Bovine mastitis, an inflammatory condition of the udder parenchyma, remains a major challenge for the global dairy industry. Characterized by pathological changes in glandular tissue and milk abnormalities, mastitis leads to significant economic losses. Escherichia (E.) coli is a leading cause of acute clinical mastitis in dairy cattle, with mammary pathogenic E. coli (MPEC) strains exhibiting virulence factors that aid in overcoming host defenses within the mammary gland. While the link between specific MPEC virulence factors and mastitis severity is still being researched, the increasing antimicrobial resistance due to widespread antibiotic use is a serious concern. A deep understanding of MPEC pathogenesis and antimicrobial susceptibility is crucial for developing improved strategies for Bovine Mastitis Diagnosis, prevention, and treatment. This article provides a comprehensive review of mastitis caused by MPEC, covering mastitis types, host immune responses, bovine mastitis diagnosis, treatment options, disease control, MPEC virulence factors, antimicrobial resistance, and the ongoing discussion about MPEC as a distinct pathotype. This information is essential for identifying knowledge gaps and guiding future research towards better diagnostic tools and therapeutic interventions for this critical disease in dairy farming.
Understanding Clinical and Subclinical Mastitis in Cattle
Bovine mastitis manifests in two primary forms: clinical and subclinical, each presenting distinct characteristics. Clinical mastitis is readily identifiable through visible signs in the udder and milk. Dairy professionals should be vigilant for udder abnormalities such as redness, swelling, heat, and pain upon palpation, alongside changes in milk consistency, including clots, flakes, or unusual discharges like serum-like or bloody milk. Systemic symptoms may also accompany clinical mastitis, indicating a more severe infection. These can include fever, loss of appetite (anorexia), depression, lethargy, and reduced grooming behavior. Milk quality is also visibly compromised, with an elevated somatic cell count (SCC) and altered milk composition, notably a decrease in lactose content. E. coli is frequently implicated in acute clinical bovine mastitis, often associated with rapid onset symptoms like weakness (dysstasia), diarrhea, and cool extremities. These infections are typically short-lived, lasting 10–30 days, and are most prevalent in the periods around calving, although reinfections can occur throughout lactation. While the host’s immune system often clears E. coli infections, severe cases with systemic involvement can be challenging to manage and carry a poorer prognosis.
Subclinical mastitis, conversely, is insidious, posing a hidden threat to dairy herds. It occurs 15–40 times more frequently than clinical mastitis and is not detectable through visual inspection of the udder or milk. This concealed nature makes subclinical mastitis particularly problematic, as farmers may be unaware of the ongoing milk quality degradation and the risk of disease transmission within the herd. Subclinical mastitis is often attributed to gram-negative bacteria such as E. coli, Klebsiella pneumoniae, and Serratia marcescens. Transmission can occur through milking equipment, contaminated hands, or udder washcloths, highlighting the importance of rigorous hygiene practices. Despite its lack of visible symptoms, subclinical mastitis is economically more damaging than clinical mastitis due to its significant impact on reducing milk production and quality.
Mammary Pathogenic Escherichia coli (MPEC): An Emerging Pathotype
Extraintestinal pathogenic E. coli (ExPEC) encompasses various subgroups, including uropathogenic E. coli (UPEC), meningitis-associated E. coli (MNEC), and sepsis-associated E. coli (SEPEC), each causing infections in different body sites. MPEC has been proposed as a novel ExPEC pathotype specifically adapted to cause bovine mastitis. Unlike typical gut commensals, MPEC strains have developed unique adaptations, including virulence factors, that enhance their ability to colonize the mammary gland’s challenging environment. This environment includes competing microorganisms, milk’s inherent antimicrobial components (like lactoferrin and lactoperoxidase), and the host’s immune response. These adaptations allow MPEC to thrive and cause infection in the mammary gland.
However, some studies suggest that E. coli isolates from mastitis cases are less genetically diverse and may lack traditional E. coli virulence factors compared to environmental strains. This observation indicates that mastitis-associated E. coli may be under selective pressure for specific traits that promote survival in the milk environment. Despite extensive genomic research on mastitis-associated E. coli, a universally agreed-upon set of virulence factors defining MPEC remains elusive. Some researchers advocate for a holistic approach, combining genomic analysis with host-pathogen interaction studies to fully understand MPEC pathogenesis. This includes considering physiological traits alongside genomic data, utilizing techniques such as dual RNA-Seq, Tn-Seq, comparative SNP analysis, proteomics, and metabolomics.
Research suggests that E. coli strains causing mastitis often originate from the bovine gastrointestinal tract, particularly phylogroups A and B1. These strains may be opportunistic pathogens, primarily infecting cattle with compromised immune systems, especially during the peripartum period when immune function is naturally suppressed. In such cases, environmental factors and the cow’s health status may be more critical determinants of mastitis severity than specific bacterial virulence factors.
Virulence Factors Influencing Mastitis Severity
The precise role of known virulence genes in determining the severity of E. coli mastitis is still under investigation. Mastitis-associated E. coli exhibits significant genotypic variability, with diverse virulence gene profiles found in both persistent and transient infections. Clinical symptoms may be influenced by undiscovered genes or host-related factors such as lactation stage, neutrophil function, SCC, age, nutritional status, and genetic resistance. Persistent infections, however, appear to be linked to specific bacterial traits, particularly the ability to adhere to and invade mammary epithelial cells for intracellular survival. In vitro studies have shown that persistent mastitis-associated E. coli strains exhibit enhanced adhesion, invasion, and survival within mammary epithelial cells compared to transient strains. These persistent strains may also modulate the host immune response, eliciting a less pronounced inflammatory reaction compared to acute strains. This subdued immune response may contribute to their persistence within the mammary gland.
Table 1 highlights various virulence genes and factors studied in E. coli isolates from bovine mastitis. These include factors involved in immune system activation (PAMPs), serum resistance, neutrophil survival, mammary epithelial cell adhesion and invasion, and milk survival and proliferation. While these virulence-associated properties contribute to E. coli survival in the mammary gland, they are not exclusive to mastitis-associated strains and are often found in other ExPEC pathotypes. The diversity in virulence characteristics among mastitis-causing E. coli underscores their genetic variability.
TABLE 1. Studies on Virulence Genes in E. coli Mastitis Isolates.
| Detected virulence genes (associated VFs/Function) | References |
|---|---|
| iucD, traT | Aslam et al., 2021 |
| type VI secretion system, type IV secretion system, type IV pili, hlyA, cnf2 | Sun et al., 2021 |
| fimH, ecpA, fimA, traT, ompT, irp2, hlyA | Guerra et al., 2020 |
| fecIRABCDE | Blum et al., 2018 |
| ompC, ompF, fimH, colV, irp2, fyuA, eaeA, ler, iucD | Zhang D. et al., 2018 |
| epr1 (type III secretion system) | Leimbach et al., 2017 |
| fecA, lpfA, Ipx, ecp | Kempf et al., 2016 |
| f17A, irp2, astA, iucD, colV | Liu et al., 2014 |
| lpfA, iss, astA | Blum and Leitner, 2013 |
| stx1, stx2, eaeA, f41 | Momtaz et al., 2012 |
| irp2, iucD, papC, iss, cva, afa8, astA, f17, cnf2, eaeA, vat, sfaD, tsh, saa | Suojala et al., 2011 |
| f17, eaeA | Momtaz, 2010 |
| eaeA, cnf1, cnf2, cs31A | Wenz et al., 2006 |
| stx1, cnf2, vt2e, eaeA | Bean et al., 2004 |
| traT, cnf1, cnf2, aer, f17, sfa, pap, afa8D, afa8E | Kaipainen et al., 2002 |
Note: See original article for full gene/VF descriptions and references.
Iron acquisition, particularly via the ferric dicitrate system (Fec), has emerged as a significant factor in mastitis-associated E. coli. The Fec system is crucial for E. coli growth in milk, which is naturally low in free iron. E. coli utilizes various iron transport systems, including siderophores and iron-regulated outer membrane proteins, to scavenge iron from milk. Studies have shown that the Fec system is essential for MPEC pathogenicity, suggesting it as a potential target for novel therapeutics, enhanced treatments, and vaccines.
Host Immune Response to Escherichia coli Mastitis
Innate and Acquired Mammary Gland Immunity
The bovine mammary gland employs both innate and acquired immune mechanisms to combat E. coli infections. Innate immunity, the first line of defense, includes physical barriers at the teat end and cellular and humoral components like neutrophils, macrophages, cytokines, NK cells, lactoferrin, and complement. Neutrophils are the predominant immune cells during the initial inflammatory response, rapidly migrating to the infection site. The speed and effectiveness of neutrophil recruitment and phagocytosis are key determinants of mastitis severity. Lactoferrin, an iron-binding glycoprotein, inhibits E. coli growth by limiting iron availability. Cytokines mediate local and systemic inflammatory responses. When innate immunity is insufficient, acquired immunity, mediated by lymphocytes and antibodies, becomes crucial. Antibodies from the bloodstream enter the milk, contributing to pathogen neutralization. The overall inflammatory response aims to eliminate the infection, neutralize toxins, and repair damaged udder tissue to restore milk production.
Immune Response Elicited by Mammary Pathogenic Escherichia coli
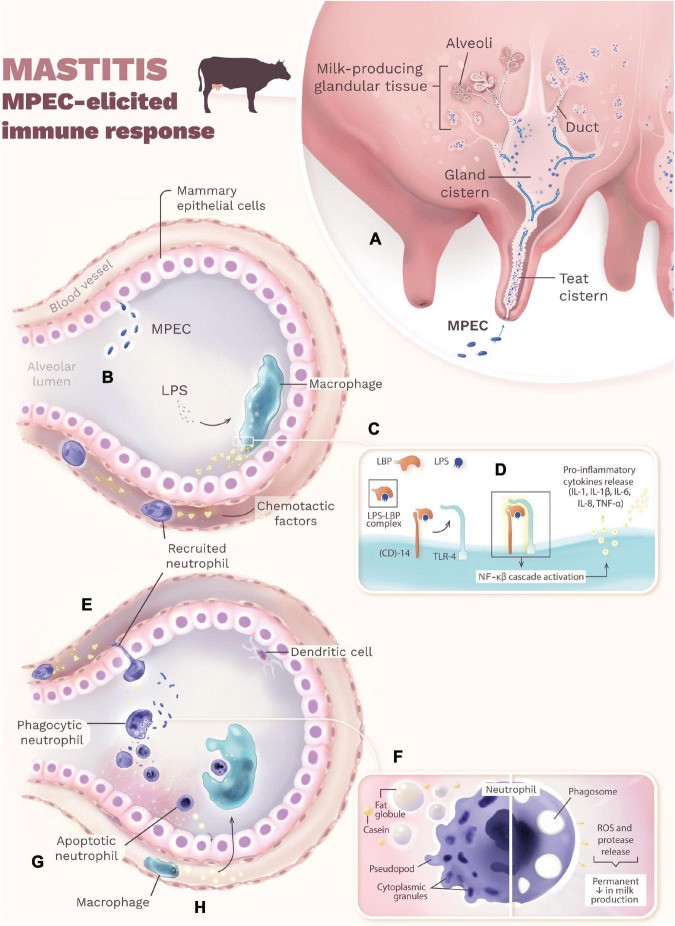
Mastitis onset begins when MPEC enters the mammary gland via the teat canal, multiplying in the teat and gland cisterns and spreading into the milk-producing tissue (Figure 1A). MPEC ferments lactose for energy, multiplying in the cisterns, ducts, and alveolar lumen without necessarily adhering to the epithelium. Some strains can adhere, leading to chronic infections. MPEC multiplication and lysis release lipopolysaccharide (LPS), a potent endotoxin, from their outer membrane (Figure 1B). LPS triggers a cascade of immune events. It binds to LPS-binding protein (LBP), and this complex interacts with CD14, leading LPS to Toll-like receptor 4 (TLR4) on macrophages (Figure 1C). TLR4 activation initiates intracellular signaling, activating nuclear factor kappa-beta (NF-κβ). This, in turn, induces macrophages and mammary epithelial cells to produce pro-inflammatory cytokines (IL-1β, IL-1, IL-6, IL-8, TNF-α) and acute-phase proteins (Figure 1D). TNF-α is a key mediator of endotoxic shock in severe coliform mastitis.
Chemotactic factors, especially IL-8, recruit neutrophils to the infection site (Figure 1E). Neutrophil efficiency in reaching the site and phagocytosing bacteria influences mastitis progression. While opsonization enhances phagocytosis, it’s not essential. Neutrophils can recognize MPEC via immunoglobulins (IgG2, IgM). However, milk components like fat globules and casein can impair neutrophil function (Figure 1F), reducing their bactericidal activity and phagocytic capacity. Neutrophils release chemicals (ROS, proteases) to kill MPEC, but these also damage mammary epithelial cells, potentially causing permanent milk production loss (Figure 1F). To limit tissue damage, neutrophils undergo apoptosis and release chemokines attracting macrophages (Figure 1G). Macrophages then phagocytose apoptotic neutrophils (efferocytosis), minimizing tissue damage (Figure 1H). Dendritic cells in the mammary alveoli are generally less responsive to MPEC infection. Successful mammary gland recovery depends on minimizing tissue damage during the immune response.
Bovine Mastitis Diagnosis: Methods and Techniques
Bovine mastitis diagnosis relies on a combination of clinical observation and laboratory tests. Clinical mastitis is often diagnosed through visual examination for udder swelling, redness, heat, pain, and abnormal milk. However, bovine mastitis diagnosis for subclinical cases requires indirect measures of inflammation. Reduced milk production is often the only visible sign of subclinical mastitis, necessitating further diagnostic tests. Somatic cell count (SCC) in milk is a primary indicator of mammary gland health. Healthy cows typically have SCC below 100,000 cells/mL, while mastitis cases show SCC exceeding 200,000 cells/mL. The California Mastitis Test (CMT) is a cow-side test estimating SCC for rapid bovine mastitis diagnosis. Electronic SCC provides a more precise measurement on bulk milk samples. Milk culture, involving microbiological analysis of milk samples, is crucial for identifying E. coli and other pathogens involved in clinical mastitis. However, bacterial culture may yield false negatives in approximately 30% of samples due to low bacterial numbers. Repeat cultures or specialized techniques may be necessary in such cases for accurate bovine mastitis diagnosis.
Advancements in Escherichia coli Mastitis Diagnosis: Addressing Challenges and Future Directions
Early and accurate bovine mastitis diagnosis is critical for minimizing the economic impact and ensuring animal welfare. While SCC and CMT are widely used, they have limitations. CMT results can be subjective, leading to potential false positives and negatives. SCC interpretation can be influenced by factors other than infection, such as lactation stage, milk yield, and stress. Genotypic approaches are increasingly used to complement phenotypic methods for improved bovine mastitis diagnosis, particularly in resolving false-negative culture results. Polymerase Chain Reaction (PCR) offers enhanced sensitivity for detecting growth-inhibited bacteria, reducing false negatives. Other molecular techniques used in pathogen identification include ribotyping, amplified fragment length polymorphism (AFLP), restriction fragment length polymorphism (RFLP), pulsed-field gel electrophoresis (PFGE), multiple-locus variable-number tandem repeat analysis (MLVA), transfer DNA intergenic spacer length polymorphism, and DNA sequencing of housekeeping genes. Microarray technology enables rapid detection of multiple mastitis-causing pathogens within hours.
Future advancements in bovine mastitis diagnosis are focused on developing rapid, cost-effective, and highly sensitive “cow-side” tests using novel biomarkers. Proteomic research is identifying protein expression patterns that can serve as diagnostic biomarkers and therapeutic targets. While promising, these advanced techniques are not yet readily available for routine bovine mastitis diagnosis. The ideal diagnostic method for routine use must be specific, sensitive, affordable, rapid, and capable of processing numerous samples. Proteomics holds potential for accurate biomarker identification for early bovine mastitis diagnosis, treatment monitoring, and the discovery of novel therapeutic targets.
Bovine Mastitis Treatment and Control Strategies
Effective bovine mastitis management requires a multifaceted approach encompassing treatment and preventative measures. Environmental management and hygiene are paramount in controlling environmental mastitis, particularly E. coli mastitis, given the ubiquitous nature of E. coli in cattle environments. Good herd health management and rigorous hygiene practices are crucial for prevention. Antibiotic therapy for coliform mastitis is not routinely recommended due to high spontaneous cure rates and the risk of antibiotic residues in milk, except in severe cases, especially during the early postpartum period. Supportive care, including fluid therapy and anti-inflammatory drugs (steroidal or non-steroidal), is often the initial treatment of choice. For severe E. coli mastitis, parenteral administration of fluoroquinolones or third-generation cephalosporins may be necessary.
Vaccination offers a proactive approach to mastitis control, particularly with E. coli J5 vaccine. Vaccination aims to enhance the animal’s specific immunity, increasing antibody levels in milk and blood to neutralize coliform toxins and facilitate bacterial clearance. The J5 vaccine, based on a mutant E. coli strain, stimulates cross-reactive antibodies against a broad range of gram-negative bacteria. Vaccination is strategically timed around periods of high mastitis risk, such as pre- and postpartum. Outer membrane protein A (OmpA) is another potential vaccine antigen under investigation. OmpA is a virulence factor involved in E. coli pathogenesis. While studies on recombinant OmpA vaccines have shown immunogenicity, further research is needed to determine its efficacy in preventing E. coli mastitis.
Antimicrobial Resistance in Mastitis-Associated Escherichia coli: A Growing Concern
Antibiotics remain essential for treating severe bovine mastitis caused by E. coli. However, antibiotic therapy is often ineffective against coliform mastitis due to spontaneous cure rates and increasing antimicrobial resistance. This has led to a rise in antimicrobial-resistant E. coli isolates and persistent mastitis cases. E. coli from mastitic milk exhibits resistance to multiple antibiotic classes, including aminopenicillins, polypeptides, lincosamides, and macrolides. Judicious antibiotic use is crucial in clinical mastitis management, reserving treatment for culture-positive cases and severe infections. Pathological changes in the mammary gland can hinder antibiotic distribution, and E. coli biofilm formation can further contribute to antibiotic resistance.
E. coli serves as a reservoir for antimicrobial resistance genes, capable of horizontal gene transfer to other bacteria. Resistance genes commonly found in mastitis-associated E. coli confer resistance to aminoglycosides, streptomycin, tetracycline, sulfonamides, ampicillin, and β-lactams. Extended-spectrum β-lactamase (ESBL)-producing E. coli is a significant concern. ESBLs confer resistance to a broad range of β-lactam antibiotics. The increasing prevalence of ESBL-producing E. coli in cattle and milk poses risks to both animal and human health due to potential transmission through unpasteurized milk and dairy products. Antimicrobial susceptibility testing, including antibiograms, disc diffusion, and minimum inhibitory concentration (MIC) tests, is essential for guiding rational antimicrobial use. MIC testing is considered more accurate than antibiograms. However, MIC tests may not accurately reflect antibiotic efficacy against biofilm-embedded bacteria.
Conclusion: Towards Improved Bovine Mastitis Diagnosis and Management
Mammary gland colonization is often a polymicrobial event, yet mastitis cases are frequently attributed to a single bacterial species, or even a single strain. E. coli‘s ability to acquire virulence genes contributes to the development of mastitis-causing strains. MPEC has been proposed as a distinct pathotype adapted to the mammary gland environment. Understanding the infection strategies of E. coli is crucial for developing novel prevention and treatment approaches for mastitis caused by this and other pathogens. With increasing restrictions on antibiotic use in farm animals, new treatment and prevention strategies are urgently needed. Improved bovine mastitis diagnosis, focusing on early and accurate detection, coupled with integrated management practices encompassing hygiene, vaccination, and judicious antimicrobial use, are essential for mitigating the impact of this costly disease on the dairy industry.
Author Contributions
DG and MM wrote the manuscript. DG developed figure and table. MM revised manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
The open access publication fees for this article were covered by the Iowa State University Library.
References
References are the same as the original article and are omitted here for brevity.