Parkinson’s disease (PD) stands as a significant neurodegenerative disorder, imposing substantial burdens on individuals and society alike. The insidious nature of PD means its pathology can begin over a decade before traditional diagnostic criteria can detect it based on symptom severity. As the quest for treatments that can alter the course of the disease intensifies, understanding the optimal timing for diagnosis becomes paramount. While current diagnostic approaches rely on established clinical criteria, the integration of additional clinical markers and biomarkers holds promise for earlier detection, particularly within research settings. This article delves into the crucial benefits of diagnosing Parkinson’s disease in its early stages, even before the emergence of classic clinical signs. However, pinpointing the “timely” moment for diagnosis is a nuanced decision, influenced by individual patient preferences, the effectiveness of available disease-modifying therapies (or their future emergence), and the capacity of healthcare systems to provide comprehensive support across all disease stages. Compelling evidence underscores the quality-of-life improvements offered by existing symptomatic treatments, bolstering the argument for diagnosis at the onset of noticeable symptoms. The advent of disease-modifying treatments would further amplify the advantages of early diagnosis. The benefits extend beyond the individual and their families, positively impacting broader society and the research community. Ultimately, shared decision-making, guided by the ethical principles of autonomy, beneficence, and non-maleficence, must be central to determining a “timely” diagnosis for each person.
Keywords: Parkinson’s disease, neurodegeneration, prodromal, disease modifying therapy, ethics, personalized medicine
Understanding Parkinson’s Disease: A Growing Global Challenge
Parkinson’s disease (PD) is a progressive, age-related neurodegenerative condition that is becoming increasingly prevalent worldwide. Currently, it affects approximately 41 out of every 100,000 individuals aged 40 to 49, with this number dramatically escalating to 1,607 per 100,000 in those over 80 years old 1– 3. Global trends paint a concerning picture, indicating that PD is on the verge of becoming a pandemic. The prevalence of PD more than doubled between 1990 and 2015, and it now affects an estimated 6.2 million people globally. Projections based on current trends and global population growth estimate that this number could surge to between 12.9 and 14.2 million by 2040 1, 2, 4. While it was once believed that PD did not shorten lifespan, accumulating evidence suggests otherwise 5, 6, with a significant reduction in “healthy life years” for many patients. This is even more pronounced in PD patients who develop dementia 7, highlighting the urgent need for effective interventions and improved management strategies.
PD is recognized as the archetypal “shaking palsy,” or “paralysis agitans,” first meticulously described by James Parkinson two centuries ago 8. The hallmark motor symptoms crucial for diagnosis are bradykinesia (slowness in initiating and executing movement, and a decrement in repetitive movements), rigidity, and tremor. However, it’s crucial to recognize that PD is far more than just a motor disorder. A wide array of non-motor symptoms frequently emerge even before motor symptoms are prominent enough for a diagnosis. These non-motor features significantly impact the quality of life of individuals with PD and their caregivers 9. They encompass a broad spectrum of issues including pain, autonomic dysfunction (such as constipation, orthostatic hypotension, and erectile dysfunction), psychiatric disturbances (including cognitive impairment, depression, anxiety, and apathy), and other debilitating symptoms like fatigue, rapid eye movement (REM) sleep behavior disorder (RBD), loss of smell (hyposmia), and excessive saliva production (hypersalivation) 9, 10.
The motor symptoms of PD arise from the progressive degeneration and death of dopamine-producing neurons, primarily in a brain region called the substantia nigra 11. Similar to other neurodegenerative diseases, the pathological process in PD involves the misfolding and aggregation of a naturally occurring protein, alpha-synuclein. This aberrant protein accumulation and subsequent neuronal dysfunction begin long before overt clinical symptoms become apparent 12– 16. By the time a clinical diagnosis is typically made, significant neuronal damage has already occurred. It is estimated that individuals have already experienced a 50 to 70% reduction in striatal dopamine levels and a 30 to 50% loss of dopaminergic neurons [17](#ref-17], [18](#ref-18]. Therefore, a major focus of Parkinson’s disease research is to develop interventions that can be initiated before substantial neuronal loss has occurred, with the aim of slowing down or halting the disease progression.
Early Versus Timely Diagnosis: A Matter of Perspective
The imperative to intervene earlier in Parkinson’s disease necessitates the ability to identify individuals at risk or in the very early stages, before they would typically meet diagnostic criteria. However, it’s essential to distinguish between “early” and “timely” diagnosis. From a purely scientific standpoint, “early” diagnosis is understood within the context of disease pathology and its clinical manifestations. Neurodegenerative diseases generally follow a predictable pattern, progressing from a pre-symptomatic or prodromal phase through defined disease stages, ultimately leading to advanced disease. This perspective is objective and essential for understanding disease mechanisms, identifying potential therapeutic targets, and evaluating treatment efficacy. Drawing parallels with advancements in infectious diseases and cancer, the principle that earlier detection leads to better outcomes is compelling, especially when effective disease-modifying treatments are available.
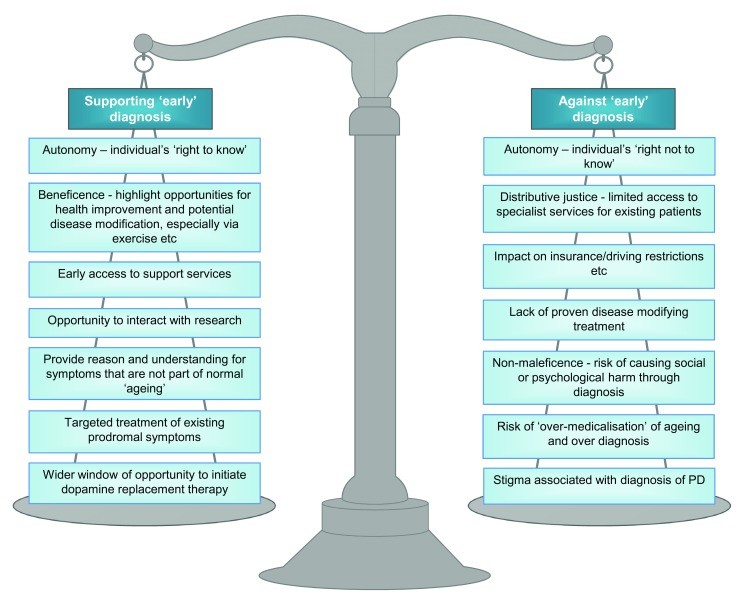
However, the concept of “timely” diagnosis introduces a more patient-centered and nuanced perspective. “Timely” diagnosis may hold different meanings for different individuals and within different societal contexts. Healthcare has evolved from a paternalistic model to a more person-centered, individualized approach. “Timely” diagnosis considers the individual’s unique priorities, acknowledging both the potential advantages and disadvantages of knowing about a diagnosis earlier in the disease process. These multifaceted arguments, relevant to PD and other conditions, have been comprehensively summarized in the context of dementia by Dhedhi et al. 19 (See Figure 1).
Figure 1. Arguments for and against “early” diagnosis of Parkinson’s Disease. Early diagnosis presents both potential benefits and risks, influencing individual well-being and healthcare approaches. Adapted from Dhedhi et al. 19, these arguments highlight the complexities surrounding the decision to pursue early diagnosis, emphasizing the need for careful consideration of individual circumstances and preferences in the context of Parkinson’s disease.
Current Diagnostic Approaches and the Window for Earlier Intervention
The definitive “gold standard” for confirming Parkinson’s disease remains postmortem pathological examination of brain tissue. This examination looks for the presence of alpha-synuclein-positive inclusions (Lewy bodies) within neurons that haven’t yet died. Clinical diagnosis of PD, performed by movement disorder specialists, is accurate approximately 85% of the time 20. The most widely used diagnostic criteria for research purposes are the United Kingdom Parkinson’s Disease Society Brain Bank Criteria. These criteria employ a three-step process: first, confirming the presence of a Parkinsonian syndrome; second, ruling out any exclusion criteria (conditions that mimic PD); and third, establishing the presence of supportive features that increase diagnostic confidence 21. Diagnosis is often an iterative process. While a diagnosis of PD can frequently be made at the initial consultation, it requires periodic review to ensure it remains the most accurate explanation for the patient’s symptoms.
Given the inherent limitations of clinical diagnosis, ongoing research is dedicated to refining diagnostic methods and identifying reliable biomarkers. The International Parkinson and Movement Disorders Society (MDS) has recently updated the clinical diagnostic criteria for PD 22. These revised criteria offer a two-tiered diagnostic approach, categorizing diagnoses as “clinically established PD” and “clinically probable PD.” The criteria utilize an algorithmic approach to confirm the presence of parkinsonism and then systematically evaluate “supportive,” “red flag” (features suggesting alternative diagnoses), and “absolute exclusion” criteria. A significant advancement of these new criteria is their explicit consideration of non-motor symptoms. However, whether these updated criteria will lead to improved overall diagnostic accuracy is still under evaluation. Remarkably, despite extensive research efforts, biomarkers—whether clinical, genetic, biochemical, or imaging-based (reviewed in 23)—currently play a minimal role in routine clinical diagnosis. Even dopamine transporter single-photon emission computed tomography (SPECT) imaging, a functional neuroimaging technique, is only mentioned in the new criteria as an absolute exclusion criterion if the scan is normal, indicating no dopaminergic deficit. Furthermore, national guidelines in the UK explicitly advise against the routine clinical use of biomarkers for PD diagnosis and specifically discourage the use of magnetic resonance imaging (MRI) to confirm PD outside of research settings 24. This underscores the ongoing need for robust and readily applicable biomarkers to enhance diagnostic accuracy and facilitate earlier intervention.
Approaching Early Diagnosis: Identifying Individuals at Risk
Recognizing the long pre-diagnostic phase of Parkinson’s disease, research has increasingly focused on characterizing this period. Numerous studies have been initiated to identify and validate risk factors, prodromal markers, and early clinical features that can predict the eventual development of PD 25– 27. Several large-scale cohort studies are actively contributing to this effort:
- The Parkinson’s Associated Risk Study (PARS): This multicenter US study compares older adults with and without hyposmia (reduced sense of smell), a known early marker of PD. Participants undergo annual physical assessments and dopamine transporter SPECT scans every two years 28.
- The PRIPS (Prospective Validation of Risk Factors for the Development of Parkinsonian Syndromes) study: Conducted across three European countries, PRIPS compares risk factors, physical examinations, olfactory function, and transcranial sonography in over 1,800 adults older than 50 years 29.
- The Tübingen Evaluation of Risk Factors for Early Detection of Neurodegeneration (TREND) cohort: This German cohort has followed nearly 700 individuals aged 50 to 85 who were enriched for potential pre-diagnostic symptoms. Enrollment criteria included having at least one of the following: depression, reduced smell, or RBD. Participants undergo biannual assessments, including motor and neuropsychological examinations, olfactory testing, quantitative gait and balance assessments, and transcranial sonography 30.
- The Parkinson’s Progression Markers Initiative (PPMI): PPMI is a large multinational cohort study with recruitment sites in North America, Europe, Israel, and Australia. It includes a longitudinal study of over 400 individuals with established PD, a control cohort of nearly 200 individuals without PD or a family history of PD, and a specific “at-risk” cohort of 65 individuals with RBD or hyposmia. PPMI also actively recruits individuals with and without PD who carry genetic mutations associated with increased PD risk, such as mutations in LRRK2, GBA, or SNCA [31](#ref-31].
- The PREDICT-PD study: Based in the UK and ongoing since 2011, PREDICT-PD has followed over 1,300 older adults (aged 60 to 80 at enrollment) without a diagnosis of PD or other neurological diseases. Annual online assessments track motor and non-motor symptoms using validated questionnaires, a tapping test to assess motor slowing 32, and objective smell testing 33, [34](#ref-34].
While these studies employ diverse methodologies, they collectively demonstrate the feasibility of identifying individuals with strong evidence of “pre-diagnostic” PD using epidemiological, clinical, imaging, and other risk markers 28, 35– 38. As these cohorts mature and “high-risk” individuals transition to a diagnosis of established PD, they provide crucial proof-of-concept data and will help refine optimal strategies for “early” detection. However, the question of whether “early” detection translates to “timely” diagnosis remains a complex consideration.
It is also important to note that a significant family history of PD or a known genetic predisposition can profoundly influence an individual’s perspective on early versus timely diagnosis. Furthermore, genetic risk factors may also present opportunities for participation in genetically targeted disease-modifying treatment (DMT) trials. For instance, Gaucher’s disease (GD), a lysosomal storage disorder caused by mutations in the GBA gene, is associated with a significantly increased risk of PD. Genetic testing in unselected PD cohorts reveals GBA mutations in up to 10% of cases, with even higher rates in certain populations, such as Ashkenazi Jewish PD cohorts 39– 42. Similarly, mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are prevalent in PD patients of Ashkenazi Jewish and Berber Arab descent, and the clinical presentation and pathology of LRRK2-related PD may differ from “idiopathic” PD 43– 48.
In parallel with revising the clinical criteria for PD diagnosis, the MDS has also developed research criteria for prodromal PD 26. These criteria calculate a combined likelihood ratio for prodromal PD based on risk factors (age, sex, occupational toxin exposure, smoking and caffeine habits, family history, and nigral hyperechogenicity on transcranial sonography) and prodromal features (RBD, abnormal dopamine transporter imaging, subtle motor impairments on quantitative testing, hyposmia, constipation, orthostatic hypotension, erectile and urinary dysfunction, and depression/anxiety disorders). A cutoff of at least 80% likelihood using these criteria defines “probable prodromal PD,” and this threshold has been validated in several studies 49– 51.
Unveiling the Benefits: Why Early Diagnosis of Parkinson’s Disease Matters
The “timeliness” of a Parkinson’s disease diagnosis is inherently subjective and depends on a complex interplay of personal and societal factors, crucially including the availability of effective treatments. Individual preferences are shaped by their desire for knowledge, their personal weighting of various risk factors and symptoms, and their overall values. This is reflected in the wide spectrum of disease severity observed at the time of initial PD diagnosis. Some individuals seek medical advice at a stage where PD cannot be diagnosed using current criteria, while others delay seeking help until they are significantly burdened by the disease (See Figure 2). In the absence of proven DMTs, some individuals, particularly those with a family history of PD or a strong personal connection to the disease, may have a strong desire for the earliest possible diagnosis. Others may welcome a diagnosis to explain subtle but concerning symptoms, while many others may prefer to be diagnosed when symptoms are more overt, aligning with current diagnostic practices.
Figure 2. The Spectrum of Timeliness in Parkinson’s Disease Diagnosis. Parkinson’s disease onset is gradual, with pathology potentially beginning a decade before diagnosis. Progressive pathology leads to a gradual accumulation of subtle motor and non-motor symptoms. The timeliness of diagnosis varies significantly among individuals due to diverse factors and personal priorities, falling broadly into four categories. As disease-modifying treatments become available, the rationale for earlier “timely” diagnosis strengthens. However, a personalized approach is essential, emphasizing shared decision-making and individualized care to determine the optimal timing for each person.
The timeliness of established PD diagnosis remains a personal perspective, significantly influenced by available treatment options. Historically, delaying the initiation of anti-Parkinsonian medication was often considered advisable to postpone the onset of treatment-related complications and due to concerns about potential levodopa toxicity. However, the ELLDOPA (Early vs. Late Levodopa) trial definitively demonstrated no clinical benefit from delaying levodopa treatment, and robust clinical evidence refutes the concept of levodopa toxicity 52. Conversely, for individuals newly diagnosed with PD, both pharmacological and non-pharmacological interventions are available that can significantly improve quality of life. Effective symptomatic treatments for PD are well-established. Observational studies have shown that individuals with PD who remain untreated (by mutual agreement with their physician) experience a significantly worse quality of life across all domains of the Parkinson’s Disease Questionnaire-39 (PDQ-39) compared to those who initiate anti-Parkinsonian medication early in their disease course 53. It is reasonable to extrapolate this observation to individuals who remain undiagnosed despite experiencing symptoms, highlighting the potential benefit of earlier diagnosis for symptom management and quality of life.
Non-pharmacological management of PD is also continually advancing. Exercise has been proven to be beneficial in both PD and Alzheimer’s disease (AD) 54– 56. Specific forms of exercise like Tai Chi, Qigong, dance, and other focused physical activities have been shown to enhance quality of life and positively impact various aspects of PD 57, 58. Furthermore, individuals in the pre-diagnostic phase of PD often seek medical attention from their primary care physicians more frequently, sometimes years before a formal PD diagnosis, suggesting that their symptoms are already impacting their well-being enough to warrant medical consultation 10. Many of these early non-motor symptoms are treatable. Therefore, earlier diagnosis can facilitate the identification and management of these often-overlooked early features of PD. Collectively, these observations strongly support the rationale for early diagnosis at a point when symptoms first begin to negatively impact a patient’s quality of life. This approach would shift the optimal timing of diagnosis to coincide with the initial presentation of motor and non-motor symptoms, with the primary goals of improving quality of life and long-term outcomes for individuals with PD.
Looking towards the future, it’s essential to acknowledge the history of unsuccessful drug trials aimed at modifying the underlying disease process in PD. Numerous trials of nutraceuticals like coenzyme Q10, green tea polyphenols, and creatine, as well as pharmaceuticals including rasagiline, pramipexole, and tocopherol, have failed to demonstrate disease-modifying effects 59– 61. However, the pursuit of DMTs is far from over and is becoming increasingly sophisticated and potentially more successful. Current research is increasingly focused on developing DMTs targeted at specific genetic subgroups of PD. Examples include ambroxol for GBA mutation carriers 40, 62, LRRK2 competitive kinase inhibitors 63, and antisense-oligonucleotide therapies 64 are entering clinical trials. The Linked Clinical Trials initiative is also playing a crucial role by identifying drugs with established safety profiles in other medical fields that have promising preclinical data to warrant further investigation in PD clinical trials 65. For instance, a link between diabetes mellitus and PD has been observed 66, and exenatide, a hypoglycemic agent used to treat diabetes, has shown encouraging results in a small randomized controlled trial in PD 67. Similarly, emerging evidence suggests potential neuroprotective effects of statins, and a randomized controlled study of high-dose simvastatin in PD is currently underway in the UK 68. Furthermore, vaccine approaches based on promising animal data are also being tested in human trials 69. If and when interventions are proven to delay the onset or slow the progression of PD, the argument for bringing forward diagnosis to a pre-symptomatic stage for high-risk individuals will become even more compelling.
Societal and Research Benefits of Timely Diagnosis
The economic burden of Parkinson’s disease is immense, impacting not only individuals and their families but also society as a whole 70. A comprehensive study analyzing the economic impact of PD in the US revealed that the disease cost the nation over USD $14 billion in 2010, and individuals with PD incurred more than twice the medical expenditure compared to a matched population without PD 71. In the UK, direct healthcare expenditure for PD is approximately double that of age-, gender-, and geographically matched controls, and these costs escalate with disease progression 72, 73. In Alzheimer’s disease, economic modeling of DMTs suggests potential net savings as high as £3.3 billion per year 74. These figures strongly suggest that from a societal perspective, particularly given the projected increase in PD prevalence, “timely” diagnosis of PD, as early as possible when disease-modifying interventions become available, is economically prudent and socially beneficial.
Beyond the economic implications, timely diagnosis is also critically important for advancing Parkinson’s disease research. The field can learn valuable lessons from recent clinical trial failures in Alzheimer’s disease. Unfortunately, numerous AD DMT trials have failed to meet their primary endpoints 75. While the reasons for these failures are complex and likely multifactorial, a recurring theme in reviews analyzing these unsuccessful AD DMT trials is that interventions may have been initiated “too little, too late” in the disease process 76, 77. Similarly, for DMTs in Parkinson’s disease to be most effective, it is likely crucial to initiate treatment before the majority of the nigrostriatal pathway is significantly damaged – that is, earlier than the current point of typical clinical diagnosis. Early diagnosis is essential for identifying and recruiting individuals in the very early stages of PD for clinical trials of novel DMTs, maximizing the chances of demonstrating efficacy and accelerating the development of effective therapies.
Navigating Timely Diagnosis: A Personalized, Ethical Approach
In alignment with the principles of personalized care for established Parkinson’s disease, the cornerstone of achieving “timely” diagnosis lies in establishing a mutual understanding between the clinician and the patient. This process involves the clinician sharing their expertise about PD, its likely progression, and available treatment options, while the patient articulates their individual understanding of their symptoms, their goals, their fears, and their personal weighting of potential benefits and risks associated with early diagnosis (both medical and psychological) 78. This shared decision-making process carefully weighs the perceived risks of early diagnosis against the potential benefits, adhering to the fundamental pillars of medical ethics: autonomy (respecting patient’s right to self-determination), beneficence (acting in the patient’s best interest), non-maleficence (avoiding harm), and justice (fair allocation of resources and access to care). While current benefits of early diagnosis primarily stem from improved symptomatic management, the increasing availability of DMT trials for willing participants is likely to shift individual perspectives on what constitutes “timely” diagnosis, potentially favoring earlier detection.
Conclusion: Embracing Personalized Timeliness in Parkinson’s Diagnosis
In conclusion, a rigid, “one-size-fits-all” approach to diagnosing Parkinson’s disease is not appropriate. “Early” diagnosis exists on a temporal and mechanistic continuum, while “timely” diagnosis is inherently personalized, tailored to the individual’s unique circumstances, priorities, social context, and the available therapeutic and healthcare system options within their environment. Beyond the crucial quantitative research focused on developing neuroprotective treatments, there is a pressing need for robust qualitative research to explore societal attitudes towards pre-diagnostic, prodromal, and pre-motor identification of PD pathology. Ultimately, a personalized approach to diagnosis, firmly grounded in the individual’s attitudes, circumstances, and available treatments, will be fundamental in optimizing care and outcomes for people at risk of or living with Parkinson’s disease.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
- Mayela Rodriguez-Violante, Movement Disorders Clinic, National Institute of Neurology and Neurosurgery, Mexico City, Mexico
- Matthew J. Farrer, Djavad Mowafhagian Centre for Brain, University of British Columbia, Vancouver, British Columbia, Canada
Funding Statement
This review was supported by grants from: Parkinson’s UK (G-1606), National Institute for Health Research University College Hospitals Biomedical Research Centre and Bart’s Charity (Preventative Neurology Grant).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
1. Dorsey ER, Bloem BR, Beck JC, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939-53. PubMed: 30219587
2. GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877-97. PubMed: 28931491
3. de Rijk MC, Tzourio C, Breteler MM, et al. Prevalence of parkinsonism and Parkinson’s disease in Europe: The EUROPARKINSON Collaborative Study. J Neurol Neurosurg Psychiatry. 1997;62(1):10-5. PubMed: 9009908
4. Bloem BR, Okun MS, Wegener B, et al. Parkinson’s disease as a public health crisis. J Parkinsons Dis. 2012;2(1):1-2. PubMed: 23939188
5. Dahodwala N, Bloem BR, Factor SA, et al. Mortality in Parkinson disease: a systematic review and meta-analysis. Neurology. 2018;91(8):e773-e785. PubMed: 30045960
6. Savica R, Grossardt BR, Ahlskog JE, et al. Survival and causes of death in patients with incident Parkinson disease: a 20-year population-based study. Arch Neurol. 2010;67(11):1343-9. PubMed: 21041581
7. Hely MA, Reid WG, Adena MA, et al. The Sydney Multicentre Study of Parkinson’s disease: progression and mortality at 20 years. J Neurol Neurosurg Psychiatry. 2008;79(3):300-6. PubMed: 17684039
8. Parkinson J. An Essay on the Shaking Palsy. London: Whittingham and Rowland; 1817.
9. Chaudhuri KR, Healy DG, Rascol O. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235-45. PubMed: 16487888
10. Schrag A, Nunn P, Wood NW, et al. ‘Pre-motor’ symptoms in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;69(1):57-61. PubMed: 10864619
11. Dauer M, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889-909. PubMed: 12971891
12. Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197-211. PubMed: 12490260
13. Hawkes CH, Tredici KD, Daniel SE. Parkinson’s disease: a dual-hit hypothesis based on selective neuroanatomy and neurophysiology. Neuropathol Appl Neurobiol. 2007;33(6):599-614. PubMed: 17941978
14. Jellinger KA. Pathology of Parkinson’s disease. J Neural Transm Suppl. 1999;56:1-29. PubMed: 10565157
15. Fearnley JM, Lees AJ. Pathology of Parkinson’s disease. Adv Neurol. 1995;65:29-37. PubMed: 7634844
16. Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Cold Spring Harb Perspect Med. 2017;8(1):a024146. PubMed: 29298897
17. Cheng HC, Upton AL, Sachdev P, et al. Loss of dopamine terminal markers in early Parkinson’s disease as determined by [18F]FE-CIT and PET. Brain. 2010;133(Pt 1):171-84. PubMed: 19965960
18. Bernheimer H, Birkmayer W, Hornykiewicz O, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical and pharmacological correlations. J Neurol Sci. 1973;20(4):415-55. PubMed: 4357981
19. Dhedhi RA, Davies N, Phipps M, et al. Arguments for and against early diagnosis of dementia: a systematic review. Alzheimers Res Ther. 2016;8(1):20. PubMed: 27221632
20. Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-4. PubMed: 1549285
21. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745-52. PubMed: 2899549
22. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-601. PubMed: 26474316
23. Tysnes OB, Storstein A. Biomarkers for Parkinson’s disease. J Parkinsons Dis. 2017;7(Suppl 1):S95-S108. PubMed: 29513691
24. NICE. Parkinson’s disease in adults. Diagnosis and management. NICE guideline [NG71]. 2017. https://www.nice.org.uk/guidance/ng71
25. Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1602-11. PubMed: 26474317
26. Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464-70. PubMed: 31538287
27. Lang AE, Postuma RB. Moving towards disease modification in Parkinson’s disease. Mov Disord. 2018;33(4):507-15. PubMed: 29575791
28. Jennings D, Dawson TM, Dawson VL. The Parkinson’s Associated Risk Study (PARS): Design and baseline data. Mov Disord. 2018;33(5):807-15. PubMed: 29533548
29. Berg D, Riess O, Rüb U, et al. Prospective Validation of Risk Factors for the Development of Parkinsonian Syndromes Study (PRIPS). Study design and baseline data. Mov Disord. 2011;26(10):1801-9. PubMed: 21887755
30. Schulz J, Becker C, Brockmann K, et al. The Tübingen evaluation of risk factors for early detection of neurodegeneration (TREND) study: study design and baseline data of 668 individuals at risk for parkinsonian syndromes. J Parkinsons Dis. 2014;4(4):597-609. PubMed: 25421862
31. Parkinson Progression Markers Initiative. www.ppmi-info.org Accessed 2018 Nov 13.
32. Heilman KM, Bowers D, Bloom J, et al. Speed-accuracy trade-off and handedness in Parkinson’s disease. Neurology. 1993;43(1):94-9. PubMed: 8420481
33. Doty RL, Shaman P, Dann MS. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502. PubMed: 6463981
34. Doty RL, Marcus A, Yousem KE, et al. The olfactory vector hypothesis of neurodegenerative disease: is there evidence in humans? Ann Neurol. 2018;83(6):1082-110. PubMed: 29774665
35. Siderowf A, Stern M, McKhann L, et al. Parkinson Progression Marker Initiative. Design, rationale, and feasibility. Mov Disord. 2011;26(8):1635-45. PubMed: 21793185
36. Simuni T, Marek K, Dawson TM, et al. Parkinson Progression Marker Initiative (PPMI)—rationale, design and overview. Prog Neurobiol. 2012;97(3):259-64. PubMed: 22285559
37. van Hilten JJ, Berg D, Fidler V, et al. Clinical detection of Parkinson’s disease in at risk populations. J Parkinsons Dis. 2017;7(s1):S71-S75. PubMed: 29513688
38. van de Vijver DA, Verbaan D, van Rooden SM, et al. Clinical features of patients with likely prodromal Parkinson’s disease: baseline data from the Dutch PRO-PD cohort. J Parkinsons Dis. 2017;7(4):711-20. PubMed: 29206675
39. Goker-Alpan O, Schulman JM, Siddique N, et al. Increased risk of Parkinson disease in individuals with Gaucher disease. Arch Neurol. 2004;61(4):494-8. PubMed: 15082588
40. Mullin S, Smith L, Sharples K, et al. Ambroxol for disease modification in Parkinson’s disease: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2020;19(12):935-48. PubMed: 33125058
41. Sidransky E, Lopez G. The link between Parkinsonism and Gaucher disease. Lancet Neurol. 2012;11(11):986-95. PubMed: 23062650
42. Gan-Or Z, Schulman JM, Bar-Shira A, et al. Mutation frequency and haplotype analysis of the glucocerebrosidase gene in Ashkenazi Jews with Parkinson disease. Arch Neurol. 2008;65(8):1071-5. PubMed: 18695082
43. Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide ancestry of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583-90. PubMed: 18550395
44. Lesage S, Durr A, Tazir M, et al. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med. 2006;354(4):422-3. PubMed: 16436774
45. Ozelius LJ, Sagi-Eisenberg R, Schreibman-Goren N, et al. LRRK2 G2019S Parkinson’s disease in Ashkenazi Jews: clinical characteristics and founder effect. Ann Neurol. 2006;60(4):402-10. PubMed: 16988948
46. Goldwurm S, Zini M, Ghidoni R, et al. Phenotype of Parkinson’s disease in carriers of the LRRK2 G2019S mutation in Italy. Neurology. 2007;68(2):107-9. PubMed: 17210840
47. Tanner CM, গোল্ডস্টেইন ডিএস. Phenotype of LRRK2 G2019S Parkinson’s disease: further clinical and pathological insights. Neurology. 2007;68(2):98-9. PubMed: 17210838
48. Sharma M, Li A, Shtilbans A, et al. LRRK2 G2019S mutation in Parkinson’s disease: evidence for a common founder in the Ashkenazi Jewish population. Ann Neurol. 2008;63(3):389-92. PubMed: 18306194
49. Iranzo A, Tolosa E, Gelb DJ, et al. Validation of the MDS research criteria for prodromal Parkinson’s disease in REM sleep behavior disorder. Mov Disord. 2016;31(8):1174-8. PubMed: 27328934
50. van de Vijver DA, ten Kate M, van Rooden SM, et al. Validation of the Movement Disorder Society criteria for prodromal Parkinson’s disease: the Dutch PRO-PD study. Mov Disord. 2018;33(2):255-63. PubMed: 29193359
51. Wijemanne S, Evans AH, Abbott R, et al. Validation of the Movement Disorder Society criteria for prodromal Parkinson’s disease in a UK population cohort. J Neurol Neurosurg Psychiatry. 2018;89(12):1305-11. PubMed: 29795915
52. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498-508. PubMed: 15590952
53. Jenkinson C, Fitzpatrick R, Peto V, et al. Health-related quality of life in patients with Parkinson’s disease: development and validation of the PDQ-39 questionnaire. Mov Disord. 1997;12(2):277-84. PubMed: 9062488
54. Goodman RA, Ford ES, Spetzler JC, et al. Prevalence of disabilities and chronic diseases among former college athletes and nonathletes. Med Sci Sports Exerc. 1995;27(1):18-27. PubMed: 7885196
55. Ahlskog JE, Geda YE, Lowe V, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876-84. PubMed: 21885773
56. Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288-95. PubMed: 21791644
57. Li F, Harmer P, Fisher KJ, et al. Tai Chi and Parkinson’s disease. Meds Sports Sci Exerc. 2012;44(5):851-60. PubMed: 22033597
58. Hackney ME, Earhart GM. Health-related physical activity and quality of life in Parkinson disease. Mov Disord. 2009;24(8):1194-9. PubMed: 19388119
59. Schapira AH, McDermott MP, Holloway RG, et al. Coenzyme Q10 and ethyl pyruvate prolong survival in cell and animal models of Parkinson’s disease. J Neurosci. 2009;29(3):711-9. PubMed: 19158345
60. Beitz D. Creatine and Parkinson’s disease: a systematic review. J Neurol Sci. 2005;233(1-2):183-90. PubMed: 15935705
61. Reichmann H, Gasser T, Gerlach M, et al. Neuroprotective strategies in Parkinson’s disease. J Neural Transm Suppl. 2002;62:1-19. PubMed: 12486995
62. Silveira I, Alves V, Almeida V, et al. Ambroxol improves lysosomal function in GBA-N370S Parkinson’s patient cells. J Parkinsons Dis. 2019;9(3):585-97. PubMed: 31294705
63. Athauda D, Barger G, Warren L, et al. Phase 1 study of safinamide in Parkinson’s disease patients with the LRRK2 G2019S mutation. Mov Disord. 2019;34(1):118-28. PubMed: 30375131
64. van Rumund A, Esselink RA, Bloem BR, et al. Antisense oligonucleotides for Parkinson’s disease: a systematic review. Mov Disord. 2017;32(12):1688-97. PubMed: 29178531
65. Williams-Gray CH, Foltynie T. Linked clinical trials for neurodegenerative diseases. Lancet Neurol. 2016;15(1):12-3. PubMed: 26687818
66. Becker C, Jick SS, Meier CR. Diabetes mellitus and risk of Parkinson disease. Mov Disord. 2018;33(4):516-24. PubMed: 29575792
67. Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1641-9. PubMed: 28712595
68. Khoo TK, Brookes CE, Hadik MA, et al. Simvastatin in Parkinson’s disease (SPARKS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019;18(3):253-63. PubMed: 30660495
69. El-Agnaf OM, Moussa CE. Alpha-synuclein immunotherapy for Parkinson’s disease. Mov Disord. 2017;32(12):1698-707. PubMed: 29178532
70. Findley LJ. The economic impact of Parkinson’s disease. Parkinsonism Relat Disord. 2007;13 Suppl 3:S8-S12. PubMed: 18178064
71. Kowal SL, Lee AJ, Lachmann S, et al. Burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(14):1908-15. PubMed: 24282187
72. Dodel R, Berger K, Oertel WH, et al. Health care costs in Parkinson’s disease: results from a German prospective cohort study (DEMDIS). Eur J Neurol. 2001;8(2):127-36. PubMed: 11240654
73. Huse DM, Grossman H, Kavanagh J, et al. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20(11):1438-44. PubMed: 16094670
74. Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence of dementia in 2005. Alzheimers Dement. 2007;3(2):81-91. PubMed: 19592147
75. Cummings JL, Morstorf J, Zhong K. Alzheimer’s disease drug-development pipeline: 2019. Alzheimers Dement (N Y). 2019;5:167-93. PubMed: 31214557
76. Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322-33. PubMed: 24450891
77. Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311-21. PubMed: 24450890
78. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-92. PubMed: 9086388