Blood Cultures
While the detection of Brucella organisms through culture is challenged by their slow growth, biosafety concerns, and decreased sensitivity in chronic cases and localized infections, bacterial isolation remains the definitive proof of brucellosis. Laboratory diagnosis through culture allows for precise species identification and genotyping, crucial for tracing infection sources, differentiating between wild and vaccine strains (19), and conducting antibiotic susceptibility testing when needed.
In Brucella Laboratory Diagnosis, blood cultures are particularly valuable in the early stages of the disease, when serological tests may still be negative or show low antibody levels (20). Furthermore, isolating brucellae from blood cultures provides a definitive diagnosis in patients where brucellosis is not initially suspected but the bacterium is incidentally recovered during routine fever workups (21, 22). This is crucial because a patient’s history of zoonotic exposure might be unclear due to brucellosis’s extended incubation period, spanning weeks or months. The clinical signs of brucellosis often mimic other conditions, including systemic and localized infections, as well as rheumatic or hematological disorders (8). Without considering brucellosis in the differential diagnosis, specific serological and nucleic acid tests might not be ordered, leading to missed diagnoses. This is especially common in regions where brucellosis is rare, awareness is low, and physicians are less likely to include it in their differential diagnoses, as demonstrated by a brucellosis outbreak in Hong Kong (23). However, even in endemic areas, brucellosis, known as the “great imitator,” can be overlooked. A study in southern Israel, a hyperendemic region, found B. melitensis in blood cultures from 21 patients with suspected brucellosis and, strikingly, in 42 cultures from 27 individuals where brucellosis was not initially considered (21). Similarly, a Turkish hospital reported that 59.1% of confirmed brucellosis patients had been previously misdiagnosed by a physician (24).
Dynamics of Brucella Bacteremia
Brucella species are highly infectious and can enter the human body via various routes, including the gastrointestinal and respiratory tracts, conjunctiva, and broken skin, or directly into the bloodstream in cases of transfusion or transplacental transmission (8, 16). Regardless of the entry point, Brucella rapidly crosses the epithelial layer and is engulfed by macrophages and dendritic cells in the mucosa (8). Initially, brucellae localize in regional lymph nodes before spreading through the bloodstream to macrophage-rich tissues such as the liver, spleen, lymph nodes, and bone marrow. Here, they establish a facultative intracellular lifestyle, evading both innate and adaptive immune responses and many antibiotics (16). Since bacteremia is always a part of human brucellosis pathogenesis, peripheral blood cultures are a valuable tool for confirming the diagnosis, although their sensitivity varies widely (10 to 90%) across different studies (16). Factors influencing Brucella recovery from blood cultures are detailed in Table 1.
TABLE 1. Factors Affecting Blood Culture Positivity in Brucellosis Diagnosis
| Category | Associated factors |
|---|---|
| Microbial | Brucella species |
| Patient | Age, symptom duration, systemic vs. focal disease, primary infection vs. relapse, prior or current antibiotic use |
| Blood culture method | Blood specimen volume, number of cultures, monitoring frequency, blood culture system sensitivity, incubation period, periodic blind subcultures, terminal blind subcultures |
Open in a new tab
Table 1: Factors that influence the success rate of blood cultures in diagnosing brucellosis. This table categorizes factors related to the microbe, the patient, and the blood culture methodology.
In early brucellosis, patients typically experience continuous, low-grade bacteremia, readily detectable with two or three separate blood culture sets. As the infection progresses, the bacteria are cleared from the bloodstream and sequestered within macrophages. Consequently, the concentration of bacteria in circulation decreases, and bacteremia becomes less consistent, making bacterial isolation more challenging (25). The importance of multiple blood cultures was highlighted in a Turkish study where Brucella was found in 83.9% of patients with paired blood cultures, compared to 58.6% with a single culture (P = 0.03) (26). The natural progression of human brucellosis and Brucella bacteremia is generally unpredictable (27). The organism can intermittently re-enter the bloodstream (25), increasing the risk of relapse and spread to distant sites (28, 29).
Patients with brucellic bacteremia often present with higher fever than those without demonstrable bacteremia (30). However, Brucella is a pathogen with relatively low virulence in humans, and the inflammatory response may be subdued. Therefore, the organism can be recovered even from patients with mild symptoms or no fever (31. Blood cultures should always be performed when brucellosis is suspected, regardless of fever presence (32).
Evaluating Blood Culture Method Performance for Brucella Detection
Brucella species are characterized by slow generation times (several hours), low bacterial concentrations in circulation, and minimal CO2 production, a key metabolite monitored by automated blood culture systems. To maximize bacterial recovery, guidelines from the American Society for Microbiology (33) and the WHO (34) recommend incubating inoculated media for 4 weeks and performing blind subcultures of apparently negative blood culture media. However, this approach is costly, labor-intensive, requires significant lab space, and delays diagnosis. Conversely, limiting incubation to the standard 5- to 7-day period in clinical microbiology labs is not advisable unless it’s proven that Brucella-containing vials are not systematically missed. Many studies employing short incubation protocols without blind subcultures have reported Brucella detection within 3 to 7 days (35–38). These reports can be misleading if they do not address the possibility of prematurely discarding vials with viable Brucella. This is particularly critical as a positive blood culture is often the primary and only definitive evidence of infection. Therefore, blood culture system sensitivity and time-to-detection must be assessed in well-designed prospective studies with prolonged incubation and blind subcultures of negative vials. Sensitivity should be calculated as the proportion of positive vials identified within the routine 5- to 7-day incubation period out of the total positive vials detected by the system and/or blind subcultures over 4 weeks.
Blood Culture Methodologies
(i) Manual Monophasic Method
Traditional microbiological approaches for Brucella isolation from blood were similar to those for other bacterial pathogens. Blood culture vials were inoculated, incubated at 35°C, and periodically checked for turbidity, indicating bacterial or fungal growth. However, due to Brucella‘s slow growth and the common practice of discarding vials after 5-7 days if not flagged by automated systems, Brucella often remained undetected. When brucellosis was suspected and communicated to the lab, vials were incubated longer, and blind subcultures were performed on agar plates.
(ii) Manual Biphasic Methods
(a) Castañeda Flask
To avoid repeated blind subcultures, Ruiz-Castañeda developed a simple, inexpensive biphasic flask in the late 1940s (39, 40). This flask contains a solid nutrient agar slant on one side and a suitable broth (like serum-dextrose or peptone-based medium) on the other. After inoculation with patient samples (blood, bone marrow, exudates, tissues, etc.) and adding 10% CO2, the flask is sealed, tilted to wet the agar slant with the blood-medium mixture, and incubated at 35°C upright. Flasks are examined every 48 hours for colony growth on agar, broth turbidity, or both (40). If no growth is seen, the flask is retilted and reincubated, repeating this for at least 35 days (39, 41). Eliminating repeated subcultures saves time and labor and reduces the risk of laboratory-acquired brucellosis. The Castañeda method is not specific to Brucella; other microorganisms can grow, requiring isolate identification. Despite being surpassed by automated systems, the Castañeda method remains widely used in resource-limited endemic countries due to its low cost and practicality (27, 42–47).
Gotuzzo et al. in Peru reported Brucella colony appearance in Castañeda flasks averaging 4.3 days for bone marrow and 6.7 days for blood, with all positives detected within 15 days (48). However, a Spanish study found most positive blood cultures required 1 to 3 weeks of incubation (49). These discrepancies may stem from differences in patient populations, Brucella strain characteristics, or variations in in-house biphasic media.
(b) TUMS Medium
A Castañeda flask variant, Tehran University of Medical Sciences (TUMS) medium, uses a solid urea agar base on the slant and brain heart infusion in the liquid phase (50). This formulation leverages Brucella‘s urease activity, indicated by a pH indicator color change, to expedite isolate identification.
(c) Hémoline Medium
Hémoline, a commercial biphasic blood culture system by bioMérieux, was evaluated by Ruiz et al. (51). Blood samples from presumptive brucellosis patients were inoculated into flasks, incubated for 3 weeks, and subjected to terminal blind subcultures. The median detection time was only 5 days, but 23.5% of positive cultures were delayed, becoming positive after 7 days (51).
(iii) Lysis-Based Blood Cultures
Given the limited sensitivity and prolonged detection times of standard liquid media blood cultures for Brucella, lysing white blood cells before plating on solid media emerged as an alternative. The rationale is that Brucella does not circulate freely in the bloodstream. After opsonization, they are readily phagocytosed by polymorphonuclear cells (52). Engulfed brucellae reside in intracellular vacuoles where most organisms eventually die, reducing culture sensitivity and delaying detection. Lysing white blood cells releases phagocytosed but still viable bacteria, which can then be plated on suitable solid media.
(a) Lysis-Filtration
In the early 1950s, Braun and Kelsh developed a membrane filtration method for culturing circulating microorganisms, tested in a brucellosis animal model (53). Blood from infected rabbits was heparinized and osmotically lysed. The lysate was filtered through a sterilized Millipore filter under negative pressure. Filter membranes were placed on petri dishes, and after incubation, trapped organisms grew as colonies. This lysis-filtration method was cumbersome, unsafe, and prone to filter clogging, preventing widespread adoption.
(b) In-House Lysis-Centrifugation Method
The lysis-filtration method was improved by separating bacteria from the blood lysate via centrifugation instead of filtration, followed by sediment plating (54, 55). Etemadi et al. compared this in-house lysis-centrifugation with the Castañeda method using 14 peripheral blood, 2 bone marrow aspirate, and 2 CSF samples (54). Lysis-centrifugation detected B. melitensis in all specimens within 2 days, while all 18 Castañeda vials remained negative after 3 weeks (54.
Mantur and Mangalgi conducted a prospective study with 148 Indian patients with acute and chronic brucellosis, diagnosed by serology (56). In 121 acute cases, lysis-centrifugation recovered Brucella in 90.9%, significantly more than Castañeda at 71.8% (P = 0.001). Detection time was also shorter for lysis-centrifugation (2.4 ± 0.9 days vs. 6.7 ± 2.2 days; P < 0.001). In 27 chronic cases, lysis-centrifugation detected 55.6% and Castañeda 33.3% (P = 0.087), with detection times of 2.7 ± 1.4 days vs. 7.2 ± 2.6 days, respectively (P = 0.001).
More recently, Mangalgi and Sajjan compared lysis-centrifugation, Castañeda, and blood clot cultures in 169 serologically positive patients (57). Lysis-centrifugation isolated B. melitensis in 43.2%, Castañeda in 24.9%, and blood clot culture in 34.9%. Lysis-centrifugation had significantly shorter detection times (4.1 ± 0.9 days vs. 9.6 ± 1.7 days vs. 5.8 ± 1.4 days, P < 0.05).
Espinosa et al. compared Etemadi lysis-centrifugation with Castañeda in 88 Peruvian patients with brucellosis symptoms and SAT titer ≥1:25 (58). Lysis-centrifugation showed better sensitivity, detecting Brucella in 43.2% versus 35.2% with Castañeda, though not statistically significant. However, lysis-centrifugation significantly reduced detection time (3.8 ± 0.8 days vs. 13.6 ± 6.5 days, P < 0.05).
Alonso et al. compared lysis-centrifugation with the Bactec 460 system (55). Equal blood volumes were processed by lysis-centrifugation or inoculated into Bactec 460 vials. Lysis-centrifugation recovered B. melitensis in 27.8% of 54 patients, while Bactec 460 was positive in 35.2%. Lysis-centrifugation detected the organism in 3.5 days (range, 2-4 days) versus 14 days (range, 7-30 days) for Bactec 460.
(c) Isolator Microbial Tube
Commercial Isolator microbial tubes (Wampole Laboratories) have replaced in-house lysis-centrifugation methods. These tubes contain sodium polyanethole sulfonate (SPS) anticoagulant and detergent. SPS prevents clotting, while detergent lyses polymorphonuclear cell membranes, releasing phagocytosed organisms. The lysate is plated onto agar. Two versions exist: a 10-ml tube for adults requiring centrifugation before plating, and a smaller 1.5-ml tube for children, plated directly.
Navas et al. inoculated 10 ml of blood into an adult Isolator tube and two 5-ml aliquots into aerobic and anaerobic Bactec NR660 vials (59). Sensitivity was similar, but lysis-concentration cultures had shorter detection times (2-5 days vs. 17-29 days, mean 20.6 days for Bactec). Although Brucella was unexpectedly detected in both aerobic and anaerobic Bactec media in one patient, the actual blood volume processed by Bactec was half that in the Isolator tube, potentially delaying detection (59.
(d) Bactec Myco/F Lytic Vial
The Bactec Myco/F lytic vial, a recent addition to Bactec 9000 media, enhances detection of intracellular pathogens like mycobacteria and fungi. It combines leukocyte lysis with automated monitoring and safety (60). A volume-controlled study comparing Myco/F lytic vials with aerobic pediatric and adult Bactec vials for B. melitensis recovery showed similar sensitivity but significantly longer time-to-positivity for the lytic medium (101.4 ± 46.7 h vs. 65.5 ± 18.9 h, P = 0.004). This suggests the Myco/F lytic vial medium may not fully meet Brucella‘s nutritional needs (60).
(iv) Blood Clot Cultures
Culturing blood clots, where leukocytes with phagocytosed organisms are trapped, is a potentially rational approach, as antibodies in brucellosis patient sera can inhibit bacterial growth. This involves collecting blood in a sterile tube, allowing it to clot, centrifuging to separate serum (for serology), and mechanically disrupting and plating the clot onto agar (56). Published data on this technique is limited and inconsistent. Escamilla et al. compared clot cultures (with taurocholate-streptokinase or bile) to whole blood cultures (61). Clot cultures showed lower sensitivity and were more labor-intensive. Taurocholate-streptokinase clot cultures isolated Brucella in 70.0%, bile-clot in 3.3%, and whole-blood in 93.3% of positive specimens. Emulsifying supplements may have negatively impacted Brucella viability, reducing sensitivity.
Mangalgi and Sajjan reported better blood clot culture results in 169 serologically confirmed brucellosis patients (57). Clot culture sensitivity was 34.9%, Castañeda 24.8%, and lysis-centrifugation 43.1%. Mean detection times were 5.8 ± 1.4, 9.6 ± 1.7, and 4.1 ± 0.9 days, respectively. Mantur et al. found clot cultures more sensitive than whole blood cultures, improving sensitivity by >20% and reducing detection time from 8.2 to 3.1 days (62). While blood clot culture is simple and low-cost, potentially improving brucellosis diagnosis in resource-limited settings, more data is needed before routine adoption.
(v) Automated Blood Culture Systems
Automated blood culture systems have rapidly advanced over the past four decades, replacing less sensitive manual monophasic methods (Fig. 1). Current systems detect bacterial or fungal metabolic activity by monitoring increasing CO2 or decreasing oxygen levels in vials. These systems detect gas composition changes before visible turbidity, shortening bacteremia diagnosis time. Many also mechanically agitate aerobic vials to enhance oxygen exposure, nutrient availability, and CO2 release.
FIG 1. Timeline of Blood Culture Methods for Brucella Isolation.
Open in a new tab
Figure 1: A timeline illustrating the historical progression of blood culture techniques used for isolating Brucella bacteria, highlighting the evolution from manual methods to automated systems.
(a) Factors Influencing Brucella Detection by Automated Systems
Automated blood culture systems vary in their methods for measuring metabolic changes. Bactec 9000 and FX series use fluorescence levels that increase with CO2 and decrease with O2. BacT/Alert systems (bioMérieux) use a colorimetric sensor for CO2. Vital instruments (bioMérieux) measure fluorescence quenching caused by medium acidification.
CO2 release depends on factors like initial bacterial load (concentration × blood volume), species replication time, metabolic activity, broth adequacy, inhibitory factors, CO2 measurement sensitivity and frequency, and positivity cutoff levels.
Brucella bacteremia often has low bacterial loads (1-5 CFU/ml) (63–65). Time-to-detection is inversely related to bacteremia magnitude, as shown in simulated blood culture studies (66, 67). Larger blood samples increase culture sensitivity; recommended volumes are 20-30 ml for adults, 2-4 ml for children under 3, and ≥10 ml for older children (68). However, a study on Myco/F lytic medium performance used mean blood volumes under 5 ml (60. A blood-to-broth ratio of at least 1:5 to 1:10 is crucial to dilute inhibitory serum factors like complement, antibodies, or antibiotics. Larger blood samples should be distributed across multiple vials to maintain this dilution effect (68).
Brucella has a longer doubling time (2.5-3.5 h) than many pathogens (65), and limited CO2 release due to carbohydrate metabolism via the pentose-phosphate pathway. Simulated blood cultures showed B. melitensis CO2 production is slower and reaches lower peaks than other bacteria (66), explaining prolonged detection times in automated systems. In a Bactec NR730 study, Brucella broth turbidity appeared about a day before CO2 monitor positivity (65), negating automated reading advantages.
Attempts to enhance Brucella CO2 release and accelerate detection by supplementing broth with glucose, erythritol, pyruvate, alanine, glutarate, urea, or pH modification were explored (65). Alanine and pyruvate slightly increased CO2. Acidifying the medium to pH 6.2 with elevated pyruvate caused a more significant increase. While medium composition changes may speed up Brucella detection, they could negatively affect growth of other bacteria in the vial.
Increasing broth volume from 30 ml (Bactec NR660) to 40 ml (Bactec 9000), reducing the blood/broth ratio, may have improved sensitivity for Brucella bacteremia detection in newer systems (69).
SPS is added to prevent blood clotting. While essential for its anticoagulant, antiphagocytic, anticomplementary, and aminoglycoside-neutralizing properties (important for brucellosis patients on antibiotics), SPS can inhibit Brucella recovery (65). SPS concentration has been reduced to 0.025% in Bactec 9000 vials, from 0.035% in Bactec NR660 and BacT/Alert media.
(b) Radiometric CO2 Detection
The semiautomated Bactec 460, introduced in the 1970s, revolutionized blood culture. A sterilized needle pierced the vial’s rubber top to aspirate and analyze the headspace gas. This headspace contained radioactive CO2 from 14C-labeled substrates in the broth. Positivity was defined by radioactivity reaching a threshold or significantly increasing between measurements. However, manual bottle loading was time-consuming, limiting CO2 monitoring to once or twice daily. Breaching vial tops risked cross-contamination (70), with serious clinical and public health implications, especially for brucellosis.
While Bactec 460 improved bacteremia diagnosis for common pathogens, its Brucella detection was suboptimal (55, 71–73). Sensitivity was lower than Castañeda flasks (73, detection times often exceeded the standard 1-week incubation (71), and Brucella presence was often missed by CO2 readings, only detected by terminal blind subcultures (72, 73).
(c) CO2 Detection by Infrared Technology
Later generations of automated systems integrated incubators, eliminating manual vial loading. Continuous CO2 monitoring offered quicker positive vial detection, potentially critical for septic patient management. However, this advantage is lost without 24/7 lab staffing or timely communication of results to physicians.
Limited data exists on Bactec NR instrument performance using infrared CO2 detection for Brucella. Evaluations are hampered by 7-day incubation periods and lack of terminal subcultures in most reports. Results were generally disappointing (21, 55, 59, 67, 74, 75). Comparative studies showed few Bactec NR detections within 1 week (67), demonstrating lower sensitivity and longer detection times than Hémoline flasks (74) and Isolator tubes (55).
The only methodologically sound Bactec NR evaluation monitored bottles for 4 weeks with weekly blind subcultures (21). In a 2-year study of 373 blood cultures from 21 Israeli patients, 7.2% grew B. melitensis. Bactec NR detected 78.8% within 1 week, but 22.2% were missed by automated reading and only detected by subculture after 2-3 weeks, indicating limited and unsatisfactory Brucella bacteremia detection capacity.
(d) Continuous Monitoring Systems
Experience with current automated blood culture systems for Brucella isolation is slowly growing. While brucellosis is still endemic in many countries, high system costs limit access in developing regions. In industrialized nations, where these systems have been available for over 30 years, zoonotic brucellosis is largely controlled, and human cases are rare.
Most evaluations of continuous monitoring systems for Brucella have been in endemic countries like Israel (60, 63, 76, 77), Turkey (26), and Saudi Arabia (78), where advanced medical facilities coexist with traditional rural lifestyles.
(e) BacT/Alert System
Published data on BacT/Alert system performance for Brucella recovery is limited and inconclusive (66, 79, 80). It successfully detected B. melitensis bacteremia in a travel-related case in 2.8 days (66, and another report showed all 9 blood cultures from 5 patients positive within 3.7 days, including a pancreatic fluid sample positive in 13.3 hours (79). However, Casas et al. reported poor BacT/Alert performance (80). Blood cultures from 6 serologically confirmed brucellosis patients were monitored for 10 days. Negative vials were incubated for another 10 days with subcultures on days 10 and 20 (80). Only one positive bottle was detected by the automated system within 3 days, while 7 were detected by day 10 subculture and one more by day 20 (80).
(f) Bactec 9000 Instruments
Studies in Middle Eastern endemic countries in the 1990s and 2000s reported successful Brucella detection from blood and sterile fluids within 10 days using Bactec 9000 series instruments. Gedikoglu et al. used Bactec 9120 for Turkish patients with suspected brucellosis, monitoring vials for 7 days (75). Thirty cultures from 15 patients were B. melitensis-positive within 84 hours. Saudi researchers using Bactec 9240 in an area endemic for both B. abortus and B. melitensis monitored vials for 3 weeks without blind subcultures (69). Over 2 years, 85 vials were B. melitensis-positive and 12 B. abortus-positive. All 97 positives were detected by day 9, with 92.7% by day 5 (69). Another Saudi study using Bactec 9240 and NR660, monitored for 6 weeks with weekly subcultures, found 8 Brucella-positive cultures, detected on average after 1 week (range, 4-14 days), but system performances were not reported separately, nor was precise detection time (78).
In a Turkish retrospective study, Durmaz et al. used Bactec 9120, incubating bottles for 1 week, with Gram stain and subculture of negative bottles (81). Twenty bottles grew B. melitensis after a mean of 30.0 hours (range, 31.2-117.5 hours; median, 69.9 hours), and no positives were missed by automated monitoring. However, Ayaşlioğlu et al. reported inferior results, with Bactec 9050 detecting 84.1% of 58 positive blood cultures within 1 week, but 8 additional cultures only detected by day 30 blind subculture (26).
Kurtoglu et al., in a rural Turkish hyperendemic area, used Bactec 9050 and 9120 for febrile patients, monitoring vials routinely for 5 days, extended to 2 weeks when brucellosis was suspected (82). Sixty Brucella-positive vials were identified within 10 days, but precise detection times were not reported. Cultures from patients without brucellosis suspicion had short incubation and no subcultures, precluding false-negative rate assessment.
Other retrospective studies in developing countries also reported Brucella recovery within days using Bactec 9000 series (35–38). However, these used short incubation protocols: 5 days (35), 1 week ([37](#B37], [41](#B41], 75), or 5 days extended to 14 days when suspected (38, 82). Terminal blind subcultures were not performed in these studies.
The Bactec 9240 system’s ability to detect Brucella within 7 days was assessed in a prospective study of febrile children in southern Israel (76). Aerobic pediatric bottles were incubated for 4 weeks, with weekly subcultures if no growth. B. melitensis was recovered in 1.6% of 2,579 blood cultures, with 97.6% detected by automated reading within 2-6 days. Only one culture was detected by week-one blind subculture. Cumulative automated detection rates were 0.0%, 23.6%, 78.9%, 86.8%, 92.1%, 97.6%, and 97.6% for days 1-7, respectively. A complementary study in seropositive adults in the same region used Bactec Plus Aerobic/F vials incubated for 28 days with blind subcultures on days 7 and 28 (77). B. melitensis was isolated from 35.2% of 88 vials from 38.0% of 50 patients. Automated monitoring detected 96.8% of 31 positive vials within 1 week; the missed vial was detected by 4-week terminal subculture, indicating low initial bacterial inoculum (77).
Ayaşlioğlu et al. in Turkey, using Bactec 9050, found 5.9% of 136 Brucella-positive vials (from 60 patients) undetected by automated reading, only identified by day 30 blind subcultures (26. Bactec 9050 differs from other Bactec 9000 models by continuous mechanical shaking, potentially enhancing bacterial growth. Similar Bactec 9050 failure in Brucella detection occurred in a 16-case B. melitensis cluster investigation by Lepe et al. in 2001 (83). Vials were monitored for 3 weeks, with blind subcultures of unflagged vials (83). 81.3% of patients had confirmable Brucella bacteremia. Automated monitoring detected bacteria in 69.2% within 7 days, in 2 more patients on days 8 and 11, and failed in 2, with recovery only in final blind subculture.
The wide performance variations in current blood culture instruments across studies are unclear. The superior Bactec performance in Israeli studies (76, 77) may be due to patients presenting early in infection with high bacteremia. Studies showing lower sensitivity often enrolled patients with longer-standing disease and lower bacterial loads, decreasing detection and prolonging time-to-positivity (76, 77.
(vi) Is Prolonged Vial Incubation Still Necessary?
Studies show that CO2 measurement evolution and liquid culture media changes have significantly improved Brucella bacteremia diagnosis recently. Modern methods enhance culture sensitivity, reduce detection time, and save time and labor. Automated systems enable hands-off processing of many bottles, nearly eliminate contamination, and ensure safe handling of dangerous bacteria.
Increased sensitivity and shorter detection times of modern systems question the necessity of prolonged incubation and periodic subculturing for Brucella detection (33, 34). Current automated systems detect acute brucellosis in children and adults within the standard 1-week incubation, often eliminating subcultures, provided samples are taken early in infection (76, 77). However, in longer-duration or focal infections, prolonged incubation and terminal subcultures may still be needed to maximize isolation (26, 83.
(vii) Superior Blood Culture System for Brucella Recovery
Despite recent reviews recommending lysis-centrifugation cultures as the preferred Brucella isolation method (8, 16), the only prospective comparison between Isolator tubes and Bactec showed statistically significant superiority for automated systems in sensitivity and detection time (63). In a volume-controlled study, pediatric patient blood aliquots were inoculated into Bactec 9240 aerobic bottles or Isolator tubes (63). Of 122 cultures, 22.8% grew B. melitensis by either method. The automated system detected all 28 positives, while lysis-centrifugation detected only 22 (78.6% sensitivity; P < 0.05). Mean detection time was also significantly shorter for Bactec (2.9 days vs. 3.9 days; P < 0.01).
Performances of Bactec 9120, Vital (bioMérieux), and Hémoline biphasic flasks were prospectively compared using blood cultures from Spanish brucellosis patients (51). Hémoline detected all 19 positives, while Bactec and Vital missed one each (94.7% sensitivity). After 5 days, detection rates were 47.4%, 78.9%, and 10.5%, respectively. By week one, rates increased to 73.7%, 94.7%, and 47.4%, with Bactec 9120 significantly faster (P < 0.05). Slow Brucella detection by Vital was confirmed in two later studies, with average detection times of 119.7 and 211.7 hours (84, 85).
A head-to-head study compared BacT/Alert and Bactec 9240 using 10-ml adult blood aliquots (86). Limitations were 7-day incubation and no blind subcultures. Results were inconclusive: similar detection times (2.5 days for BacT/Alert vs. 2.8 days for Bactec 9240), with BacT/Alert detecting 52.9% and Bactec 9240 82.3% of positives (P = 0.067).
More studies with larger culture-proven brucellosis patient cohorts are needed to determine the optimal blood culture system for Brucella bacteremia detection. However, labs typically use only one automated system, making direct comparisons unlikely. System selection is a costly strategic decision based on professional and economic factors, not solely Brucella isolation capability.
(viii) Blood vs. Bone Marrow Cultures as Gold Standard
Before advanced automated systems in the mid-1990s, peripheral blood Brucella recovery was often suboptimal. Culturing bone marrow aspirates (27, 40, 41, 87–89), liver biopsies (90, 91), or lymph nodes (92) was advised to improve detection. The rationale was Brucella multiplication and concentration in the reticuloendothelial system, potentially increasing recovery from macrophage-rich tissues (16). Despite this, the optimal specimen for brucellosis diagnosis remains debated. Ganado and Bannister reported that in one-fifth of bone marrow culture-positive patients, blood cultures were negative (87). Gotuzzo et al. found bone marrow positive in 92.0% of patients and blood in only 70.0% (48). Mantur et al. reported bone marrow isolating Brucella in 82.5% versus 45.6% by blood cultures (P < 0.001) (27. Özkurt et al. prospectively inoculated blood and bone marrow into BacT/Alert vials and in-house liquid medium (93). Bone marrow yielded Brucella in 70.0% versus 48.0% for blood (P < 0.05) (93, 94. In 13 patients with paired samples, automated instrument detection in blood was 3-7 days (mean 4.3 days) versus 2-4 days (mean 2.6 days) for bone marrow (P < 0.05) (94.
Consistently, studies show higher sensitivity and faster detection with bone marrow cultures (27, 48, 74, 93–95, in both acute and chronic cases (27).
Conversely, Magill and Killough found peripheral blood cultures positive in 90% of culture-positive cases, and bone marrow in only 40% (96). Shehabi et al. reported sensitivities of 44.4% and 27.7%, respectively (97. Iseri et al., using Bactec 9050, found blood culture detection rates of 48.0% and bone marrow 34.3% (95. Wang et al. recovered Brucella from 62.5% of blood cultures and only 18.8% of bone marrow specimens (89.
While optimal specimen choice is debated, blood samples are easier to obtain and repeat, less invasive and painful, and allow larger volumes. Furthermore, blood cultures can detect brucellosis in patients where it’s not initially considered, while bone marrow aspiration requires a priori suspicion.
Brucella Isolation from Non-Blood Specimens
Traditional Culture Methods
Initial Brucella hematogenous spread leads to organ seeding and focal infections, allowing isolation from various specimens like blood, bone marrow, genital exudates, bone tissue, synovial fluid, or CSF (32). Collect samples aseptically and transport to the lab promptly (within 1-2 hours). For longer transport, keep specimens moist and cooled (2-8°C) (98). Brucella grows well on standard clinical microbiology solid media like Trypticase soy agar with hemoglobin (blood agar) and chocolate agar. They do not grow on MacConkey agar, and selective media is generally unnecessary for human specimens. For optimal detection, incubate inoculated plates for up to 14 days in 5%-10% CO2-enriched atmosphere at 35°C aerobically. Seal plates, and perform all procedures in a class II biosafety cabinet (see “Brucella Cultures and Laboratory Safety” below).
In infected animals, Brucella can be isolated from vaginal secretions, placental and fetal tissues, milk, semen, and other samples often containing commensal flora and environmental bacteria/fungi (99). Rapidly growing contaminants can overgrow agar, hindering slow-growing Brucella detection. Veterinary labs use selective media like Farrel medium and modified Thayer-Martin medium (MTM) to inhibit contaminants (99). Farrel medium is not common in human clinical labs, but MTM agar is routinely used for Neisseria gonorrhoeae isolation. Unexpected B. melitensis growth on MTM from female genital specimens led to significant lab personnel exposure in an Israeli lab (100).
Blood Culture Methods for Other Specimens
Blood culture techniques, both manual and automated, have been used for Brucella isolation from pus (41, bone marrow (27, 93, 96, liver tissue (27, lymph nodes (27, synovial fluid (101, 102, testicular aspirates (42), pancreatic exudates (79, and CSF (35. Automated blood culture systems offer continuous monitoring, labor and time savings, and safety. Results are generally comparable to or better than solid media cultures, with shorter detection times. Synovial fluid in aerobic Peds Plus bottles in Bactec 9240 for 4 weeks yielded B. melitensis in 15 vials, with 14 detected by automated reading within 3-7 days (101). One culture with only 1.3 CFU/ml showed nonsignificant CO2 readings (102. Akcam et al. compared aerobic pediatric bottles in Bactec 9240 (1-week monitoring) to solid media for sterile fluids other than blood (41). Automated monitoring detected all 5 B. melitensis-positive specimens missed by conventional cultures (41).
From Detection to Identification
Conventional Methods
Prompt and accurate Brucella identification from blood culture bottles or agar plates is critical for timely diagnosis and preventing lab personnel infection. When Brucella isolation is suspected clinically, epidemiologically, or based on isolate characteristics, strict safety measures are essential (see “Brucella Cultures and Laboratory Safety” below).
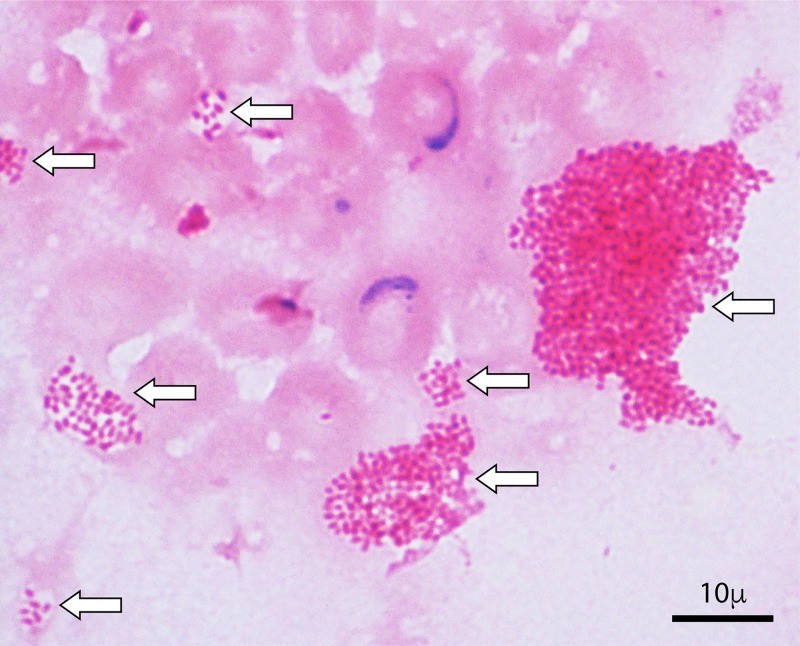
Initial identification involves Gram staining. Brucella typically appears as very small, faintly staining Gram-negative coccobacilli resembling fine sand, possibly as microcolonies in liquid media (Fig. 2). Except with Castañeda method, positive broth should be streaked for isolation on solid media. MacConkey agar inclusion is recommended, as Brucella‘s inability to grow on it is distinctive. After 2-4 days, punctate Brucella colonies may appear, developing into small (0.5-1 mm), convex, nonpigmented, nonhemolytic colonies with entire edges. Presumptive identification is based on Gram stain, capnophilia, positive oxidase, catalase, and urease, no sugar fermentation, and non-motility.
FIG 2. Gram Stain of Brucella melitensis Microcolonies.
Open in a new tab
Figure 2: Microscopic view of a Gram stain from a positive aerobic Bactec blood culture vial, revealing Brucella melitensis microcolonies, indicated by arrows. These microcolonies are a characteristic feature in Brucella-positive blood cultures.
Phenotypic Brucella identification is slow and exposes lab technicians to a highly infectious bacterium. Commercial systems can misidentify Brucella as Ochrobactrum anthropi (103, 104), Ochrobactrum intermedium (105), Haemophilus influenzae (106), Bergeyella zoohelcum (107), Bordetella bronchiseptica (108), or Psychrobacter phenylpyruvicus (109), leading to lab-acquired outbreak (110). Presumptive Brucella identification needs molecular confirmation (see Nucleic Acid Amplification Tests below) or positive slide agglutination with specific anti-O-LPS antiserum. However, due to O-LPS sharing with other Gram-negatives, serodiagnostics should only be used after phenotypic criteria are met (Gram stain, biochemical profile) and never as a shortcut for uncharacterized isolates. Smooth Brucella spp. can dissociate in culture into smooth and rough colonies; rough mutants lack O-polysaccharide and won’t agglutinate with regular antiserum, requiring anti-rough LPS reagent.
For rapid Brucella presumptive identification (or exclusion) from blood isolates, Rich et al. proposed subculturing positive Bactec 9240 broth on urea slants (111). In 33 vials with Gram-negative coccobacilli and 32 without organisms, 84.1% of 44 Brucella-growing slants showed urease activity within 4 hours, and the rest overnight. Only two Haemophilus influenzae blood cultures showed delayed positive urease, demonstrating good specificity. Maleknejad et al. used acridine orange and Gram stains of positive broth and urea slant inoculation (112). The test was positive within 4 hours in all 41 Brucella cultures and negative in 61 with other bacteria.
Brucella species-level identification is epidemiologically important due to species-host associations (2). Conventional phenotypic species identification for human pathogenic Brucella is summarized in Table 2, while molecular methods are discussed in Nucleic Acid Amplification Tests below.
TABLE 2. Phenotypic Features of Human Pathogenic Brucella Species
| Species | Dye Growth at Routine Dilution | H2S Production | Urease Test (Max Positivity Time)a | Lysis with Phage: |
|---|---|---|---|---|
| Fuchsin | Thionine | Safranin | Tb | |
| B. melitensis | Yes | Yes | Yes | No |
| B. abortus | Yesb | No | Yes | Yesc |
| B. suis | Nod | Yes | No | No |
| B. canis | Variable | Yes | No | No |
| Marine speciese | Yes | Yes | Yes | No |
Open in a new tab
Table 2: Differentiating Brucella species pathogenic to humans based on phenotypic characteristics. The table outlines key tests like dye tolerance, H2S production, urease activity, and phage lysis patterns, which are crucial for species-level identification.
aMany strains show lack of correlation with species.
bExcept biotype 2.
cExcept biotype 5.
dExcept biotype 3.
eB. pinnipedialis and B. ceti.
fLysis occurs in a few strains of B. pinnipedialis.
In 1992, Wong et al. used the Biolog microtiter plate system (Biolog) for Brucella species identification (113). This method uses differential oxidation of carbon source substrates, reducing a tetrazolium dye and producing a color reaction. After 24 hours at 35°C in 5% CO2, it unambiguously identified B. melitensis, B. abortus, and B. suis (113). Despite species-level identification, it wasn’t widely adopted, likely due to aerosol generation (113).
A miniaturized semiautomated system (Taxa Profile) using 570 metabolic reactions was evaluated for Brucella identification and speciation (2), revealing high biodiversity within Brucella. 196 reactions were stable across cultures of the same strain and reliably discriminated between 23 reference Brucella strains, also differentiating Brucella from Ochrobactrum. Based on consistent species/biovar-specific reactions, a 96-well plate (Micronaut; Merlin Diagnostika GmbH) was designed and tested with 113 Brucella isolates and related organisms. While Brucella species and biovars showed distinct metabolic patterns, Micronaut could not separate B. canis from B. suis biovar 3, and B. melitensis isolates were too homogeneous for biovar resolution. The system is reagent-free, has user-friendly software, and can detect novel Brucella species/biovars (114). However, its discriminatory power exceeds routine clinical lab needs as Brucella subtyping is not needed for therapy. Micronaut is better suited for referral labs, replacing or complementing time-consuming tube tests, especially for atypical Brucella strains (13).
MALDI-TOF Technology
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF) significantly changed microbial identification in clinical labs. MALDI-TOF provides rapid, accurate, reproducible, and cost-effective species-level identification, replacing tedious biochemical tests. Its simplicity makes it suitable for busy labs with less-skilled technicians (115, 116).
MALDI-TOF can be used directly on bacterial colonies or positive blood culture broth (117. To avoid Brucella exposure, bacterial inactivation with ethanol is typically followed by protein extraction with formic acid and acetonitrile (118–120).
Early MALDI-TOF evaluations for Brucella were inconclusive. Some studies showed precise genus-level identification of ATCC Brucella type strains in simulated blood cultures, species-level identification, and even B. suis biovar differentiation (115, 116. However, Vitek MS (bioMérieux) misidentified B. melitensis as O. anthropi (121. Bruker systems (Bruker Daltonics) showed unreliable Brucella species discrimination, suggesting the protein profile did not accurately reflect genetic evolution (117, 120). A recent study improved Vitek MS reference database using 590 proteomic spectra from 84 Brucella strains, including rare isolates (122). This modified database clearly differentiated Brucella from Ochrobactrum and precisely identified major zoonotic species: B. melitensis, B. abortus, and B. suis. Independent validation with diverse wild-type strains is needed. MALDI-TOF equipment remains expensive and inaccessible in most brucellosis-endemic countries, limiting data on its performance despite low per-identification cost.
Brucella Cultures and Laboratory Safety
Brucella is the most common cause of laboratory-acquired infections, accounting for 2% of global human brucellosis cases (122). Genus-specific features facilitate lab transmission: low infectious dose (101-102 cells); multiple entry routes relevant to lab work (respiratory mucosa, conjunctivae, GI tract, broken skin) (123); viability on surfaces for weeks or months (123, 124; and aerosol generation during manual procedures.
Brucella can infect any organ or tissue, so diverse clinical specimens may contain viable brucellae, with blood cultures being the most common. Low Brucella bacteremia levels (63) mean blood samples pose lower contagion risk unless safety protocols are severely breached. Modern blood culture instruments monitor growth without vial piercing, reducing aerosolization. However, contagion risk rises exponentially during and after solid and liquid media incubation. Tissue homogenization, centrifugation, vortexing, subculturing, and biochemical testing can disperse bacteria, contaminate the lab, and cause unintentional worker transmission (125). The catalase test, strongly positive in Brucella, is particularly hazardous due to bubbling and aerosolization.
In endemic regions, high Brucella-positive culture numbers increase lab transmission risk. In a Turkish lab processing ~400 Brucella cultures annually, 18% of workers were infected, an 8% annual risk per employee (126). Two studies at Soroka University Medical Center (SUMC) in southern Israel (B. melitensis endemic area) found Brucella in 3.2% of 3,974 positive aerobic Bactec bottles and 8.7% of 126 Isolator cultures in 1997 (127, and in 2.5% of 20,620 positive Bactec vials from 2002-2009 (128). Positive culture prevalence was significantly higher from April-September (3.3%) than October-March (0.9%) (P < 0.001) (128).
While CDC recommends class II biosafety cabinets for all live Brucella manipulations (129), incautious media handling often occurs before Brucella suspicion/confirmation, potentially exposing technicians.
Due to nonspecific brucellosis symptoms, clinicians often miss the diagnosis, failing to alert labs to potential Brucella presence, increasing technician exposure risk. Early recognition and correct Brucella identification are lab responsibilities. Small Gram-negative coccobacilli growing on blood and chocolate agar but not MacConkey agar should be processed cautiously in biosafety cabinets, following a “rule-out-or-refer” policy with basic biochemical testing under strict safety.
Following a 1997 SUMC lab-acquired brucellosis outbreak (7 cases), a strict infection control policy was implemented (127). All positive blood culture vials are processed in safety cabinets until Brucella is ruled out. Isolator tube use for suspected brucellosis, routine antibiotic susceptibility testing of Brucella, and aerosol-generating procedures were discontinued. Since these enhanced safety practices, no lab staff infections have occurred in over two decades, despite increasing isolations (128). In brucellosis-endemic areas, processing all positive blood culture vials in safety cabinets initially, pending organism identification, is prudent. Presumptively identified Ochrobactrum, Psychrobacter phenylpyruvicus, Bordetella bronchiseptica, or Bergeyella zoohelcum isolates should be similarly managed until Brucella is excluded.
Conclusions
Despite long-standing serological tests and sensitive molecular assays, isolating the causative organism remains clinically and epidemiologically relevant in brucella laboratory diagnosis. Historically, slow Brucella growth hampered recovery, requiring prolonged vial incubation and blind subcultures or Castañeda flasks/lysis-centrifugation. Automated blood culture systems monitoring CO2 production have improved sensitivity and shortened detection times in the last four decades. Currently, over 95% of acute brucellosis blood cultures detect the organism within 1 week without subcultures. Prolonged incubation and blind subcultures are still needed for longer-duration or focal infections. MALDI-TOF and nucleic acid tests enable rapid, precise, and safe Brucella identification and species determination.