Introduction
Ovarian tumors represent a significant portion of gynecological disorders encountered in clinical practice. Among these, ovarian cancer ranks as the seventh most prevalent cancer among women worldwide. A critical challenge in managing ovarian tumors is the preoperative differentiation between primary ovarian cancers and metastatic tumors, which account for 5% to 10% of malignancies involving the ovaries. Accurate differentiation is paramount as it dictates the most effective treatment strategies. However, this distinction can be clinically challenging, particularly when metastatic ovarian tumors are detected before the primary tumor site is identified. The diagnostic dilemma is further compounded by the similar imaging appearances of primary and metastatic ovarian tumors.
While some studies suggest that metastatic ovarian tumors are frequently bilateral, and bilaterality could be a differentiating feature from primary tumors, other research presents conflicting findings. Notably, common primary ovarian cancers like serous and undifferentiated carcinomas also exhibit bilateral involvement in a substantial number of cases. The ambiguity surrounding bilateral ovarian tumors necessitates further investigation into their pathological and morphological characteristics to refine diagnostic accuracy. Currently, there is a gap in comprehensive data guiding the differential diagnosis of bilateral ovarian tumors specifically detected via magnetic resonance (MR) imaging. This article aims to address this gap by exploring the diverse pathological types of bilateral ovarian tumors identified on MR imaging and to pinpoint useful diagnostic markers for distinguishing between these types, including primary ovarian tumors and metastases.
Materials and Methods
This study was conducted retrospectively, analyzing data from 457 consecutive patients who presented with ovarian tumors and underwent MR imaging followed by pathological diagnosis. The MR examinations were performed between January 2005 and March 2015. Patients with endometriotic cysts and functional cysts were excluded based on MR and ultrasound findings and follow-up evaluations. From the initial cohort, 371 patients were excluded due to unilateral tumors on MR imaging, leaving a final study group of 86 patients with bilateral ovarian tumors. Ethical approval was obtained from the ethics committee of Tottori University, with a waiver for informed consent (1606A027).
MR Imaging Technique
MR imaging was performed using a combination of 3.0-T and 1.5-T MR systems, employing phased array coils. Standard imaging protocols included axial and sagittal T1-weighted and T2-weighted images in all cases. Due to the study’s duration, scan parameters and sequences varied slightly. Representative pelvic MR imaging sequences and parameters included: axial and sagittal T1-weighted spoiled gradient recalled (SPGR) images, axial and sagittal T2-weighted fast SE images, and axial and/or sagittal SPGR images with fat suppression. Axial diffusion-weighted (DW) imaging was also performed. In a majority of cases (73 patients), contrast-enhanced MR images were acquired, with dynamic imaging in almost all of these. Contrast agent (gadolinium) was administered intravenously, and images were taken pre-contrast, immediately post-contrast, and at multiple time points up to 120 seconds post-injection. Antiperistaltic medication (butyl-scopolamine) was administered intramuscularly to patients imaged on 3.0T systems to minimize motion artifacts, unless contraindicated.
Image Analysis
MR images were independently reviewed by two radiologists with extensive experience in gynecological MR imaging to confirm bilaterality. Consensus was reached in cases of disagreement. A single radiologist then analyzed the MR images of bilateral ovarian tumors, blinded to clinical and pathological data, focusing on: (i) morphology (predominantly cystic or solid); (ii) maximum tumor diameter (summed diameters for bilateral tumors, or diameter of fused mass); (iii) ascites (none, pelvic only, or beyond pelvis); and (iv) peritoneal implants. Solid components were defined as thickened septa, vegetations, and enhancing solid portions on contrast-enhanced T1-weighted images. Morphology, ascites, and peritoneal implants were assessed across all imaging sequences, including DW imaging.
Statistical Analysis
Statistical analyses were conducted to compare serous carcinoma versus metastasis, and primary malignant ovarian tumors versus metastasis. Fisher’s exact test and Chi-squared test were used for categorical variables (morphology, peritoneal implants). Mann-Whitney U test was applied to continuous variables (maximum tumor diameter, ascites, CA 125 serum levels). Receiver operating characteristic (ROC) curve analysis was utilized to evaluate diagnostic performance. Statistical analyses were performed using SPSS version 21.0. Optimal cutoff values were determined by maximizing the Youden index.
Results
Eighty-six patients with bilateral ovarian tumors on MR imaging were identified (mean age 54 years, range 15-86 years). Pathological examination revealed that in five of these patients, the tumors were actually unilateral despite bilateral appearance on MR imaging. The pathological diagnoses for the remaining 81 patients are detailed in Table 1. In the vast majority (77 cases), the bilateral tumors exhibited the same pathology on both sides. Four cases showed different pathologies in each ovary (Figure 1). The most frequent diagnoses were serous carcinoma (n = 36), mature teratoma (n = 20), and metastasis (n = 12). The primary origins of metastasis were diverse, including appendiceal, colon, cervical, bile duct, gastric, kidney cancers, peritoneal mesothelioma, and uterine malignant lymphoma (Figures 2-4). Given the straightforward MR imaging diagnosis of mature teratomas, further analysis concentrated on differentiating serous carcinoma, other primary malignant ovarian tumors, and metastasis.
Table 1: Pathological Diagnosis of Bilateral Ovarian Tumors
| Pathological Type | N |
|---|---|
| Primary Benign | 26 |
| Mature Teratoma | 20 |
| Mature Teratoma/Serous Adenoma | 1 |
| Mature Teratoma/Struma Ovarii | 1 |
| Serous Adenoma | 3 |
| Fibroma | 1 |
| Primary Benign and Malignant | 1 |
| Serous Adenoma/Mucinous Borderline Tumor | 1 |
| Primary Malignant | 42 |
| Serous Carcinoma | 36 |
| Clear Cell Carcinoma | 3 |
| Carcinosarcoma | 2 |
| Borderline Endometrioid Tumor/Endometrioid Carcinoma | 1 |
| Metastasis | 12 |
| Appendiceal Cancer | 3 |
| Cervical Cancer | 2 |
| Colon Cancer | 2 |
| Bile Duct Cancer | 1 |
| Gastric Cancer | 1 |
| Kidney Cancer | 1 |
| Peritoneal Mesothelioma | 1 |
| Uterine Malignant Lymphoma | 1 |
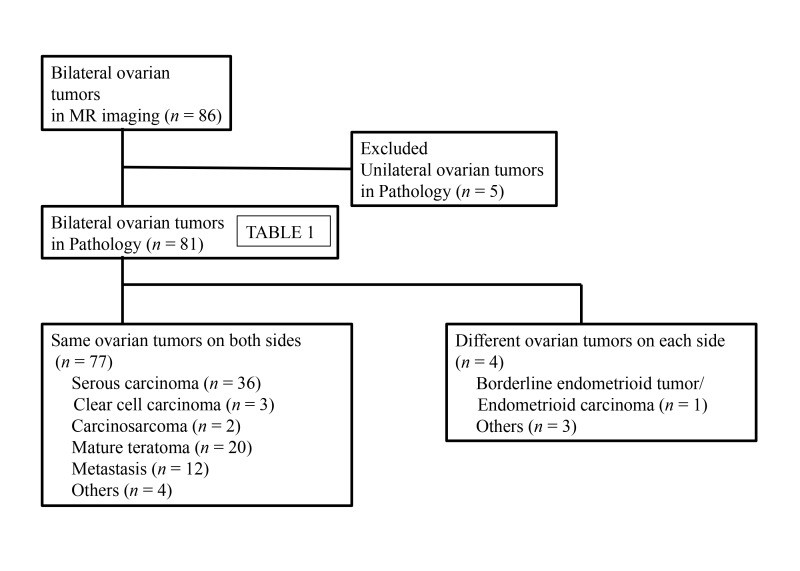
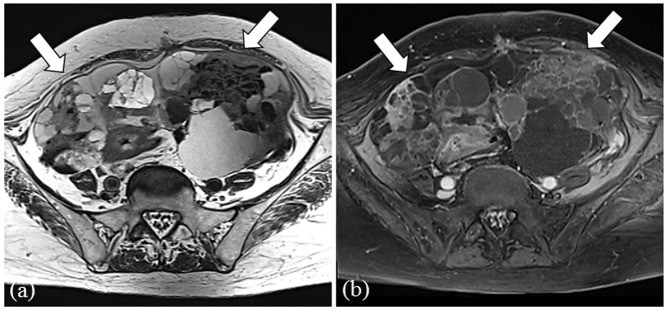
Figure 1.
Flow diagram illustrating the patient selection process for bilateral ovarian tumor analysis via MR imaging.
Figure 2.
Representative MR images of a 50-year-old female with serous carcinoma. (a) T2-weighted image showing bilateral multilocular cystic ovarian tumors with solid components, massive ascites and peritoneal implants (arrows). (b) Contrast-enhanced T1-weighted image with fat-suppression highlighting solid components and peritoneal implants (arrows). (c) Diffusion-weighted image showing high signal intensity in solid components of ovarian tumors and peritoneal implants (arrows).
Figure 4.
MR images illustrating metastasis from sigmoid colon cancer in a 57-year-old female. (a) T2-weighted image showing bilateral multilocular ovarian cystic tumors with thick internal septae (arrows). (b) Contrast-enhanced T1-weighted image with fat-suppression demonstrating the tumor structure.
Table 2 and 3 summarize the findings for CA 125 levels, maximum tumor diameter, morphology, ascites, and peritoneal implants. Initial analyses focused on distinguishing serous carcinoma from metastasis. CA 125 serum levels were significantly higher in serous carcinomas compared to metastases (P < 0.0001). Maximum tumor diameter was significantly smaller in serous carcinomas than in metastases (P = 0.0012). However, morphology (P = 0.32), ascites (P = 0.26), and peritoneal implants (P = 0.09) did not show significant differences. A cutoff value of 683.3 U/mL for CA 125 and 144 mm for maximum tumor diameter were determined by ROC analysis for differentiating serous carcinoma from metastasis. When CA 125 was below 683.3 U/mL or maximum tumor diameter exceeded 144 mm, the sensitivity and specificity for diagnosing metastasis were 90% and 58%, respectively.
Similar analyses were conducted to differentiate primary malignant ovarian tumors from metastasis. Again, CA 125 levels were significantly higher in primary malignant ovarian tumors (P < 0.0001), while maximum tumor diameter was significantly smaller (P = 0.0048). Morphology (P = 0.42), ascites (P = 0.19), and peritoneal implants (P = 0.24) showed no significant differences. Using a CA 125 cutoff of 683.3 U/mL and a maximum tumor diameter cutoff of 144 mm for differentiating primary malignant ovarian tumors from metastasis yielded a sensitivity of 90% and a specificity of 52% for metastasis.
Table 2: CA 125 and Maximum Tumor Diameter in Bilateral Ovarian Tumors
| Mean CA 125 (U/mL) | Mean Maximum Tumor Diameter (mm) | |
|---|---|---|
| Serous Carcinoma (N=36) | 3261.0 (103.2–31550) | 120 (28–304) |
| Primary Malignant Ovarian Tumor (N=42) | 2834.1 (40.4–31550) | 124 (28–304) |
| Metastasis (N=12) | 389.7 (23.3–1720) | 170 (68–360) |
(Range values are provided in parentheses).
Table 3: MR Findings of Primary Malignant Ovarian Tumors and Metastasis
| N | Morphology (n) | Ascites (n) | Peritoneal Implant (n) | |
|---|---|---|---|---|
| Solid | Cystic | ++ | ||
| Primary Malignant Ovarian Tumors | 42 | 23 | 19 | 21 |
| Serous Carcinoma | 36 | 21 | 15 | 19 |
| Clear Cell Carcinoma | 3 | 1 | 2 | 2 |
| Carcinosarcoma | 2 | 1 | 1 | 0 |
| Endometrioid Borderline Tumor/carcinoma | 1 | 0 | 1 | 0 |
| Metastasis | 12 | 5 | 7 | 8 |
| Appendiceal Cancer | 3 | 1 | 2 | 3 |
| Cervical Cancer | 2 | 1 | 1 | 1 |
| Colon Cancer | 2 | 1 | 1 | 0 |
| Bile Duct Cancer | 1 | 0 | 1 | 1 |
| Gastric Cancer | 1 | 1 | 0 | 1 |
| Kidney Cancer | 1 | 0 | 1 | 1 |
| Peritoneal Mesothelioma | 1 | 0 | 1 | 1 |
| Malignant Lymphoma | 1 | 1 | 0 | 0 |
(Ascites+: not beyond the pelvic cavity; Ascites++: beyond the pelvic cavity)
Figure 3.
Representative MR images of a 73-year-old female with serous carcinoma. (a) T2-weighted image showing bilateral solid ovarian tumors, massive ascites, and peritoneal implants (arrows). (b) Contrast-enhanced T1-weighted image with fat-suppression highlighting tumor and implants. (c) Diffusion-weighted image showing high signal intensity in ovarian tumors and peritoneal implants.
Discussion
Our findings indicate that serous carcinoma, mature teratoma, and metastasis are the most prevalent pathological types among bilateral ovarian tumors detected by MR imaging. These entities should therefore be considered as the primary differential diagnoses in cases of bilateral ovarian tumors. While mature teratomas are known to present bilaterally in 10-15% of cases, they were excluded from further comparative analysis due to their straightforward diagnosis based on fat components and chemical shift artifacts on MR imaging. Serous carcinomas were the predominant primary malignant bilateral ovarian tumors in our cohort. Given the critical clinical need to differentiate between primary and metastatic ovarian tumors, our study focused on comparing metastasis with serous carcinomas, and subsequently with all primary malignant ovarian tumors.
The results clearly demonstrate that CA 125 serum level and maximum tumor diameter are valuable parameters in differentiating metastasis from serous carcinoma or other primary ovarian malignancies. CA 125, a high molecular weight glycoprotein, is a well-established marker for epithelial ovarian cancers, with elevated serum levels in approximately 80-85% of patients. Our study identified a CA 125 cutoff value of 683.3 U/mL as a significant differentiator between primary malignant ovarian tumors and metastasis. This contrasts with some previous studies suggesting limited diagnostic value of CA 125 in this differentiation, potentially due to variations in tumor stage, histological subtypes, and study populations. CA 125 levels are known to correlate with FIGO stages in ovarian cancer, and bilateral tumors often represent advanced stages. Serous and undifferentiated carcinomas, which were prevalent in our study, are associated with higher CA 125 levels compared to other subtypes, reinforcing the utility of CA 125 in predicting primary malignant ovarian tumors, particularly serous carcinomas, in the context of bilateral ovarian masses.
Maximum tumor diameter also emerged as a useful differentiating factor, with metastases tending to be larger than serous carcinomas or primary malignant ovarian tumors. The combination of a CA 125 level below 683.3 U/mL or a maximum tumor diameter exceeding 144 mm showed a high sensitivity (90%) for metastasis diagnosis, albeit with moderate specificity (52-58%). This suggests that these criteria can effectively flag cases where metastasis should be strongly considered, prompting further diagnostic evaluations and potentially offering a cost-effective diagnostic strategy. This finding contrasts with some prior research reporting larger primary ovarian tumors compared to metastases, which may be attributable to differences in study design and patient selection. Our study’s focus on bilateral ovarian tumors detected on MR imaging, rather than pathologically confirmed ovarian carcinomas in general, likely reflects a more clinically relevant scenario in daily practice.
Conversely, MR imaging features such as overall tumor morphology, presence of ascites, and peritoneal implants were not found to be reliable differentiators in our study, aligning with previous reports. The morphological variability observed across serous carcinomas, primary ovarian tumors, and metastases – with nearly half exhibiting predominantly solid patterns – underscores the diagnostic challenge. Serous carcinomas are known for their diverse appearances, encompassing both cystic and solid components, potentially due to their varied developmental pathways, distinguishing low-grade from high-grade serous carcinomas. Metastatic tumors also presented with heterogeneous morphologies, possibly reflecting the diverse primary sites of origin, as exemplified by the differing appearances of Krukenberg tumors versus metastases from colon or appendiceal cancers. Thus, relying solely on morphological features from MR imaging is insufficient for reliably differentiating bilateral ovarian tumors.
Similarly, ascites and peritoneal implants, while frequently observed in both primary malignant ovarian tumors and metastases in our study, did not provide significant discriminatory value. This is consistent with some previous studies but contrasts with others. The high prevalence of ascites and peritoneal implants in our primary ovarian tumor cohort is likely due to the predominance of serous carcinoma, particularly high-grade serous carcinoma, which are known to commonly present with these features. However, ascites and peritoneal implants are also common in carcinomatous peritonitis, a frequent manifestation in advanced cancers with ovarian metastasis, thus explaining their presence in both groups and limiting their utility in differential diagnosis.
Prior studies have suggested other differentiating factors, including patient age, tumor multilocularity, locule uniformity, and enhancement patterns. However, the complex and variable nature of ovarian tumors, particularly serous carcinomas with their microcystic components, necrosis, and solid areas, makes precise evaluation of multilocularity and locule uniformity challenging. Furthermore, our study did not assess enhancement patterns or ADC values due to the use of different MR devices over the study period, which is a limitation.
Limitations
Our study has several limitations. The use of multiple MR devices and the relatively small sample size, especially for metastasis and non-serous primary malignant tumors, are notable. Future research with larger cohorts and standardized imaging protocols is needed to validate these findings. Additionally, our serous carcinoma group may include both ovarian and peritoneal carcinomas, reflecting the evolving understanding of high-grade serous carcinoma origins. We also did not specifically evaluate endometriotic cysts, which, while not classified as ovarian tumors in the WHO classification used, can present diagnostic challenges. Furthermore, tumor-like lesions such as hyperreactio luteinalis and stromal hyperplasia, which can mimic bilateral ovarian tumors, were not considered. Finally, the CA 125 levels in metastasis are influenced by the primary site, and a different distribution of primary sites could potentially yield different results.
Conclusion
In conclusion, this study highlights serous carcinoma, mature teratoma, and metastasis as the most common diagnoses for bilateral ovarian tumors detected on MR imaging. Crucially, CA 125 serum level and maximum tumor diameter emerge as valuable and practical markers for differentiating between metastasis and serous carcinoma or other primary malignant ovarian tumors. These markers can aid in refining the differential diagnosis of bilateral ovarian tumors, thereby guiding appropriate clinical management strategies.
Conflict of Interest: The authors declare no conflict of interest.