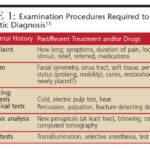
Prostate cancer is frequently initially detected through screening procedures. (Refer to Screening Tests for Prostate Cancer for detailed information.) Early stages of prostate cancer often present without noticeable symptoms, while more advanced cases may be identified due to the symptoms they manifest.
If prostate cancer is suspected based on screening test results or the emergence of symptoms, further diagnostic tests are essential to confirm the diagnosis. Upon consulting a primary care physician, individuals may be referred to a urologist, a specialist in treating conditions of the urinary and genital tracts, including prostate cancer.
The definitive diagnosis of prostate cancer is achieved through a prostate biopsy, as elaborated upon below. Among the various diagnostic tools, a bone scan plays a critical role in specific scenarios, particularly in assessing the spread of prostate cancer. This article delves into the importance of bone scans in the diagnosis of prostate cancer, especially when metastasis to the bone is suspected.
Initial Steps in Prostate Cancer Diagnosis
When prostate cancer is suspected, the diagnostic process typically begins with a comprehensive evaluation that includes medical history, physical examination, and specific blood tests.
Medical History and Physical Exam
During the initial consultation, your doctor will inquire about any symptoms you may be experiencing, such as urinary or sexual dysfunction, and the duration of these symptoms. Furthermore, they will assess potential risk factors for prostate cancer, including family history and other relevant medical conditions.
A physical examination is a crucial part of the initial assessment. This usually includes a digital rectal exam (DRE), where the doctor inserts a gloved and lubricated finger into the rectum to palpate the prostate gland. This allows the detection of any irregularities, such as bumps or hardened areas, which might indicate the presence of cancer. The DRE can also provide insights into the extent of the cancer, such as whether it is confined to one or both sides of the prostate or if it has potentially spread to surrounding tissues. The doctor may also examine other areas of the body as part of a comprehensive physical assessment.
Following the physical examination, further tests are typically ordered to investigate the suspicion of prostate cancer.
PSA Blood Test
The prostate-specific antigen (PSA) blood test is a cornerstone in prostate cancer detection. PSA is a protein produced by both normal and cancerous cells within the prostate gland. While predominantly found in semen, a small quantity of PSA circulates in the bloodstream.
Role of PSA Test in Suspected Prostate Cancer
The PSA blood test serves a dual purpose: screening for prostate cancer in asymptomatic men and as an initial diagnostic test for men presenting with symptoms suggestive of prostate cancer.
PSA levels in the blood are quantified in nanograms per milliliter (ng/mL). It’s crucial to understand that while the likelihood of prostate cancer increases with higher PSA levels, there isn’t a definitive PSA threshold that definitively confirms or excludes prostate cancer.
While many clinicians use a PSA cutoff of 4 ng/mL as a trigger for further investigation, some may recommend additional testing at lower levels, such as 2.5 or 3 ng/mL, depending on individual risk factors.
- The majority of men without prostate cancer exhibit PSA levels below 4 ng/mL. However, it’s important to note that a PSA level under 4 ng/mL does not completely rule out the possibility of prostate cancer.
- Men with PSA levels between 4 and 10 ng/mL (the “borderline range”) have approximately a 25% chance of having prostate cancer. Notably, a significant proportion of these cancers are low-grade and may not necessitate immediate treatment.
- When PSA levels exceed 10 ng/mL, the probability of prostate cancer rises to over 50%. Similar to the borderline range, some of these may also be low-grade cancers that may not require aggressive intervention.
Elevated PSA levels warrant further diagnostic evaluation to determine the presence of prostate cancer. For a comprehensive understanding of the PSA test, including factors influencing PSA levels, specialized PSA tests, and subsequent steps for abnormal PSA results, refer to Screening Tests for Prostate Cancer.
PSA Test in Men Diagnosed with Prostate Cancer
For individuals already diagnosed with prostate cancer, the PSA test remains a valuable tool for several reasons:
- In newly diagnosed cases, the PSA level, in conjunction with DRE findings and tumor grade (determined from biopsy), aids in deciding the necessity of additional tests like CT scans or bone scans.
- PSA levels are integral in determining the stage (extent) of the cancer. In cases where cancer hasn’t spread, PSA levels help categorize the cancer into risk groups, influencing treatment strategy.
- Monitoring PSA levels is critical in assessing treatment efficacy and detecting potential cancer recurrence post-treatment. (Refer to Following PSA Levels During and After Treatment for detailed information).
Image alt text: A medical professional performing a Digital Rectal Exam (DRE) to check the prostate gland, a key early detection method for prostate cancer.
Prostate Biopsy: Confirming the Diagnosis
If PSA blood test, DRE, or other findings raise suspicion of prostate cancer, a prostate biopsy is typically the next crucial step.
A biopsy involves extracting small tissue samples from the prostate gland for microscopic examination. The core needle biopsy is the standard method for diagnosing prostate cancer, usually performed by a urologist.
During the biopsy, the urologist utilizes imaging guidance, such as transrectal ultrasound (TRUS), MRI, or a fusion of both, to visualize the prostate. A thin, hollow needle is swiftly inserted into the prostate, either through the rectal wall (transrectal biopsy) or the skin between the scrotum and anus (transperineal biopsy). Upon withdrawal, the needle retrieves a small core of prostate tissue. This process is repeated multiple times, typically yielding around 12 core samples from different regions of the prostate.
While the procedure may sound uncomfortable, each biopsy sample acquisition usually causes only brief, mild discomfort due to the use of a spring-loaded biopsy instrument that rapidly inserts and retracts the needle. Local anesthesia is often administered to numb the area around the prostate to minimize discomfort.
The biopsy procedure itself takes approximately 10 minutes and is generally performed in a doctor’s office. Prophylactic antibiotics are usually prescribed before and sometimes after the biopsy to reduce the risk of infection.
Post-biopsy, some soreness in the area, blood in urine, and light rectal bleeding (especially if hemorrhoids are present) are common. Blood in semen or rust-colored semen may also occur and can persist for several weeks, depending on ejaculation frequency.
Interpreting Biopsy Results
Biopsy samples are sent to a pathology lab where a specialized physician, a pathologist, examines them microscopically to detect cancer cells. Pathology reports, detailing the findings, usually take 1 to 3 days, but sometimes longer. Results are typically categorized as:
- Negative for cancer: No cancer cells detected in the samples.
- Positive for cancer: Cancer cells identified in the samples.
- Suspicious or atypical: Abnormalities observed, but not definitively cancerous. Further investigation may be required.
Negative Biopsy Result
A negative prostate biopsy, in conjunction with a low probability of cancer based on PSA levels and other tests, may preclude the need for further diagnostic procedures beyond routine PSA tests and DREs at later intervals.
However, biopsies can occasionally miss cancer, even with multiple samples, if the needles fail to traverse cancerous areas, resulting in a false-negative result. If clinical suspicion for prostate cancer remains high (e.g., very elevated PSA), the doctor may recommend:
- Additional lab tests (blood, urine, or biopsy sample analysis) like Prostate Health Index (PHI), 4Kscore test, PCA3 tests (e.g., Progensa), and ConfirmMDx to refine cancer risk assessment. These are discussed in What’s New in Prostate Cancer Research?
- Prostate MRI (if not already performed) to identify suspicious areas warranting biopsy.
- Repeat prostate biopsy, potentially targeting previously unsampled areas or using imaging guidance like MRI to precisely target suspicious regions.
Prostate Cancer Grade: Gleason Score and Grade Groups
If prostate cancer is diagnosed via biopsy, it is assigned a grade, reflecting the cancer’s aggressiveness based on microscopic appearance. Higher-grade cancers appear more abnormal and exhibit a greater propensity for rapid growth and spread. Prostate cancer grading primarily utilizes two systems: the Gleason score and Grade Groups.
Gleason Score
The Gleason system, a long-established grading method, assigns grades from 1 to 5 based on the resemblance of cancer cells to normal prostate tissue.
- Grade 1: Cancer cells closely resemble normal prostate tissue.
- Grade 5: Cancer cells are highly abnormal.
- Grades 2-4: Represent intermediate degrees of abnormality.
Grades 1 and 2 are rarely used in practice; most prostate cancers are graded 3 or higher.
Prostate cancers often exhibit areas with varying grades. The Gleason score is derived by adding the grades of the two most prevalent cancerous patterns. For example, a Gleason score of 3+4=7 indicates that the majority of the tumor is grade 3, with a lesser component of grade 4, summing to 7.
While typically based on the two predominant grades, modifications exist for cases with extensive high-grade cancer or the presence of three grades including high-grade, to reflect the aggressive nature of the cancer.
Theoretically, Gleason scores range from 2 to 10, but scores below 6 are uncommon.
Based on Gleason scores, prostate cancers are broadly categorized into:
- Gleason score ≤ 6: Well-differentiated or low-grade, typically slow-growing and less likely to spread.
- Gleason score 7: Moderately differentiated or intermediate-grade.
- Gleason score 8-10: Poorly differentiated or high-grade.
Grade Groups
Recognizing limitations in the Gleason score’s granularity, particularly in predicting patient outcomes, the Grade Group system was developed. It provides a more refined grading from 1 (least aggressive) to 5 (most aggressive):
- Grade Group 1 = Gleason 6 (or less)
- Grade Group 2 = Gleason 3+4=7
- Grade Group 3 = Gleason 4+3=7
- Grade Group 4 = Gleason 8
- Grade Group 5 = Gleason 9-10
Grade Groups are increasingly favored for their improved prognostic accuracy and are likely to gradually replace the Gleason score, though both may appear on pathology reports currently.
Additional Pathology Report Information
Besides cancer grade, pathology reports often include:
- Number of biopsy cores containing cancer (e.g., “7 out of 12”).
- Percentage of cancer within each core.
- Laterality (unilateral or bilateral) of cancer within the prostate.
Suspicious, Atypical, and Other Biopsy Results
Biopsy findings may sometimes be inconclusive, showing abnormalities that are not definitively cancerous.
Prostatic intraepithelial neoplasia (PIN): Characterized by altered prostate cell appearance without invasion into surrounding tissue. PIN is classified into:
- Low-grade PIN: Cells appear nearly normal. Not considered a significant prostate cancer risk factor. Follow-up is usually standard.
- High-grade PIN: Cells exhibit more significant abnormalities. Associated with increased prostate cancer risk, necessitating careful monitoring and potential repeat biopsy or further risk assessment tests (PHI, 4Kscore, PCA3, ConfirmMDx), especially if multifocal or if initial biopsy was limited.
Intraductal carcinoma: Cancer cells present within prostate ducts. Often associated with high-grade prostate cancer nearby, typically warranting aggressive treatment like surgery or radiation therapy.
Atypical small acinar proliferation (ASAP): Also termed glandular atypia or atypical glandular proliferation, or simply “suspicious for cancer.” Indicates cells suggestive of cancer but insufficient for definitive diagnosis. High likelihood of concurrent prostate cancer, often prompting repeat biopsy within a few months.
Proliferative inflammatory atrophy (PIA): Prostate cells smaller than normal with inflammation. Not cancerous, unclear if it directly leads to high-grade PIN or prostate cancer.
Genetic and Molecular Testing in Prostate Cancer
Further tests on prostate cancer cells and genetic testing may be recommended in certain situations to refine treatment strategies and understand individual risk profiles.
Testing Prostate Cancer Cells for Gene or Protein Changes
Analysis of cancer cells from biopsy samples for specific gene or protein alterations can inform treatment decisions.
- In localized prostate cancer, molecular or genomic tests (Decipher, Oncotype DX Prostate, Prolaris, Promark) can assess cancer aggressiveness, aiding in decisions regarding active surveillance versus definitive treatment (surgery or radiation). See Risk Groups and Lab Tests to Help Determine Risk for Localized Prostate Cancer for details.
- In metastatic prostate cancer, identifying specific gene or protein changes can guide targeted therapy selection. For example, BRCA gene mutations or defects in DNA repair genes may indicate potential benefit from PARP inhibitors.
Genetic Testing for Inherited Gene Changes
Genetic counseling and testing for inherited gene mutations is recommended for men with prostate cancer in specific scenarios:
- Family history of known relevant gene mutations (e.g., BRCA, Lynch syndrome).
- Strong family history of prostate or other related cancers.
- Personal history of other cancers, especially breast cancer.
- Ashkenazi Jewish ancestry.
- Metastatic prostate cancer.
- High-risk or intraductal carcinoma prostate cancer.
- Gene alterations in prostate cancer cells (e.g., BRCA) that might be inherited.
Refer to Genetic Counseling and Testing for Prostate Cancer Risk for more information.
Imaging Tests for Prostate Cancer: Detecting Spread and Guiding Procedures
Imaging tests play a crucial role in prostate cancer diagnosis and management. They utilize various technologies to visualize internal body structures, aiding in:
- Detecting prostate cancer.
- Guiding procedures like biopsy and treatment.
- Assessing cancer spread (staging).
The choice of imaging test depends on the clinical scenario. TRUS and/or MRI are commonly used to guide prostate biopsies. In diagnosed prostate cancer, imaging for metastasis may be necessary, though men with low-risk localized disease may not require extensive staging imaging.
Common imaging modalities for prostate cancer include:
Transrectal Ultrasound (TRUS)
TRUS involves inserting a finger-width probe into the rectum. Sound waves emitted from the probe generate echoes that are converted into a prostate image.
The procedure is quick (under 10 minutes), typically done in-office, and may cause mild pressure but is generally not painful. Local anesthesia may be used.
TRUS applications include:
- Detecting suspicious prostate areas in men with abnormal DRE or PSA (though sensitivity is limited).
- Guiding prostate biopsies.
- Measuring prostate size for PSA density calculation (see Screening Tests for Prostate Cancer).
- Guiding certain treatments like brachytherapy and cryotherapy.
Advanced TRUS techniques like color Doppler and micro-ultrasound are emerging with potentially improved diagnostic utility. (See What’s New in Prostate Cancer Research?)
Magnetic Resonance Imaging (MRI)
MRI scans use radio waves and magnets to create detailed soft tissue images, providing clear visualization of the prostate and surrounding areas. Gadolinium contrast may be used intravenously to enhance image clarity.
MRI applications in prostate cancer:
- Assessing need for prostate biopsy in men with abnormal screening or symptoms (multiparametric MRI).
- Guiding prostate biopsy by targeting suspicious areas (MRI/ultrasound fusion biopsy).
- Directing needles during prostate biopsy.
- Determining cancer extent (stage) by assessing spread beyond the prostate. Typically not needed for low-risk localized cancers.
An endorectal coil may be inserted into the rectum during MRI to improve image quality, which can cause discomfort. Sedation may be offered.
Multiparametric MRI (mpMRI): Combines standard MRI with techniques like diffusion weighted imaging (DWI), dynamic contrast enhanced (DCE) MRI, and MR spectroscopy to characterize prostate tissue more comprehensively, assessing both anatomy and functional parameters.
mpMRI results are often reported using the Prostate Imaging Reporting and Data System (PI-RADS), categorizing suspicious areas from PI-RADS 1 (very low suspicion) to PI-RADS 5 (high suspicion of clinically significant cancer).
MRI/ultrasound fusion-guided prostate biopsy: Integrates pre-biopsy MRI findings with real-time TRUS during biopsy, allowing targeted sampling of MRI-identified suspicious areas.
Bone Scan: Detecting Prostate Cancer Spread to Bones
When prostate cancer metastasizes, bone is a frequent site of spread. A bone scan is crucial for identifying bone metastases.
This procedure involves injecting a small amount of radioactive material that preferentially accumulates in areas of bone damage. A special camera detects the radioactivity and generates an image of the skeleton.
Bone scans can suggest bone metastases, but findings are not always specific to cancer, as benign conditions like arthritis can produce similar appearances. Further tests like X-rays, CT scans, MRI, or bone biopsy may be needed for confirmation.
The Role of Bone Scan in Prostate Cancer Diagnosis and Staging
A bone scan, also known as bone scintigraphy, is a nuclear medicine imaging technique particularly sensitive in detecting areas of increased bone turnover, which is often associated with bone metastases. In the context of prostate cancer, a bone scan is not a primary diagnostic tool for detecting the initial prostate tumor itself. Instead, its crucial role lies in staging prostate cancer and determining if the cancer has spread beyond the prostate gland to the bones.
When is a Bone Scan Necessary for Prostate Cancer?
Bone scans are not routinely performed for all men diagnosed with prostate cancer. They are typically recommended in specific situations where there is a higher risk of bone metastasis. These situations include:
- High-risk prostate cancer: Men with high Gleason scores (8 or higher) or Grade Group 4 or 5 cancers are at increased risk of metastasis.
- Elevated PSA levels: Significantly high PSA levels (typically >20 ng/mL) can suggest a higher likelihood of cancer spread, including to the bones.
- Symptoms suggestive of bone metastasis: Bone pain, especially persistent or worsening back, hip, or pelvic pain, unexplained fractures, or elevated alkaline phosphatase levels in blood tests can raise suspicion of bone metastases and warrant a bone scan.
- Staging intermediate- to high-risk prostate cancer: To accurately stage the cancer and guide treatment decisions, a bone scan may be ordered in men with intermediate or high-risk prostate cancer, even in the absence of bone-related symptoms.
How Does a Bone Scan Help in Prostate Cancer Diagnosis and Management?
- Detecting Bone Metastases: The primary purpose of a bone scan in prostate cancer is to detect bone metastases. Prostate cancer cells, when they spread to the bone, disrupt normal bone metabolism, leading to increased bone turnover. The radioactive tracer used in bone scans accumulates in these areas of active bone remodeling, highlighting potential sites of metastasis.
- Staging Prostate Cancer: A positive bone scan, indicating bone metastases, significantly impacts the staging of prostate cancer. It signifies that the cancer is no longer localized to the prostate and has spread distantly (stage M1 disease). This advanced stage often necessitates systemic therapies rather than localized treatments.
- Guiding Treatment Decisions: The presence or absence of bone metastases, as determined by a bone scan, is a critical factor in treatment planning. For localized prostate cancer without bone metastases, treatment options may include surgery, radiation therapy, or active surveillance. However, if bone metastases are detected, treatment strategies shift towards systemic approaches like hormone therapy, chemotherapy, immunotherapy, or radiopharmaceuticals, often in combination, to manage the widespread disease.
- Monitoring Treatment Response and Recurrence: Bone scans can also be used to monitor treatment response in men with bone metastases and to detect recurrence or progression of bone metastases over time.
Limitations of Bone Scans:
While bone scans are sensitive for detecting bone metastases, they have limitations:
- Specificity: Bone scans are not highly specific for cancer. Benign conditions like arthritis, fractures, infections, and bone islands can also cause increased bone turnover and lead to false-positive results on bone scans.
- Early Metastases: In very early stages of bone metastasis, when bone changes are minimal, bone scans may not be sensitive enough to detect the cancer spread, leading to false-negative results.
- “Cold” Lesions: Rarely, some types of bone metastases may be “cold” lesions, meaning they do not show increased tracer uptake on bone scans, potentially leading to missed diagnoses.
Due to these limitations, if a bone scan is positive or equivocal, further imaging tests, such as plain X-rays, CT scans, MRI, or even a bone biopsy, are often necessary to confirm the presence of bone metastases and differentiate them from benign conditions.
Image alt text: A bone scan image displaying areas of increased radiotracer uptake, indicative of potential bone metastases in a prostate cancer patient.
Positron Emission Tomography (PET) Scan
PET scans are similar to bone scans, using radioactive tracers, but PET tracers are designed to accumulate more specifically in cancer cells.
Standard FDG-PET scans, using a type of sugar tracer, are not very effective for prostate cancer detection. However, newer PET tracers show promise.
Newer PET Tracers for Prostate Cancer:
More effective PET tracers for prostate cancer include:
- Fluciclovine F18
- Sodium fluoride F18
- Choline C11
PSMA PET Scans: Tracers targeting prostate-specific membrane antigen (PSMA), highly expressed on prostate cancer cells, are particularly useful:
- Ga 68 PSMA-11 (Ga 68 gozetotide, Locametz, Illuccix, Gozellix)
- 18F-DCFPyl (piflufolastat F 18 or Pylarify)
- 18F-rhPSMA-7.3 (flotufolastat F 18 or Posluma)
These newer PET scans are valuable when the location of prostate cancer spread is unclear, such as in ambiguous bone scan results or rising PSA after treatment. PSMA PET scans can also guide radiopharmaceutical therapy targeting PSMA.
PET scans offer whole-body cancer detection but less detailed images than MRI or CT. Combined PET-MRI or PET-CT scans offer both functional and anatomical information.
Clinical applications and availability of newer PET scans are still evolving.
Computed Tomography (CT) Scan
CT scans use X-rays to create detailed cross-sectional images. CT scans are generally not essential for initial diagnosis of localized prostate cancer. However, they can be helpful in assessing lymph node involvement and local recurrence after treatment.
MRI is superior for prostate gland imaging compared to CT.
Lymph Node Biopsy
Lymph node biopsy, or lymphadenectomy, involves removing lymph nodes to check for cancer spread. It is not routinely performed for prostate cancer but may be necessary to assess lymph node metastasis.
Lymph Node Removal During Prostate Cancer Surgery
During radical prostatectomy for prostate cancer treatment, pelvic lymph nodes may be removed if there is a significant risk of spread (based on PSA, Gleason score). (See Surgery for Prostate Cancer).
Lymph node and prostate tissue are analyzed post-surgery.
Lymph Node Biopsy as a Separate Procedure
Lymph node biopsy as a separate procedure is rare. It may be considered when radical prostatectomy is not planned (e.g., radiation therapy) but lymph node status is critical.
Needle biopsy guided by imaging (MRI or CT) is the typical approach, involving local anesthesia and lab analysis of the sample.
Conclusion
Diagnosing prostate cancer involves a multi-faceted approach, starting with screening tests and clinical evaluation, progressing to biopsies for definitive diagnosis, and utilizing various imaging modalities for staging and treatment planning. While bone scans are not used to diagnose the primary prostate tumor, they are indispensable in assessing for bone metastases, particularly in men with higher-risk disease or symptoms suggestive of bone spread. Understanding the role and limitations of bone scans, alongside other diagnostic tools, is crucial for effective prostate cancer management and informed patient care.
- Written by
- References
The American Cancer Society medical and editorial content team
Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as editors and translators with extensive experience in medical writing.
American College of Radiology. PI-RADS® Prostate Imaging – Reporting and Data System. Version 2.1. 2019. Accessed at https://www.acr.org/-/media/ACR/Files/RADS/Pi-RADS/PIRADS-V2-1.pdf?la=en on January 30, 2020.
Benway BM, Andriole GL. Prostate biopsy. UpToDate. 2023. Accessed at https://www.uptodate.com/contents/prostate-biopsy on July 12, 2023.
Epstein JI. An update of the Gleason grading system. J Urol. 2010;183:433-440.
Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur Urol. 2016;69(3):428-435.
National Comprehensive Cancer Network (NCCN). Practice Guidelines in Oncology: Prostate Cancer Early Detection. Version 1.2023. Accessed at https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf on July 25, 2023.
Nelson WG, Antonarakis ES, Carter HB, DeMarzo AM, DeWeese TL. Chapter 81: Prostate Cancer. In: Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology. 6th ed. Philadelphia, Pa: Elsevier; 2020.
Preston MA. Screening for prostate cancer. UpToDate. 2023. Accessed at https://www.uptodate.com/contents/screening-for-prostate-cancer on July 12, 2023.
Postma R, Schröder FH, van Leenders GJ, et al. Cancer detection and cancer characteristics in the European Randomized Study of Screening for Prostate Cancer (ERSPC)–Section Rotterdam. A comparison of two rounds of screening. Eur Urol. 2007;52(1):89-97
Taplin ME, Smith JA. Clinical presentation and diagnosis of prostate cancer. UpToDate. 2023. Accessed at https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-prostate-cancer on July 12, 2023.
Zelefsky MJ, Morris MJ, Eastham JA. Chapter 70: Cancer of the Prostate. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 11th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2019.
Last Revised: March 21, 2025
American Cancer Society medical information is copyrighted material. For reprint requests, please see our Content Usage Policy.