Introduction
Lung cancer remains the deadliest cancer globally, characterized by a concerningly low overall 5-year survival rate of just 17% post-diagnosis. This grim statistic is largely attributed to late-stage diagnoses, where treatment options are limited and prognosis is poor. Conversely, patients diagnosed at an early, localized stage of lung cancer exhibit significantly improved 5-year survival rates exceeding 70%. This stark contrast underscores the critical importance of early lung cancer detection to enhance patient outcomes. Flexible bronchoscopy, a minimally invasive procedure, has become a cornerstone in the diagnostic workup for individuals suspected of lung cancer. However, traditional white light bronchoscopy (WLB) alone has limitations, detecting only a fraction of early-stage tumors – approximately 29% of carcinoma in situ (CIS) and 69% of microinvasive tumors. To overcome these diagnostic challenges and improve early detection rates, the field of bronchoscopy has witnessed remarkable advancements over the past two decades. Innovative techniques such as autofluorescence bronchoscopy (AFB), narrow band imaging (NBI), and high magnification bronchovideoscopy (HMB) have emerged, aiming to increase diagnostic yield and accuracy. Furthermore, to address the limitations of visualizing only the proximal bronchial tree, probe-based technologies including radial endobronchial ultrasound (R-EBUS), optical coherence tomography (OCT), confocal laser endomicroscopy (CLE), and laser Raman spectroscopy (LRS) have been developed. This article delves into these cutting-edge bronchoscopic technologies, evaluating their role in enhancing lung cancer diagnosis and their potential to improve patient prognosis. While tissue biopsy remains the gold standard for confirming diagnoses, these bronchoscopic advancements represent the safest and most precise tools currently available for comprehensive evaluation of both central and distal airway mucosa.
Autofluorescence Bronchoscopy (AFB)
Autofluorescence bronchoscopy (AFB) leverages the natural fluorescence properties of bronchial tissues to differentiate between normal and abnormal areas. Healthy bronchial tissue contains endogenous fluorophores, such as tryptophan, collagen, elastin, and porphyrins. When illuminated with violet or blue light, these fluorophores emit a green fluorescence. In contrast, abnormal tissues, including dysplastic and cancerous lesions, exhibit altered concentrations of these fluorophores, resulting in reduced green fluorescence and the emission of red, purple, or magenta light. This difference in fluorescence emission allows for visual distinction between healthy and potentially malignant tissues during bronchoscopy.
Multiple studies, including meta-analyses, have consistently demonstrated the advantages of AFB, particularly when used in conjunction with WLB, over WLB alone for lung cancer and precancerous lesion detection. These studies indicate that AFB enhances sensitivity, albeit at the cost of slightly reduced specificity. A meta-analysis by Sun et al. highlighted a significant improvement in sensitivity with WLB + AFB compared to WLB alone, with a relative sensitivity of 2.04 for intraepithelial lesions and 1.15 for invasive cancer. However, the relative specificity was reported to be 0.65, indicating a higher rate of false positives with AFB. The lower specificity of AFB stems from its susceptibility to producing false positive results in cases of inflammation, infection, or trauma. These conditions can alter tissue metabolism and blood flow, leading to decreased green autofluorescence and mimicking the fluorescence patterns of cancerous lesions.
Different AFB systems have been developed, each with unique features. The Laser-induced fluorescence endoscopy (LIFE) system, utilizing a helium-cadmium laser emitting blue light, was one of the earliest systems. Clinical trials evaluating the LIFE system in combination with WLB demonstrated a substantial increase in sensitivity for detecting early intraepithelial neoplastic lesions compared to WLB alone. However, subsequent studies, such as one by Tremblay et al. exploring the use of AFB (Onco-LIFE system) in lung cancer screening alongside low-dose computed tomography (LDCT), did not show a significant incremental benefit. This suggests that AFB may not be justified as a primary screening tool in the general high-risk population.
Later AFB systems, such as the SAFE 1000, SAFE 3000 (both by Pentax), and autofluorescence imaging (AFI) bronchovideoscope system (Olympus), have incorporated advancements in light sources and imaging processing. The AFI system, for instance, integrates autofluorescence, reflected green, and red light signals. Chiyo’s pioneering study comparing AFI, LIFE, and WLB demonstrated superior sensitivity of AFI (80%) and LIFE (96.7%) for detecting dysplasia compared to WLB (53.3%). While AFI showed better specificity (83.3%) than LIFE (36.6%), WLB specificity was comparable to AFI (50%). Further studies have largely corroborated the enhanced sensitivity of AFI over WLB.
Beyond lesion detection, AFB has shown promise in other clinical scenarios. Studies in animal models suggest its potential in detecting post-surgical complications like airway ischemia, enabling early intervention and preventing stricture formation. AFB may also be valuable in the follow-up of patients with known severe dysplasia or CIS in the central airway, guiding surgical resection and potentially reducing postoperative recurrence rates.
Figure 1: Bronchoscopic Views of Squamous Cell Carcinoma with Different Imaging Modalities
This image demonstrates the visual differences in detecting invasive squamous cell carcinoma using autofluorescence imaging (A), white light bronchoscopy (B), and narrow band imaging (NBI) (C). Autofluorescence imaging highlights the abnormal tissue more distinctly compared to white light, while NBI focuses on vascular patterns.
Narrow Band Imaging (NBI)
Narrow band imaging (NBI) is an advanced endoscopic technology that enhances the visualization of endobronchial microvasculature, crucial for identifying early cancerous and precancerous changes. NBI achieves this enhanced visualization by utilizing specific narrow wavebands of light. Blue narrow band light (390–445 nm) is strongly absorbed by capillaries in the superficial mucosal layer, while green narrow band light (530–550 nm) penetrates deeper and is absorbed by hemoglobin in submucosal blood vessels. This spectral differentiation allows NBI to highlight the microvascular architecture of the bronchial wall.
The vascular patterns visualized by NBI are particularly informative in differentiating various stages of squamous cell carcinoma progression, from angiogenic squamous dysplasia (ASD) and carcinoma in situ (CIS) to micro-invasive and invasive squamous cell carcinoma. These patterns, termed “Shibuya’s descriptors,” are based on the angiogenic switch, a process of new blood vessel formation that is a hallmark of tumorigenesis. NBI also aids in distinguishing between adenocarcinoma and squamous cell carcinoma based on vascular morphology: adenocarcinoma typically presents with dotted blood vessels, while squamous cell carcinoma exhibits tortuous blood vessels and abrupt-ending vessel patterns.
A comprehensive meta-analysis by Iftikhar et al., encompassing multiple studies, evaluated the diagnostic performance of NBI alone and in combination with AFI. The analysis indicated that NBI outperforms AFI in detecting premalignant airway lesions, demonstrating a pooled sensitivity of 80%, specificity of 84%, and a diagnostic odds ratio of 31.49%. Interestingly, the combination of AFI and NBI did not significantly improve diagnostic performance compared to NBI alone.
Beyond detection, NBI plays a crucial role in assessing tumor extension and guiding surgical strategies, particularly for central airway lesions. By clearly delineating the microvasculature, NBI assists surgeons in determining the precise extent of the tumor and planning resection margins. While NBI’s efficacy in detecting premalignant lesions and early lung cancer is well-established, ongoing research explores its synergistic potential with other bronchoscopic techniques like HMB, endocytoscopy, and CLE to further enhance diagnostic accuracy.
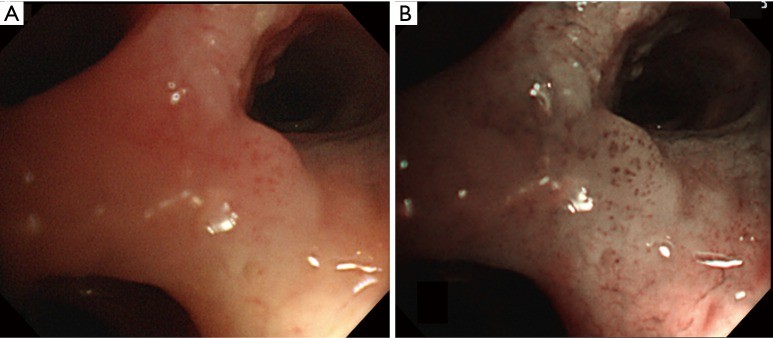
Figure 2: Carcinoma in situ Visualized with White Light and Narrow Band Imaging
This figure contrasts white light bronchoscopy (A) and narrow band imaging (B) views of squamous cell carcinoma in situ in the central airway. NBI (B) clearly highlights the abnormal vascular patterns characteristic of CIS, which are less apparent under white light (A).
High Magnification Bronchovideoscopy (HMB)
High magnification bronchovideoscopy (HMB) is a white light bronchoscopy system designed to provide magnified views of the bronchial mucosa, enabling detailed observation of vascular networks. HMB achieves magnifications of 55 to 110 times on a video monitor through a specialized video observation system and a fiber observation system for bronchoscope tip orientation. With an outer diameter of 6 mm and an observation depth of 1–3 mm, HMB allows for close-up examination of mucosal details.
While HMB has been extensively used in gastrointestinal endoscopy, Shibuya pioneered its application in bronchoscopy for detecting bronchial dysplasia at sites identified by abnormal fluorescence. Shibuya’s study demonstrated that HMB was more accurate than fluorescence bronchoscopy alone in detecting dysplasia, achieving a sensitivity of 71.4% and a specificity of 90.9%. The study identified increased vessel growth and complex tortuous vessel networks as key indicators of bronchial dysplasia, with a significantly higher vascular area ratio in premalignant lesions compared to bronchitis.
Subsequent studies have focused on the combined use of NBI and HMB to enhance the detection of premalignant bronchial lesions and early lung cancer by leveraging vascular irregularities. These studies have shown a progressive increase in capillary blood vessel diameter from angiogenic squamous dysplasia (ASD) to invasive squamous cell carcinoma. Notably, NBI combined with HMB has been shown to detect the onset of angiogenesis during lung carcinogenesis, initially in bronchial dysplastic lesions identified by fluorescence bronchoscopy and subsequently in CIS, microinvasive carcinoma, and invasive carcinoma even without prior fluorescence guidance.
Figure 3: High-Resolution Views of Angiogenic Squamous Dysplasia
This image compares white light (A) and narrow band imaging (B) bronchoscopic findings of biopsy-proven angiogenic squamous dysplasia in the central airway. NBI (B) provides a clearer visualization of the abnormal vasculature associated with ASD compared to white light (A).
Furthermore, research has explored the impact of smoking on bronchial microvessels using HMB. Yamada et al.‘s study using HMB revealed that smokers exhibited decreased vessel density in the large airway compared to non-smokers, suggesting that smoking affects not only lung parenchyma but also systemic angiogenesis.
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) is a non-invasive optical imaging technique providing high-resolution cross-sectional images of the airway wall. Based on the properties of light waves, OCT offers microscopic resolution (1–20 µm) and a penetration depth of up to 2–3 mm, allowing for detailed visualization of the airway luminal wall structure. Although analogous to B-mode ultrasound, OCT does not require physical contact between the optical probe and the tissue and provides 20 times higher resolution than ultrasound, enabling real-time delineation of cellular and extracellular structures.
Clinical studies have investigated OCT’s effectiveness in differentiating between normal and pathological bronchial tissue, indicating its potential as a valuable tool for early diagnosis of neoplastic lesions. Tsuboi et al.‘s pioneering report on endobronchial OCT in clinical practice demonstrated distinct OCT findings for normal bronchi (layered structure) and lung tumors (high backscattering areas with loss of layered structure and glandular tissues). Hariri et al.‘s ex-vivo study using OCT to diagnose primary lung cancer subtypes showed an overall accuracy of 82.6%, with sensitivity and specificity ranges of 80.3–85.7% and 87–97.6%, respectively. However, the study concluded that OCT could not replace tissue biopsy for definitive diagnosis.
Subsequent research has explored the combined use of OCT with AFB to distinguish invasive cancer from CIS and dysplasia from benign conditions by quantitatively measuring epithelial thickness. Pahlevaninezhad et al. recently reported the combination of Doppler OCT with AFI to guide biopsies of pulmonary nodules, demonstrating its potential for evaluating pulmonary parenchyma. OCT has also found applications in other bronchoscopic settings, such as real-time measurements of airway dimensions and assessment of elastic airway wall remodeling in chronic obstructive pulmonary disease (COPD), showing greater sensitivity than CT scans in detecting these changes.
In summary, OCT is a safe and feasible non-invasive imaging method providing detailed microanatomical information of the central airway, both as a standalone technique and in combination with AFB. It holds promise for in vivo imaging of preneoplastic bronchial lesions and airway wall changes in COPD patients. However, despite encouraging diagnostic yields, OCT remains largely investigational, and further studies are needed to fully determine its sensitivity and specificity in evaluating endobronchial and peripheral pulmonary lesions during routine diagnostic bronchoscopy.
Confocal Laser Endomicroscopy (CLE)
Probe-based confocal laser endomicroscopy (pCLE) is an innovative optical technology that allows for real-time, non-invasive histological imaging at the cellular level during bronchoscopy. Utilizing a 488 nm wavelength laser directed into the alveolar space through a thin, semi-flexible probe inserted via the bronchoscope’s working channel, pCLE achieves a lateral resolution of 3 µm and a depth of focus of 50 µm, providing three-dimensional endomicroscopic visualization. CLE leverages the autofluorescence properties of elastin fibers in the tracheobronchial tree, often without the need for contrast agents typically used in gastrointestinal endoscopy.
Sorokina et al.‘s ex-vivo study on lobectomy specimens identified distinctive pCLE features for adenocarcinoma, squamous cell carcinoma, and small-cell lung cancer. pCLE images revealed differences among these subtypes based on stromal and parenchymal components, demonstrating its ability to identify lung carcinoma in ex-vivo samples. Building on this, Wellikoff et al. described in-vivo pCLE changes for lung cancer diagnosis, showing that elastin composition disruption varied with tumor differentiation, ranging from mild in lepidic-type to significant in poorly differentiated adenocarcinoma.
Researchers have also explored the use of contrast agents like fluorescein and acriflavine hydrochloride to enhance epithelial bronchial cell detection during CLE bronchoscopy in suspected lung cancer cases. Fuchs et al. demonstrated that acriflavine-aided CLE’s diagnostic value was comparable to lung biopsy histology, predicting neoplastic changes with high sensitivity (96%), specificity (87.1%), and accuracy (91%).
Furthermore, pCLE’s capabilities extend beyond elastin fiber detection to include blood vessels, alveolar macrophages, and neutrophils. This broader visualization has led to its application in diagnosing conditions like pulmonary alveolar proteinosis, invasive pulmonary aspergillosis, and amiodarone-related pneumonia, where pCLE revealed highly fluorescent lipo-proteinaceous complexes and macrophages in distal bronchioles and alveolar lumens. However, current literature on CLE’s diagnostic yield remains limited, necessitating further ex-vivo and in-vivo studies to validate existing findings and refine image interpretation criteria.
Laser Raman Spectroscopy (LRS)
Laser Raman spectroscopy (LRS) is an experimental probe-based system that offers real-time in vivo diagnosis by analyzing light scattered from tissue, which provides a unique spectral fingerprint reflecting its molecular composition. LRS’s ability to differentiate various biomarkers makes it a promising tool for enhancing the specificity of early lung cancer detection. Consequently, researchers have explored combining LRS with AFB and WLB to reduce the false-positive rates associated with AFB alone.
Short et al. demonstrated improved diagnostic accuracy for detecting lung cancer and preneoplastic lesions when LRS analysis was added to AFB + WLB imaging, achieving a sensitivity of 96% and specificity of 91%. McGregor et al.‘s recent study further supported LRS’s potential, showing that it could differentiate high-grade dysplasia and malignant lung lesions from benign lesions and normal tissue with high sensitivity (90%) and good specificity (65%).
Despite its growing promise, LRS remains an investigational technology. Further research is crucial to validate its effectiveness in early lung cancer diagnosis and to establish standardized protocols for its clinical application.
Endobronchial Ultrasound (EBUS)
Endobronchial ultrasound (EBUS) integrates bronchoscopy with ultrasound technology to visualize mediastinal structures and parabronchial anatomy, improving the accuracy of transbronchial sampling and reducing biopsy errors. EBUS is currently utilized in lung cancer staging, evaluation of endobronchial tumors, peripheral pulmonary lesions (PPLs), and mediastinal masses.
Two main types of EBUS are used clinically: radial-probe EBUS (RP-EBUS) and convex-probe EBUS (CP-EBUS). RP-EBUS, introduced in 1992, is valuable for assessing the tracheo-bronchial wall structure and guiding transbronchial needle aspiration (TBNA), as well as for detecting PPLs in conjunction with navigation bronchoscopy. CP-EBUS, developed later in 2004, enables real-time TBNA of mediastinal and hilar lymph nodes, crucial for lung cancer staging.
Accurate assessment of bronchial wall tumor involvement is essential for determining optimal treatment strategies, particularly when considering photodynamic therapy (PDT) for central-type early-stage lung cancer versus surgery or radio-chemotherapy for tumors extending beyond the cartilaginous layer.
Figure 4: Radial Probe Endobronchial Ultrasound for Tumor Depth Assessment
This image illustrates the use of radial probe endobronchial ultrasound (RP-EBUS) in evaluating tumor invasion depth. White light bronchoscopy (A) reveals a lesion at the bronchial orifice. RP-EBUS (B) demonstrates the tumor’s extent and confirms no invasion into the cartilaginous layer, which is critical for treatment planning.
RP-EBUS employs a 20 MHz rotating probe passed through a standard bronchoscope, generating 360° images with high spatial resolution (sub-millimeter) and a penetration depth of 5 cm. Combined with a balloon sheath, RP-EBUS allows for detailed visualization of the central airway’s multilayer structure and the extent of lung cancer compression or infiltration, including relationships with adjacent lymph nodes or vessels.
Kurimoto et al.‘s prospective study demonstrated a strong 95.8% correlation between EBUS and histopathological findings in determining tumor invasion depth. Miyazu et al.‘s study utilizing EBUS to guide treatment decisions for squamous cell carcinoma patients showed that EBUS-guided therapy (PDT for intra-cartilaginous tumors, surgery/chemo-radiotherapy for extra-cartilaginous tumors) resulted in long-term complete remission in the PDT group. Similarly, Li et al.‘s study reported high sensitivity (88.89%), specificity (100%), and accuracy (90%) of RP-EBUS in evaluating tracheobronchial wall involvement. Furthermore, EBUS has been shown to improve specificity in predicting lung cancer in small lesions detected by AFB but not visible under WLB, increasing specificity from 50% to 90%.
Conclusions
Early detection of lung cancer within the airway is paramount to preventing its progression to invasive stages. While tissue biopsy remains the definitive diagnostic method, bronchoscopic technologies stand as the safest and most accurate means of evaluating both central and distal airway mucosa. Current evidence strongly supports the use of AFB and NBI for central airway evaluation and screening in individuals with suspected or known lung cancer, primarily due to their high sensitivity. However, their limitations in specificity and proximal airway visualization necessitate the exploration of adjunctive techniques like OCT, CLE, and LRS. Although these newer technologies require further ex-vivo and in-vivo validation to refine data interpretation and establish clinical utility, they hold immense promise. In the future, these advanced bronchoscopic tools may enable bronchoscopists to perform “optical biopsies,” providing high-resolution, three-dimensional virtual images of bronchial structures. These advancements have the potential to revolutionize the diagnostic and therapeutic landscape of airway disease, fulfilling Dr. Shigeto Ikeda’s vision: “There is more hope with the bronchoscope.”
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.