detection model combines rule-based analysis with a binary convolutional neural network, trained on Spanish EHRs and tested against extensive reference standards. This model categorizes clinical entities as affirmative or non-affirmative based on context. Temporality detection uses an NLP module with layers that assign dates to clinical entities. It identifies date mentions in EHR text and uses a Bi-LSTM-based model to determine if a date relates to a clinical entity. A normalization layer standardizes date formats from EHR text. Post-processing involves quality control and integrates NLP module outputs into a final database.
Following cNLP processing, three authors validated the tool’s results and technology performance to confirm EHRead® technology’s accuracy in identifying “COPD” records and related variables. A manual review of 560 documents established a gold standard for accuracy. Savana’s performance was evaluated against this gold standard, measuring its precision in identifying disease presence and related variables. Performance metrics included precision (P), recall (R), and the F-score, the harmonic mean of precision and recall.
Precision, indicating result reliability, was calculated as P = tp/(tp + fp). Recall, showing retrieved information quantity, was R = tp/(tp + fn). The F-score, F = 2 × precision × recall/(precision + recall), measured overall retrieval performance. True positives (tp) were correctly identified records, false negatives (fn) unidentified records, and false positives (fp) incorrectly retrieved records.
Previous findings indicated metric values above 0.9, confirming adequate diagnosis identification for the study population. F-scores for analyzed terms ranged from 0.92 to 0.97.
Statistical analysis: SPSS software (version 25.0; IBM, Armonk, NY, USA) and OpenEpi (https://www.OpenEpi.com accessed February 6, 2023) were used for statistical analysis. Standard descriptive statistics were employed. Qualitative variables are presented as frequencies and percentages, while quantitative variables are shown as means, 95% confidence intervals, and standard deviations. Numerical variables were analyzed using the Student’s t-test for independent measures, and the Chi-squared test was used for association and proportion comparisons in qualitative variables. Significance, assessed using a Chi-squared 2 × 2 contingency table controlling for sex and age biases, was set at p < 0.05. Savana orders events by odds ratio (observed vs. expected frequency), with significant differences defined by a p-value < 0.05.
3. Results
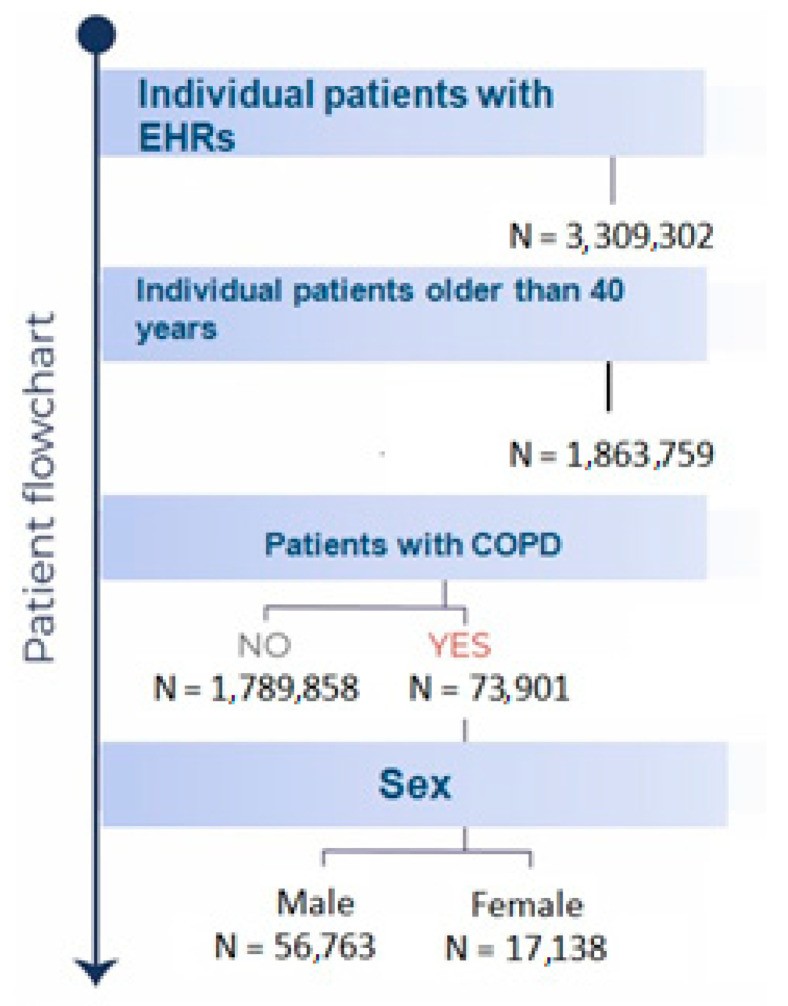
During the study period from January 1, 2011, to January 14, 2021, a total of 73,901 patients diagnosed with COPD received treatment within the Castilla-La Mancha Public Healthcare Services (SESCAM). The Average Age For Copd Diagnosis in this cohort was 73 years (95% CI: 72.9–73.1), with a significant majority, 76.8% (56,763), being male. Figure 1 provides a detailed patient inclusion flowchart for this study.
Table 1. Basal characteristics of the study population.
| Male COPD Population (n = 56,763) | Female COPD Population (n = 17,138) | p | |
|---|---|---|---|
| Age, years (95% CI) | 72.9 (72.8–73) | 72.3 (72.1–72.5) | |
| Comorbidities | |||
| Arterial hypertension (%) | 70.0 | 72.3 | |
| Dyslipidemia (%) | 49.6 | 52.5 | |
| Diabetes (%) | 37.9 | 38.5 | |
| Smoking (%) | 41.7 | 35.9 | |
| Obesity (%) | 23.9 | 32.7 | p < 0.05 |
| Heart failure (%) | 37.3 | 48.3 | p < 0.05 |
| Atrial fibrillation (%) | 19.4 | 18.4 | |
| Ischemic cardiopathy | 14.4 | 7.7 | p < 0.05 |
| Obstructive sleep apnea (%) | 13.5 | 10.8 | |
| Depression (%) | 10.0 | 27.2 | p < 0.05 |
| Hiatal hernia (%) | 12.7 | 17.3 | p < 0.05 |
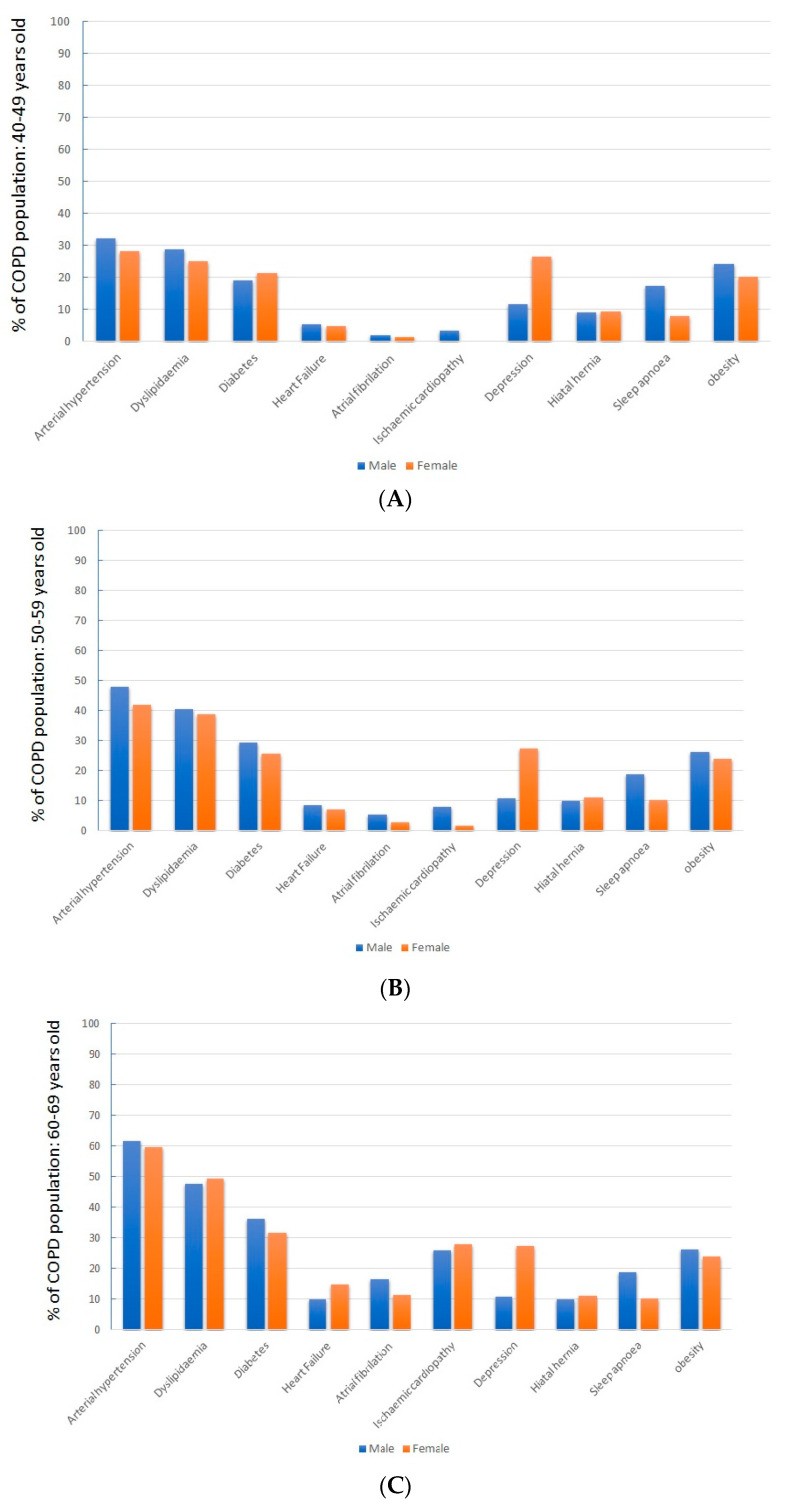
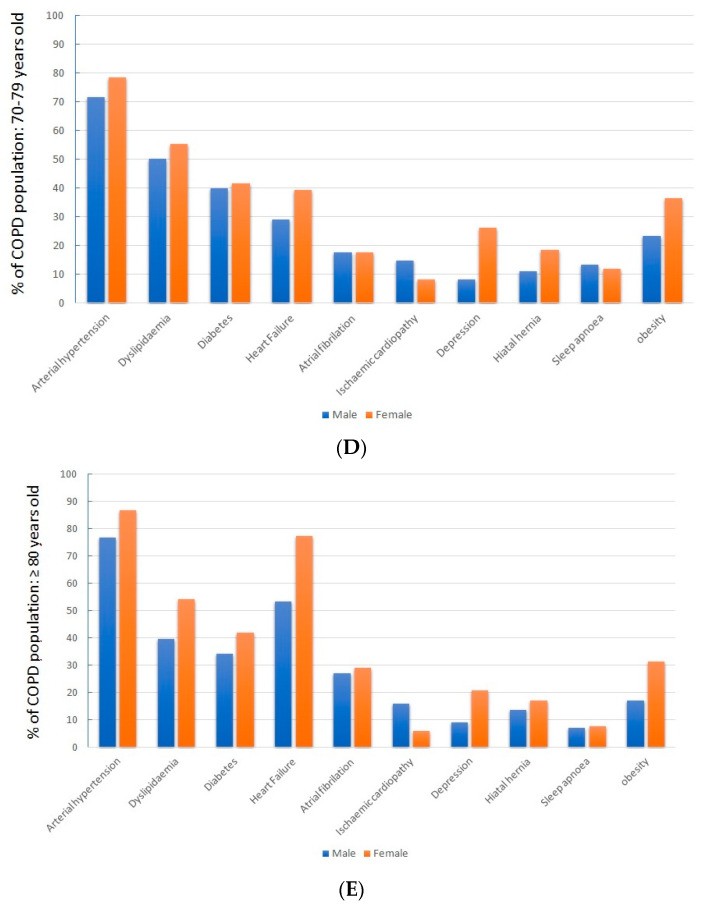
Analyzing patients by sex revealed that women showed a notably higher prevalence of obesity, heart failure, depression, and hiatal hernia (p < 0.05 for each).
When examining patients across different age ranges, a clear trend of increasing cardiovascular risk factors with age emerged. Concurrently, there was a progressive rise in associated diseases, particularly cardiovascular conditions, as detailed in Table 2.
Table 2. Comorbidities in COPD by age.
| Age Range, Years Mean (CI 95%) | >40 without COPD 62.1 (62–62.1) | Total COPD Population > 40 73 (72.9–73.1) | p | COPD 40–49 45 (44.9–45.1) | COPD 50–59 54 (54.7–54.8) | COPD 60–69 64 (64.3–64.4) | COPD 70–79 74.4 (74.4–74.5) | >COPD 80 85.3 (85.3–85.4) |
|---|---|---|---|---|---|---|---|---|
| Sex, male (%) | 51.4 | 76.8 | 66.7 | 71.2 | 79.4 | 82.1 | 77.1 | |
| Comorbidities | ||||||||
| Arterial hypertension (%) | 29.9 | 70.5 | p < 0.05 | 30.8 | 46.2 | 61.1 | 72.7 | 78.7 |
| Dyslipidemia (%) | 21.0 | 50.3 | p < 0.05 | 27.4 | 39.9 | 47.8 | 51.0 | 47.2 |
| Diabetes (%) | 14.4 | 38.1 | p < 0.05 | 19.6 | 28.2 | 35.3 | 40.0 | 37.2 |
| Smoking (%) | 11.2 | 40.3 | p < 0.05 | 70.3 | 68.2 | 52.0 | 32.1 | 17.7 |
| Obesity (%) | 8.2 | 25.9 | p < 0.05 | 22.8 | 25.5 | 26.2 | 25.7 | 20.4 |
| Heart failure (%) | 6.9 | 40.1 | p < 0.05 | 5.1 | 8.0 | 10.6 | 30.9 | 58.7 |
| Atrial fibrillation (%) | 4.5 | 19.1 | p < 0.05 | 1.9 | 4.5 | 9.2 | 17.4 | 27.5 |
| Ischemic cardiopathy | 2.7 | 12.9 | p < 0.05 | 2.6 | 6.4 | 10.3 | 13.5 | 14.7 |
| Obstructive sleep apnea (%) | 2.5 | 12.9 | p < 0.05 | 14.1 | 16.3 | 15.4 | 11.0 | 7.1 |
| Depression (%) | 6.9 | 14.0 | p < 0.05 | 16.4 | 15.5 | 12.0 | 11.2 | 11.7 |
| Hiatal hernia (%) | 5.1 | 13.8 | p < 0.05 | 9.1 | 10.2 | 10.8 | 12.4 | 14.3 |
Notably, heart failure was highly prevalent in older patients, affecting 30.9% of those aged 70–79 and 58.7% of those over 80. Compared to the general population aged 40 and above, COPD patients demonstrated a significantly higher occurrence of cardiovascular risk factors, cardiovascular disease, depression, and hiatal hernia.
Table 3 illustrates the impact of age on disease burden, assessed by hospital admissions and mortality rates.
Table 3. Burden of the disease evaluated by hospitalizations and hospital mortality.
| Age Range, Years | 40–49 | 50–59 | 60–69 | 70–79 | >80 |
|---|---|---|---|---|---|
| % of total COPD population | 3.2 | 11.9 | 20.8 | 28.0 | 36.1 |
| % COPD patients that required hospitalization for acute deterioration | 11.9 | 14.7 | 19.9 | 24.2 | 30.7 |
| Number of hospitalizations among patients that required hospitalization | 2.3 | 2.5 | 2.9 | 3.0 | 3.0 |
| In-hospital death | 3.7 | 2.6 | 3.2 | 4.3 | 6.2 |
The data indicates a significant increase in hospital admissions and mortality with age, representing a substantial portion of the COPD population.
Sex-related differences in comorbidities were consistent across all age groups, as shown in Figure 2.
4. Discussion
The findings of this study reinforce that COPD patients often face a complex web of health conditions, largely linked to comorbidities that become more prevalent with age. As COPD patients advance in age, shifts in their health profile are linked to increased healthcare utilization, particularly hospitalizations, and higher mortality rates.
COPD should be viewed not as a singular disease entity, but rather as a syndrome encompassing both functional and structural lung alterations that manifest in recognizable chronic symptoms. This arises from the interplay of environmental and genetic factors, with aging acting as a significant contributor. The ultimate clinical outcomes resulting from genetic-environmental interactions, along with prior cumulative exposures—affecting both the patient and potentially their parents—highlight the critical role of time, or aging [13]. Understanding the significance of this age factor in COPD could pave the way for identifying new targets for early therapeutic and preventive interventions.
Certain comorbidities, notably cardiovascular diseases like heart failure, can significantly influence COPD symptoms. Agustí et al., using data from the ECLIPSE study, suggested a shared underlying mechanism [20]. However, current data cannot definitively confirm a causal link or merely an association due to overlapping risk factors. Regardless, our study robustly demonstrates a high prevalence of comorbidities in COPD patients, which can critically shape the clinical expression of COPD. Some of these comorbidities are significantly associated with increased morbidity and mortality [21]. Prior observational studies have also noted a higher comorbidity prevalence in COPD patients compared to the general population, a finding echoed in our study [8,22]. The key contribution of this study is quantifying the extent of this issue in a real-world setting, free from the selection biases common in many prior observational studies, and determining its magnitude adjusted for age.
Several studies have indicated an inverse relationship between a patient’s health status and the presence of comorbidities, especially when three or more are present, irrespective of lung function [23,24,25]. Furthermore, the risk of exacerbations, hospitalizations, mortality, and the economic burden of COPD on healthcare systems are all linked to the number of comorbidities [26,27,28].
Some research indicates that COPD patients in real-world settings have a higher incidence of obesity, depression, obstructive sleep apnea (OSA), and hiatal hernias compared to the general population [29,30,31]. These conditions do not necessarily increase in frequency with patient age. However, other comorbidities, particularly cardiovascular diseases, show a significant age-related increase. Heart failure, common in COPD patients, becomes particularly critical in those over 70, potentially worsening baseline conditions and mimicking or exacerbating COPD flare-ups.
Our real-world data confirms COPD as a disease predominantly affecting older individuals, with this age group comprising a larger proportion of patients and exhibiting more complex disease presentations, thus placing a greater demand on healthcare resources. Utilizing big data methods and artificial intelligence has provided a realistic view of patient treatment needs within a specific region. It reveals that over 50% of COPD patients in our study were over 70. These findings align with the EPISCAN2 study, which recently highlighted COPD prevalence in Spain [32]. That study found only 6.03% of COPD patients were aged 40–49, while 30.08% were 70–79. Notably, the over-80 age group, often overlooked, constituted 36% of our total COPD patient population.
A limitation of this study is the potential inclusion of patients whose COPD diagnosis wasn’t always confirmed by post-bronchodilator spirometry, particularly relevant in those over 80. However, as these patients are diagnosed and treated for COPD, their significant impact on treatment needs is evident. Given that most prior studies exclude this demographic, assessing specific strategies for older age groups is crucial. Personalized treatment approaches that consider individual patient needs are essential for effective management.
Multimorbidity and comorbidity represent aspects of complexity but may not fully capture a patient’s overall situation. Some patients with a single disease may require intricate management, while others with multiple conditions may be relatively straightforward to manage. Therefore, comorbidity assessment should not replace functional evaluation in diagnosis, prognosis, or therapeutic planning, especially in older populations where the impact of individual diseases is less distinct within the accumulation of multiple physiological system impairments. The strength and novelty of this study lie in its big data approach, which can overcome the historical exclusion of elderly patients from chronicity and multimorbidity studies, despite this group having the highest disease prevalence and healthcare expenditure. They represent the real patient base of our healthcare systems [33,34].
5. Conclusions
The primary conclusion of this study, strengthened by its large-scale, real-world patient population, is that COPD is now predominantly a disease affecting older adults, who frequently have comorbidities that significantly influence the disease’s progression. A holistic understanding of the patient, beyond just the disease itself, is vital for effective COPD management. Utilizing frailty assessment tools developed by geriatric specialists can enhance our understanding of older COPD patients, enabling tailored care measures. The oldest age group bears the greatest disease burden, affecting both individual patients and the public healthcare system. Understanding the role of time in COPD (GETomics [13]) could lead to new early therapeutic and preventive strategies, complementing established genetic and environmental factors.
Author Contributions
D.M., J.L.I., J.R. and J.M.R. were responsible for the design, development, data extraction, analysis, and preparation of the manuscript. The remaining authors, J.R., J.C., M.B. and A.P., participated in the data extraction, analysis, and correction of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Guadalajara’s Hospital for studies involving humans.
Informed Consent Statement
Informed consent was not required because the study is anonymous, observational, and retrospective.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available because they belong to the National Health System of Castilla La Mancha.
Conflicts of Interest
We declare there are no real or perceived conflict of interests or financial compensations related to this submission, and that we have no links to tobacco manufacturers and/or the tobacco industry.
Funding Statement
This project was funded by the Chair of Inflammatory Diseases of the Airways, University of Alcalá.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available because they belong to the National Health System of Castilla La Mancha.
[1] Miravitlles, M.; Barrecheguren, M. Time to individualise COPD prevention and treatment. Lancet 2013, 382, 1977–1988. [CrossRef] [PubMed]
[2] Vestbo, J.; Hurd, S.V.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [CrossRef] [PubMed]
[3] Calverley, P.M.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W.; Yates, J.C.; Vestbo, J.; HURXHAM Study Team. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007, 356, 775–789. [CrossRef] [PubMed]
[4] Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M.; UPLIFT Study Team. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [CrossRef] [PubMed]
[5] Wedzicha, J.A.; Calverley, P.M.; Rabe, K.F. Roflumilast: A new oral therapy for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 1187–1194. [CrossRef] [PubMed]
[6] Celli, B.R.; Decramer, M.L.; Kesten, S.; Lystig, T.; Tashkin, D.P.; Yates, J.; Wise, R.; UPLIFT Study Team. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with COPD. Eur. Respir. J. 2009, 34, 1295–1301. [CrossRef] [PubMed]
[7] Mahler, D.A.; Decramer, M.; D’Urzo, A.; Gon Y, E.; Jørgensen, L.; клеп, С.I.; Kramer, B.;зова, Т.; Maltais, F.; et al. Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: The BLAZE study. Eur. Respir. J. 2011, 38, 759–769. [CrossRef] [PubMed]
[8] van den Bemt, L.; Vrieze, A.; Brusse-Keizer, M.; Kerkhof, M.; Movig, K.; Wesseling, G.; de Jong, Y.; Taxis, K. Prevalence of polypharmacy and potential drug-drug interactions in COPD patients: A cross-sectional study in the Netherlands. Int. J. Chron. Obstruct. Pulmon. Dis. 2012, 7, 295–302. [CrossRef]
[9] Agusti, A.; Hogg, J.C. Update on Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1260. [CrossRef]
[10] Fabbri, L.M.; Luppi, F.; Beghé, B.; Dal Negro, R. COPD and comorbidities. Respir. Med. 2008, 102, 1223–1231. [CrossRef]
[11] പുരുഷോത്തമൻ, I.; ബെഹെ, ബി.; ലുപ്പി, എഫ്.; ഡാൽ നെഗ്രോ, ആർ.; ഫാബ്രി, എൽ.എം. Chronic obstructive pulmonary disease and comorbidities. Eur. Respir. Rev. 2007, 16, 1–6. [CrossRef]
[12] മിറവിറ്റില്ലെസ്, എം.; സോൾട്ടൻ, എ.; കോസ്റ്റ, സി.; മെനെൻഡെസ്, ആർ.; പിന്റോ-പ്ലാറ്റ, വി.; എഡ്വേർഡ്സ്, എൽ.ഡി.; അഗസ്റ്റി, എ.; വെസ്റ്റോ, ജെ.; ഹൺസിങ്കർ, എം.; ഡോനെറി, എം.; മറ്റുള്ളവർ. Towards the understanding of phenotype-genotype correlations in COPD: A population-based study. Respir. Res. 2009, 10, 51. [CrossRef] [PubMed]
[13] ലോፔസ്-കാമ്പുസ്, ജെ.എൽ.; ടോറസ്-പിപി, ജെ.വി.; കാംപോസ്-ഹെറേറോ, എ.; അഗസ്റ്റി, എ.ജെ.എ. COPD in the 21st century: Time to move from genes and environment to time. Lancet Respir. Med. 2017, 5, 717–719. [CrossRef]
[14] വോൺ എൽം, ഇ.; അൽറ്റ്മാൻ, ഡി.ജി.; എഗ്ഗർ, എം.; പോക്കോക്ക്, എസ്.ജെ.; ഗോർസ്കി, പി.സി.; ഒ’റൂർക്കെ, ഡി.; ഷ്ട്രോബ് ഇനിഷ്യേറ്റീവ്. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. ജെ. ക്ലിൻ. എപ്പിഡെമിയോൾ. 2007, 60, 605–608. [CrossRef]
[15] റോഡ്രിഗസ്-ഗോൺസാലസ്, എം.; ഇനോജോസ-എസ്ക്യുറോല, എ.; ലോപേസ്-ഗുവേറ, എ.; ലേഗസ്പൈൻ, പി.; റിയോസ്, എ.; ടോബിയാസ്, എ.; റോഡ്രിഗസ്, ജെ.എം.; ഡാമിയൻ, ജെ.; മിറവിറ്റില്ലെസ്, എം. Identification of patients with chronic obstructive pulmonary disease (COPD) through automated analysis of electronic health records. പ്ലോസ് വൺ 2020, 15, e0238991. [CrossRef]
[16] ലേഗസ്പൈൻ, പി.; റോഡ്രിഗസ്-ഗോൺസാലസ്, എം.; റോഡ്രിഗസ്, ജെ.എം.; റിയോസ്, എ.; ഗാർസിയ-ഗുടേറെസ്, എസ്.; മിറവിറ്റില്ലെസ്, എം. Identification of patients with asthma in primary care using electronic health records and natural language processing. ജെ. ആസ്ത്മ 2021, 58, 1582–1590. [CrossRef]
[17] ഡാമിയൻ, ജെ.; റോഡ്രിഗസ്-ഗോൺസാലസ്, എം.; മാർട്ടിനെസ്-ഗാർസിയ, എം.എ.; മിറവിറ്റില്ലെസ്, എം. Characterization of patients with COPD and depression using artificial intelligence and electronic health records. ജെ. സൈക്കിയാട്രിക് റെസ്. 2021, 138, 319–325. [CrossRef]
[18] മെനാൻഡെസ്, ആർ.; സെബല്ലോസ്, ജെ.പി.; റോഡ്രിഗസ്-ഗോൺസാലസ്, എം.; റിയോസ്, എ.; ടോബിയാസ്, എ.; റോഡ്രിഗസ്, ജെ.എം.; മിറവിറ്റില്ലെസ്, എം. Identification of patients with pneumonia in primary care using electronic health records and natural language processing. യൂറോപ്പ്. റെസ്പിർ. ജെ. 2021, 58, 2003845. [CrossRef]
[19] സ്റ്റെറിൽ, പി.; കാംബ്രിഡ്ജ്, ജെ.; ക്യാമ്പ്ബെൽ, ജെ.ആർ. SNOMED Clinical Terms: Overview of development, structure, and clinical use. ജെ. എം.ഐ.എ. 2011, 18, 93–98. [CrossRef]
[20] അഗസ്റ്റി, എ.; കാൽവർലി, പി.എം.; സിയെന്നി, ജി.; ഡോണേരി, എം.; ഹഫ്നാഗൽ, സി.; ഹോഗ്, ജെ.സി.; വെൽറ്റെ, ടി.; ബാരെച്ചെഗുറെൻ, എം. Systemic effects of chronic obstructive pulmonary disease. യൂറോപ്പ്. റെസ്പിർ. ജെ. 2017, 50, 1700344. [CrossRef]
[21] ഡെക്ക്രേമർ, എം.; കാൽവർലി, പി.എം.; ഡൂഹാൻ, ജെ.; ലോഹ്റൈച്ച്, എ.; മക്നൈ, കെ.; റോനെക്കർ, ജെ.; സാഞ്ചെസ്-ആയ്ല, എ.; സെൽനർ, സി.; ടോമസ്, എം.; വാൻ ഡെ പോൾ, എം.; മറ്റുള്ളവർ. Roflumilast and exacerbations in severe chronic obstructive pulmonary disease. യൂറോപ്പ്. റെസ്പിർ. ജെ. 2017, 49, 1602560. [CrossRef]
[22] മല്ലെസ്കോട്ട്, എം.എൽ.; ലേബ്ലാൻക്, എം.; ഓസ്റ്റെബോ, ആർ.; വെറ്റെർഗ്രെൻ, ജെ.; ബാജേ, എഫ്.; ലാഫോൺ, പി.സി.; ക്ലാപ്പിയർ, ജെ.; റൗലിയർ, എ.; ബൗലിയക്, ജെ.; ലൂയിസ്, പി.; മറ്റുള്ളവർ. Burden of COPD in France: Results from the nationwide, population-based CONSTANCES cohort. റെസ്പിർ. റെസ്. 2021, 22, 67. [CrossRef]
[23] ഹുബെർ, എം.; വാൻ വിയറ്റ്, എം.; ഗ്രിൻഹുയിസെൻ, ജെ.; സ്മിറ്റ്, എച്ച്.; ക്രെമർ, എ.; പോളാർഡ്, ബി. Towards a ‘patient-centred’ operational definition of health: A mixed methods study. ബി.എം.ജെ. ഓപ്പൺ 2016, 6, e010236. [CrossRef]
[24] വാൻ ലിൻഡൻ, സി.ജെ.; ഹുബെർ, എം.; ലോബ്രിച്ച്, എം.; എസ്സെലിങ്ക്, ആർ.എ.; സ്റ്റെർക്സ്, വൈ.ജെ.ജെ.; വെർഹാർ, എച്ച്.ജെ.എം.; വാൻ റോസം, എ.സി.എം. Why do older people with chronic diseases not function optimally? Towards an interdisciplinary, dynamic model. ഇന്റർനാഷണൽ ജെ. ഇന്റഗ്രേറ്റഡ് കെയർ 2013, 13, e001. [CrossRef]
[25] സ്മിറ്റ്, എം.; ക്രെമർ, ഇ.എ.എം.; ലുപ്പൻ, എം.ജെ.; ഒലഡാപോ, ഒ.ജെ.; വാൻ ഒഎച്ച്, എ.എ.എം.; പോളാർഡ്, ബി. The first years of ‘positive health’: A mixed methods study into experiences of general practitioners and other healthcare professionals. ബി.എം.ജെ. ഓപ്പൺ 2017, 7, e017089. [CrossRef]
[26] ഡെജോംഗെ, സി.; ഡെകോർട്ട്, എ.സി.; ഡെഗ്രൂട്ട്, വി.; ബൗസ്ക്വെറ്റ്, ജെ.; സ്റ്റാല്ലിയോ-ലാബൗരി, എം.എച്ച്. Impact of comorbidities on health status and burden in COPD. റെസ്പിർ. മെഡ്. 2015, 109, 575–584. [CrossRef]
[27] മാർട്ടിനെസ്-ഗാർസിയ, എം.എ.; സോൾട്ടൻ, എ.; അൽവേഴ്സ്-സലാസ്, ഇ.; റാമോൺ-ടോറെല്ലെസ്, ജെ.എൽ.; സെക്കീറോ, പി.; മസ്കറെൽ, ബി.; ലോപേസ്-കാമ്പുസ്, ജെ.എൽ.; മിറവിറ്റില്ലെസ്, എം. Comorbidity and mortality in COPD patients attending primary care. യൂറോപ്പ്. റെസ്പിർ. ജെ. 2011, 38, 55–61. [CrossRef]
[28] ഖാന്നൂച്ചി, എ.; കാൽവർലി, പി.എം.; ഹെർനാൻഡസ്, പി.; ജാക്കോബ്സ്, എം.; ലാമെർസ്, ജെ.ഡബ്ല്യു.ജെ.; മെക്കൈ, കെ.; റിച്ചി, സി.; വാൻ ഡെ പോൾ, എം.; വെസ്റ്റോ, ജെ.; വിസ്നേവ്സ്കി, ടി.; മറ്റുള്ളവർ. Roflumilast in patients with COPD and history of hospitalisation for exacerbation: A subgroup analysis of the randomised clinical trials. ലാൻസെറ്റ് റെസ്പിർ. മെഡ്. 2018, 6, 435–444. [CrossRef]
[29] അഫോൺസോ, വി.; ഫ്രെറെസ്, എഫ്.; മുനോസ്, എൽ.; അബാഡൽ, ജെ.എം.; മസ്കറെൽ, ജെ.; മൊൻസോ, ഇ.; കാസനോവ, സി.; മിറവിറ്റില്ലെസ്, എം.; കെയർബോം, COPD-സ്പാനിഷ് കോഹോർട്ട്. Clinical phenotypes and mortality in COPD patients: Results from the Spanish COPD cohort. യൂറോപ്പ്. റെസ്പിർ. ജെ. 2014, 43, 709–716. [CrossRef]
[30] ഹെൻഡ്രിക്സ്, എ.എം.; വാൻ റോസം, എ.സി.എം.; ബ്രൂസ്സെ-കെയ്സർ, എം.; വാൻ ഇംസ്വെല്ലെർ, സി.എച്ച്.ഇ.; വാൻ ഡെൻ ബാംറ്റ്, എൽ.; ഹുയിജെൻസ്, എം.എച്ച്.എ.; വാൻ ഡെൻ ബീർട്ട്-വൺ ഡെർ സ്ലൂയിസ്, ആർ.; വെസ്സെലിംഗ്, ജി.; മെൽസെൻ, ജെ.ഡബ്ല്യു.; ഡെ ജംഗ്, വൈ.; മറ്റുള്ളവർ. Prevalence of comorbidities in COPD in primary care: A cross-sectional study. ഇന്റർനാഷണൽ ജെ. ജനറൽ മെഡ്. 2015, 8, 93–100.
[31] മോർഗൻ, എ.ഡി.; സ്റ്റിവാർഡ്, എ.എൽ.; ബാസ്, എ.; കിം, വി.; ക്രോൺ, ആർ.ഇ.; വാങ്, ഇ.; കമ്മൻസ്, ഡി.എസ്. Factors associated with hiatal hernia in patients with COPD. തോറാക്സ് 2012, 67, 1014–1015. [CrossRef]
[32] സോൾട്ടൻ, എ.; അൽവാർ ഗുസ്മാൻ, എ.; മിറവിറ്റില്ലെസ്, എം. COPD prevalence and attributable risk factors in Spain: EPISCAN II study. യൂറോപ്പ്. റെസ്പിർ. ജെ. 2021, 58, 2100984. [CrossRef]
[33] ഫോർട്ട്സിൻസ്കി, ആർ.എച്ച്.; മക്കീൻ, ജെ.; എമേരി, സി.എഫ്.; ഫ്രോഹ്ലിച്ച്, വൈ.; ഗുഡ്ലിൻ, എസ്.ജെ.; ഹാൻ, ജെ.; മക്ഡൊനോഫ്, കെ.എച്ച്.; മോറിസ്, പി.ഇ.; ഓട്ട്, സി.; സ്മിത്ത്, പി.ജെ.; മറ്റുള്ളവർ. Contemporary review of chronic obstructive pulmonary disease and cardiovascular disease: JACC state-of-the-art review. ജെ. ആം. കോൾ. കാർഡിയോൾ. 2023, 82, 92–111. [CrossRef]
[34] അഗസ്റ്റി, എ.ജെ.എ.; ബേലർ, ബി.; ബാരെച്ചെഗുറെൻ, എം.; ബുസെറ്റോ, പി.; കാർസൺ, കെ.; കോൺനെല്ലി, കെ.എ.; ഫോർസ്റ്റർ, കെ.; ഹെർനാൻഡസ്, എ.എഫ്.; ഹഫ്നാഗൽ, സി.; ജാക്കോബ്സ്, എം.; മറ്റുള്ളവർ. Integrated care in chronic obstructive pulmonary disease: A blueprint from the FRESH AIR synergy project. ലാൻസെറ്റ് റെസ്പിർ. മെഡ്. 2023, 11, 256–272.