Introduction
Bilateral parotid swelling, while not the most frequent presentation of parotid gland disorders, is a clinical scenario that presents diagnostic complexities for both clinicians and radiologists. The parotid glands, the largest of the salivary glands, are susceptible to a wide range of pathological conditions. When swelling occurs on both sides, it signals a distinct set of potential underlying causes compared to unilateral presentations. This article provides a detailed pictorial review of the diverse conditions that can manifest as bilateral parotid swelling, emphasizing a multimodality imaging approach to aid in accurate differential diagnosis.
While some causes of bilateral parotid swelling are readily diagnosed clinically and may not necessitate imaging, others require detailed radiological investigation for definitive diagnosis. Current salivary gland imaging techniques encompass a variety of modalities, including Magnetic Resonance Imaging (MRI), Computed Tomography (CT), ultrasonography, scintigraphy, and sialography. The selection of the appropriate imaging modality is guided by the patient’s clinical presentation. This review showcases imaging characteristics of histologically confirmed cases of bilateral parotid swelling, highlighting key imaging features that facilitate diagnostic differentiation.
Differential Diagnosis of Bilateral Parotid Swelling
Bilateral parotid swelling can stem from a broad spectrum of conditions, broadly categorized as inflammatory/infectious, autoimmune, granulomatous, miscellaneous, neoplastic, and pseudolesions. A structured approach to differential diagnosis is crucial for effective patient management.
| Category | Specific Conditions |
|---|---|
| Inflammation or Infection | |
| Bacterial | Acute Suppurative Parotitis (Bilateral presentation) |
| Viral | Mumps, other viral parotitis |
| HIV Sialopathy | Diffuse Infiltrative Lymphocytosis Syndrome (DILS), BLEL cysts |
| Chronic Sialadenitis | |
| Chronic Recurrent Parotitis | |
| Autoimmune Diseases | Sjögren’s Syndrome |
| Granulomatous Diseases | Sarcoidosis, Wegener’s Granulomatosis, Kimura’s Disease |
| Miscellaneous | Sialadenosis, Polycystic Disease, Pneumoparotid, Radiation Sialadenitis |
| Neoplastic Diseases | Warthin Tumor (Papillary Cystadenoma Lymphomatosum), MALT Lymphoma |
| Pseudolesion | Masseteric Hypertrophy |
Acute Suppurative Parotitis
Acute suppurative parotitis is a bacterial infection of the parotid gland, characterized by acute, painful, and diffuse swelling. While typically unilateral, bilateral involvement occurs in 15–25% of cases, particularly in individuals who are debilitated, dehydrated, or have poor oral hygiene. The infection often arises from an ascending ductal route.
During the acute phase, sialography is contraindicated due to the risk of exacerbating the infection. CT imaging reveals dilated central ducts, ductal wall enhancement, and glandular enlargement. MRI findings vary based on the predominant tissue component; T2-weighted images may show low or high signal intensity depending on whether edema or cellular infiltrates are more prominent. Post-contrast MRI typically demonstrates diffuse glandular enhancement.
Chronic Sialadenitis
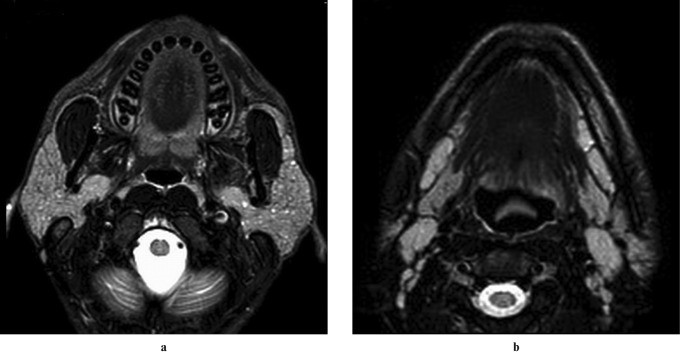
Chronic inflammation of the parotid gland can disrupt the gland’s drainage system, predisposing it to recurrent infections and gradual enlargement. Obstruction of the salivary ducts leads to progressive dilatation and subsequent gland swelling (Figure 1). Clinically, chronic sialadenitis presents with intermittent, often painful parotid swelling, which may or may not be associated with meals.
Figure 1: Imaging of Bilateral Chronic Parotitis in an 8-Year-Old. Axial T2-weighted, HASTE axial, and HASTE sagittal oblique MRI images of the right parotid gland demonstrate bilaterally dilated main parotid duct and intraglandular branches (arrows), indicative of chronic sialadenitis.
Viral Parotitis (Mumps)
Mumps, caused by the paramyxovirus, is the most prevalent cause of viral parotitis. It primarily affects children under 15 years old. Mumps typically involves the parotid glands, with bilateral involvement in approximately 75% of cases, although unilateral presentation and involvement of submandibular or sublingual glands can also occur. Diagnosis is usually clinical, and imaging is generally not required. However, if imaging is performed, CT may show non-specific parotid gland enlargement with increased attenuation, while MRI may reveal increased T2 signal intensity.
HIV Sialopathy
Parotid enlargement is observed in about 5% of individuals with human immunodeficiency virus (HIV). Diffuse infiltrative lymphocytosis syndrome (DILS), a subset of HIV-associated conditions, is characterized by persistent CD8 lymphocytosis, diffuse lymphocytic infiltration of organs (commonly the lungs), bilateral parotid swelling, and cervical lymphadenopathy. Parotid swelling in HIV sialopathy results from lymphoproliferation within intraparotid lymph nodes. Benign lymphoepithelial lesion (BLEL) cysts are a common late-stage development.
Ultrasonography in HIV sialopathy may show large anechoic or hypoechoic areas with debris and septa, representing lymphoepithelial cysts, alongside oval hypoechoic areas indicating enlarged intraparotid nodes. CT and MRI findings in BLEL are non-specific, demonstrating multiple cystic and solid masses within enlarged parotid glands (Figure 2), as well as potential tonsillar hypertrophy and reactive cervical adenopathy.
Figure 2: HIV-Associated Lympoepithelial Cysts in a 40-Year-Old Male. Axial T2-weighted MRI images (a,b) reveal enlarged bilateral parotid glands with multiple tiny hyperintense foci, more prominent on the left side. Enlarged cervical lymph nodes are also noted in the caudal section, characteristic of HIV sialopathy.
Chronic Recurrent Parotitis
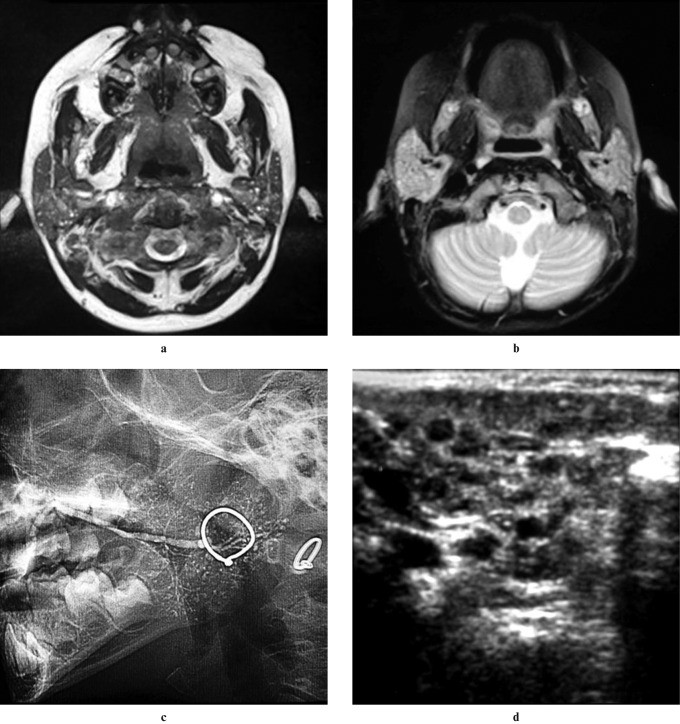
Chronic recurrent parotitis (CRP) is a rare inflammatory condition of unknown cause, characterized by repeated episodes of unilateral or bilateral parotid swelling, with or without pain. Onset typically occurs between 2 and 7 years of age. Ultrasound imaging in CRP reveals multiple minute (1–3 mm) hypoechoic or anechoic focal lesions, corresponding to punctate sialectasis seen on sialography (Figure 3). The differential diagnosis for CRP includes benign lymphoepithelial cysts and juvenile Sjögren syndrome.
Figure 3: Chronic Recurrent Parotitis in a 6-Year-Old Female. (a) Axial T2-weighted MRI and (b) 3D CISS MIP images demonstrate multiple high-signal foci in bilateral parotid glands. (c) Digital sialography of the right parotid shows scattered punctate collections of contrast material. (d) Axial ultrasound reveals multiple hypoechoic areas in the enlarged glands, collectively indicative of chronic recurrent parotitis of childhood.
In adults, CRP may arise spontaneously or represent a continuation of childhood CRP. Adult CRP tends to exhibit more significant inflammation. Imaging in adults may reveal sialectasis (cavitatory or destructive) and “sausage-like” changes in the main duct, typically seen in advanced stages.
Sjögren’s Syndrome
Primary Sjögren’s syndrome (SS) is an autoimmune disorder characterized by chronic lymphocytic infiltration of exocrine glands. It can occur as primary SS or secondary SS in association with other connective tissue disorders. Major salivary gland enlargement is seen in 25–66% of primary SS patients. Predominantly affecting middle-aged women, diagnosis relies on clinical confirmation of dry eyes and mouth, labial minor salivary gland biopsy, and detection of autoantibodies like anti-Ro (SSA) and anti-La (SSB).
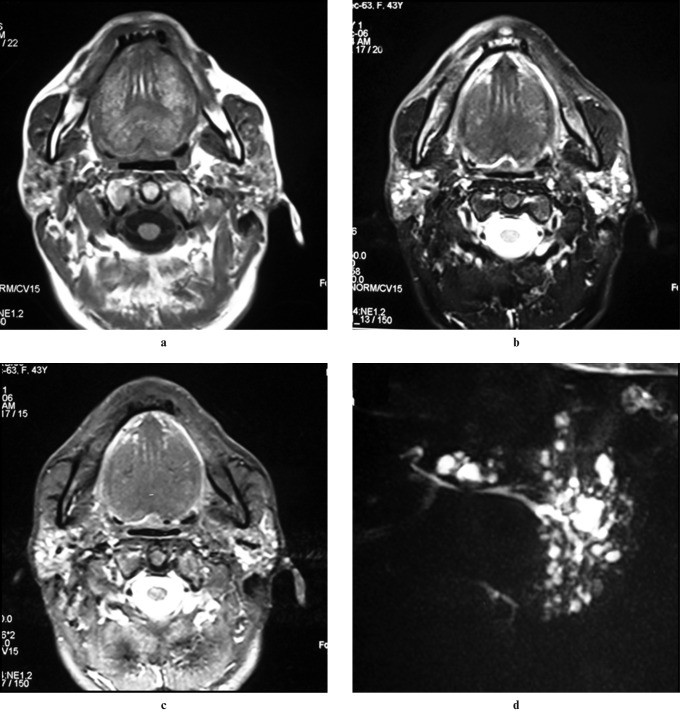
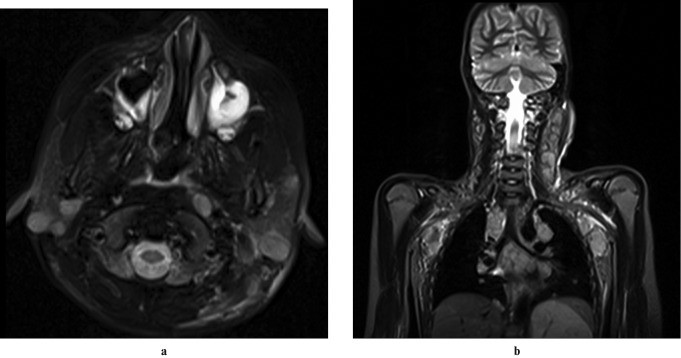
Sialography remains a sensitive diagnostic method for SS, with early changes showing numerous 1 mm punctate sialectasis uniformly distributed throughout the gland. Acinar atrophy leads to globular contrast collections and eventually cavitary sialadenitis. Initially, the central ductal system is normal, dilating in advanced stages. Early SS may show normal cross-sectional imaging. Advanced SS on ultrasound may show inhomogeneous glands with scattered small, well-defined hypoechoic or anechoic areas and increased blood flow. CT findings are non-specific, showing enlarged, dense glands with potential nodules, abnormal enhancement, and small low-attenuation cystic areas (honeycomb appearance). MRI in advanced SS often shows a “salt and pepper” or “honeycomb” appearance due to mixed high and low signal intensity areas on both short and long TR sequences. Premature fat deposition in parotid glands, demonstrable on STIR and fat-saturation imaging (Figure 4), is also characteristic of SS. MR sialography has comparable accuracy to digital subtraction sialography for SS diagnosis and staging. Notably, SS increases the risk of parotid lymphoma by 44-fold (Figure 5), typically marginal zone B-cell neoplasms.
Figure 4: Sjögren Syndrome in a 30-Year-Old Female. Axial T1-weighted, fat-suppressed T2-weighted, and STIR images show multiple small foci in bilateral parotid glands. Hyperintensity on T1-weighted and hypointensity on T2-weighted-FS and STIR sequences suggest fatty infiltration. Multiple uniform-sized foci with hypointense signal on T1-weighted and hyperintense on T2-weighted images indicate sialectasis.
Figure 5: Sjögren Syndrome with Secondary Lymphoma in a 65-Year-Old Male. (a) Axial contrast-enhanced CT shows a homogeneously enhancing mass in the right parotid gland, with multiple small hypoattenuating foci in the left. (b) Axial T1-weighted MRI reveals a large hypointense mass in the right parotid superficial lobe and hyperintense foci in the remaining right and left parotid glands, suggesting fatty infiltration. (c) Axial T2-weighted MRI shows adenopathy in the right jugular and accessory chain.
Wegener’s Granulomatosis
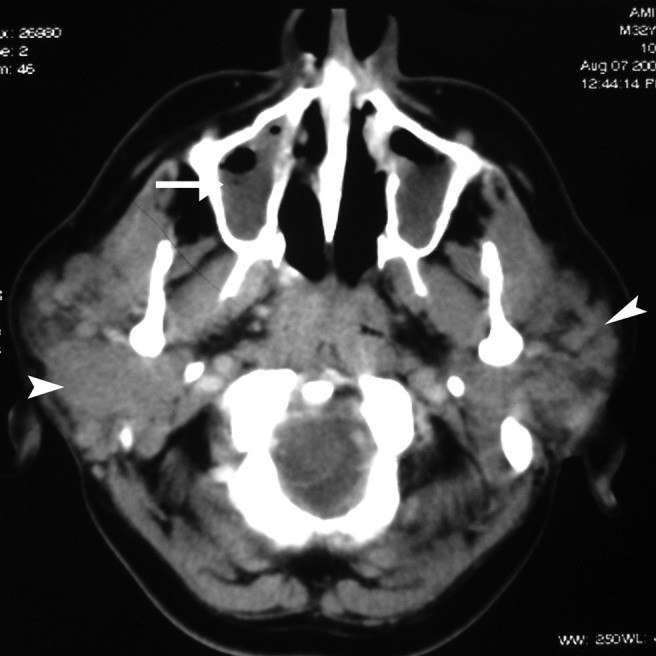
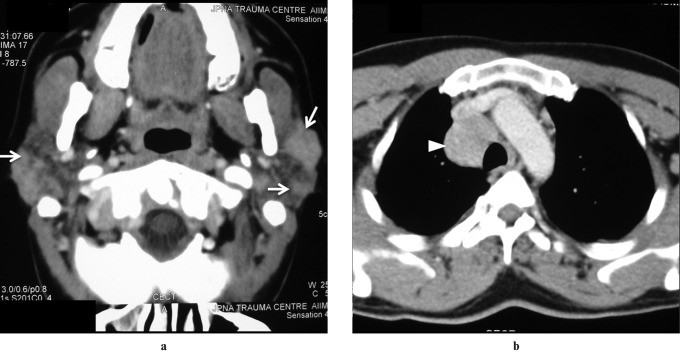
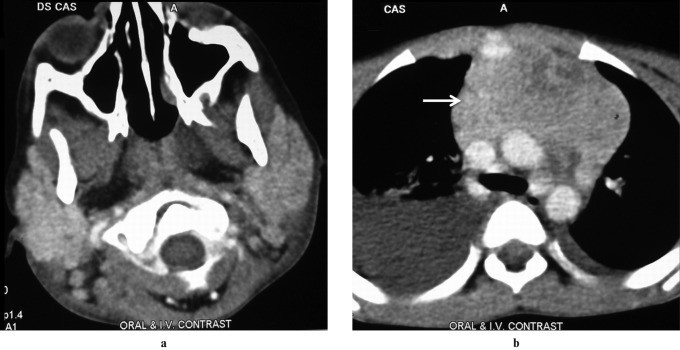
Wegener’s granulomatosis, now known as Granulomatosis with Polyangiitis (GPA), is characterized by necrotizing granulomatous inflammation, vasculitis, and classically involves the upper and lower respiratory tracts and kidneys. Salivary gland involvement is rare and not typically isolated. Parotid involvement, either unilateral or bilateral, is non-specific on imaging (Figure 6), and is always accompanied by nasal, ear, or lung symptoms. Diagnosis is based on clinical presentation, histological evidence of necrotizing granulomatous vasculitis, and positive c-ANCA assay.
Figure 6: Wegener’s Granulomatosis in a 30-Year-Old Female. Axial contrast-enhanced CT image shows enlarged bilateral parotid glands with multiple focal lesions (arrow). Bilateral maxillary sinusitis is also evident, findings consistent with but not specific for Wegener’s granulomatosis.
Sarcoidosis
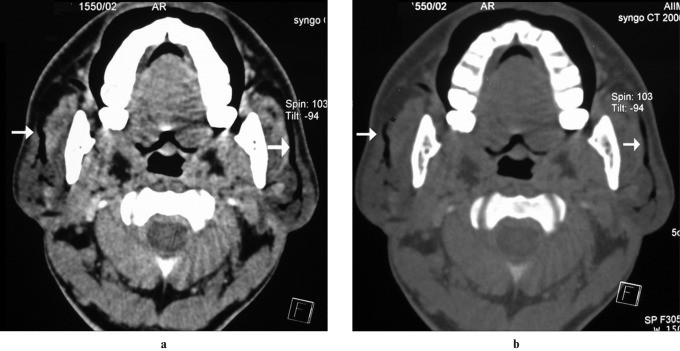
Sarcoidosis is a systemic disorder of unknown etiology, commonly affecting young to middle-aged adults. Diagnosis is based on clinical and radiological findings, supported by histological findings of non-caseating granulomas with epithelioid cell proliferation. Painless parotid enlargement occurs in 10–30% of patients, typically bilaterally (83%). Parotid enlargement can be multinodular or, less commonly, diffuse.
Imaging findings are non-specific. Multiple non-cavitatory masses representing enlarged intraparotid nodes may be seen, appearing hypoechoic on ultrasound and hypodense on contrast-enhanced CT (Figure 7). Bilateral cervical lymphadenopathy is also common. Lymphoma is a primary differential diagnosis. Diffuse sarcoidosis presents with symmetrical parotid enlargement and increased T2 signal intensity with intense gadolinium enhancement. Simultaneous enlargement and homogeneous enhancement of lacrimal and parotid glands is a classic sarcoidosis feature (Figure 8). Gallium-67 citrate scintigraphy may show the “panda sign” due to increased uptake in lacrimal and parotid glands.
Figure 7: Sarcoidosis in a 32-Year-Old Male. (a) Axial contrast-enhanced CT (CECT) image shows symmetrically enlarged parotid glands with multiple focal hyperdense lesions (arrow). (b) Axial CECT of the thorax shows a homogeneously enhancing right paratracheal node (arrowhead), supporting the diagnosis of systemic sarcoidosis.
Figure 8: Sarcoidosis in a 40-Year-Old Female. (a) Axial T2-weighted and (b) post-contrast T1-weighted MRI images show symmetrically enlarged parotid glands with increased signal intensity and diffuse glandular enhancement. (c) Axial T1-weighted post-contrast image at the orbit level shows diffuse enhancement of bilateral lacrimal glands, a classic presentation of sarcoidosis.
Kimura’s Disease
Kimura’s disease is an immune-mediated inflammatory disorder characterized by painless subcutaneous masses in the head and neck, eosinophilia, and elevated serum IgE. Salivary gland involvement can be unilateral or bilateral. Imaging findings are non-specific. CT may show ill-defined, homogeneously enhancing intraparotid masses, cervical lymphadenopathy, and/or subcutaneous masses. Other sites include submandibular glands, auricle, scalp, orbit, and oral mucosa. MRI signal intensity varies, but lesions typically show mixed T1 signal and T2 hyperintensity.
Sialadenosis
Sialadenosis is a bilateral, persistent, painless, non-inflammatory swelling, primarily of the parotid glands. Causes include diabetes mellitus, endocrinopathies (hypothyroidism), malnutrition, medications (thiourea, diuretics), alcohol abuse, and heavy metals. Onset is usually between 20 and 60 years. Histologically, it involves acinar hypertrophy, fatty replacement, and fibrosis. Imaging is rarely necessary and often unhelpful due to variable echotexture, attenuation, and signal intensity. Sialography may show enlarged parotid glands with normal but splayed ducts (Figure 9). Diagnosis is usually clinical, based on patient history and setting.
Figure 9: Sialadenosis in a 34-Year-Old Female with Hypothyroidism. (a) Digital sialogram of the right parotid gland shows attenuated main duct and intraparenchymal branches. (b) Axial T2-weighted MRI demonstrates symmetrically enlarged parotid glands without focal lesions, consistent with sialadenosis.
Polycystic (Dysgenetic) Disease
Polycystic disease of the parotid glands is a rare disorder, mostly in females, and often bilateral. Patients present with recurrent painless swelling. Histologically, numerous cysts of varying sizes lined by cuboidal or squamous epithelium are seen, interspersed with normal serous acini. Inflammation is typically absent. Ultrasound, CT, and MRI clearly show bilateral parotid enlargement with multiple cystic areas replacing glandular parenchyma.
Pneumoparotid
Pneumoparotid occurs in individuals generating high intraoral pressure, such as wind instrument players, during coughing, or dental procedures. It typically presents as unilateral or bilateral facial swelling over the parotid region, which may be painful or painless, and may be associated with warmth and erythema. Crepitus may be palpable in 50% of cases, and frothy saliva or air bubbles may emanate from Stensen’s duct upon gland massage.
Plain radiographs may show air in soft tissues and ductal outlines. Sialography may reveal air bubbles in the duct. Ultrasound and CT can demonstrate air within the parotid gland and duct system (Figure 10). CT elegantly visualizes air in both the duct and gland.
Figure 10: Pneumoparotid in a 30-Year-Old Male. (a,b) Axial CT images demonstrate air within bilateral main and intraparenchymal ducts, diagnostic of pneumoparotid, likely due to air insufflation.
Radiation Sialadenitis
Radiation sialadenitis follows radioactive iodine (I131) treatment for thyroid carcinoma due to salivary glands’ iodine concentration. Parotid glands are most affected, with transient bilateral swelling usually within 24 hours of iodine ingestion, resolving within a week. Sour candies and pilocarpine can reduce severity and duration. Diagnosis is clinical, and imaging is unnecessary.
Lymphoma
Primary salivary gland lymphoma is rare, often MALT lymphoma. Secondary lymphoma is slightly more common. Most are B-cell non-Hodgkin lymphomas. Imaging features vary, presenting as focal masses (Figure 11) or diffuse glandular infiltration (Figure 12). Focal disease is more common, showing enlarged intraparotid lymph nodes with altered echogenicity and increased Doppler flow on ultrasound. CT shows mildly enhancing enlarged intraparotid nodes. T1-weighted MRI best visualizes lesions as homogeneous intermediate signal intensity masses. Diffuse lymphoma causes variable, non-specific imaging features across modalities. Extraparotid nodal disease can suggest lymphoma.
Figure 11: Non-Hodgkin Lymphoma in a 6-Year-Old Child. (a) Axial T2-weighted MRI shows multiple focal hyperintense lesions in bilateral parotid glands. (b) Coronal T2-weighted MRI reveals multiple nodes involving the left jugular chain, bilateral axilla, and mediastinum, indicating systemic lymphoma.
Figure 12: Non-Hodgkin Lymphoma in an 8-Year-Old Child. (a) Axial contrast-enhanced CT (CECT) shows symmetrically enlarged parotid glands without focal lesions. (b) Axial CECT of the thorax demonstrates a large anterior mediastinal mass (arrow) with right pleural effusion, highlighting the systemic nature of lymphoma.
Warthin’s Tumor
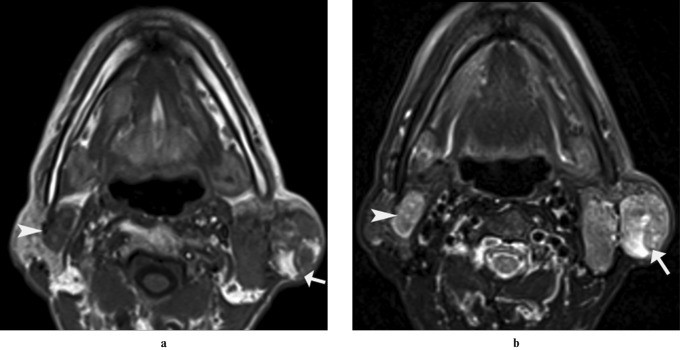
Warthin’s tumor (papillary cystadenoma lymphomatosum) is a benign epithelial tumor and the most common salivary neoplasm to present bilaterally, either synchronously or metachronously. It manifests as a painless, slow-growing mass, bilateral in up to 10% of cases. Ultrasound typically shows a well-defined hypoechoic mass, often with small cystic areas, or sometimes a predominantly cystic mass. MRI reveals a well-defined, partially cystic mass with fluid signal intensity in cystic components and intermediate signal intensity in solid components. Multiple or bilateral parotid or periparotid masses strongly suggest Warthin’s tumor (Figure 13).
Figure 13: Bilateral Warthin’s Tumor in a 60-Year-Old Male. (a) Axial T1-weighted and (b) T2-weighted MRI images show multiple well-defined focal lesions involving bilateral parotid glands. The left parotid lesion is heterogeneous with solid and cystic components, while the right is solid. The cystic component is hyperintense on both T1- and T2-weighted images.
Benign Masseteric Hypertrophy
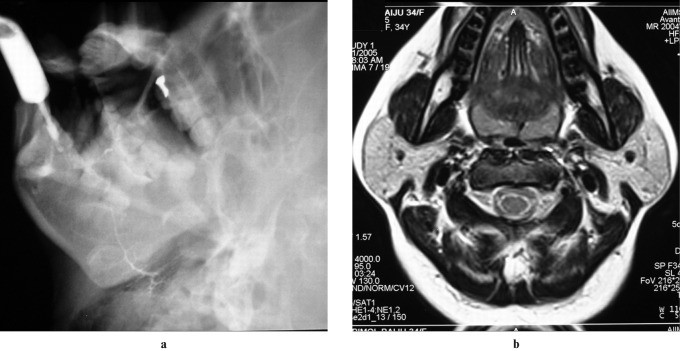
Bilateral masseteric hypertrophy can mimic parotid enlargement, as the superficial parotid lobe overlies the posterior masseter muscle. Clinically, the face appears rectangular. Radiographically, bony hyperplasia occurs at the masseter’s mandibular insertion with mandibular angle flaring. Sialography shows lateral Stensen’s duct displacement and narrowing by the enlarged masseter muscle (Figure 14). Cross-sectional imaging reveals enlarged masseter muscles with normal CT attenuation and MRI signal intensity.
Figure 14: Bilateral Masseteric Hypertrophy in a 20-Year-Old Male. (a) Clinical photograph shows broad cheek bulge, more pronounced on the right. (b) Digital sialogram shows narrowing of the main parotid duct (arrow) where it crosses the masseter muscle. (c) Axial CT shows bilateral masseter muscle enlargement with normal attenuation, suggestive of benign masseteric hypertrophy.
Conclusion
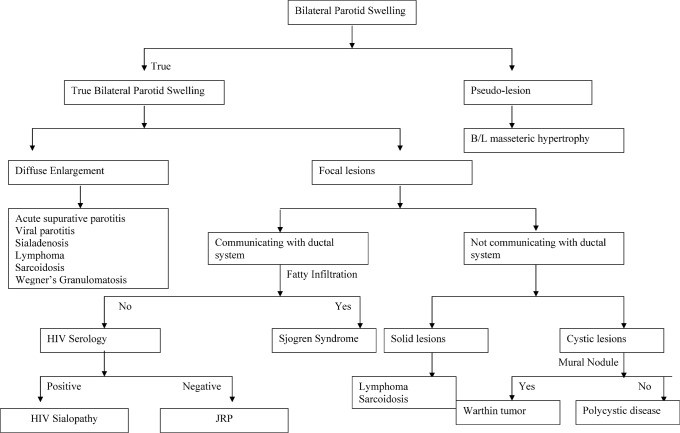
Bilateral parotid swelling is a multifaceted clinical presentation with a wide range of potential etiologies. Imaging plays a crucial role in the diagnostic process of salivary gland diseases (Figure 15). It is instrumental not only in confirming or excluding the presence of masses but also in suggesting the nature of the underlying condition based on distinctive imaging features. Conditions such as chronic recurrent parotitis, Sjögren’s syndrome, HIV sialopathy, Warthin’s tumor, lymphoma, and sarcoidosis often exhibit characteristic imaging patterns that aid in diagnosis. Pseudo-lesions like masseteric hypertrophy can be confidently identified through imaging, whereas conditions such as sialadenosis and Wegener’s granulomatosis may present with more non-specific imaging findings.
Figure 15: Algorithmic Approach to Bilateral Parotid Swelling. This flowchart illustrates a diagnostic algorithm for evaluating patients with bilateral parotid swelling, integrating clinical assessment and imaging modalities to reach a differential diagnosis.
