Cardiac masses, a diagnostic challenge frequently encountered in cardiology, encompass a spectrum from benign to malignant primary tumors and secondary metastatic lesions. Echocardiography often serves as the initial and crucial imaging modality for detection. However, differentiating these masses and formulating an effective therapeutic strategy necessitates a comprehensive approach. Malignant cardiac tumors, in particular, carry a grave prognosis, underscoring the importance of accurate and timely diagnosis to facilitate appropriate interventions and potentially improve patient outcomes. This article delves into the echocardiographic characteristics of both benign and malignant cardiac tumors, aiming to guide differential diagnosis and inform subsequent diagnostic and therapeutic decisions.
Keywords: cardiac masses, cardiac tumors, differential diagnosis, echocardiography, multimodality imaging, cardiac tumor diagnosis
Abbreviations
CDI: Color Doppler imaging
CT: Computed tomography
HF: Heterogeneity factor
MRI: Magnetic resonance imaging
PET: Positron emission tomography
SDI: Spectral Doppler imaging
SUVmax: Maximum standardized uptake
TOE: Transesophageal echocardiography
TTE: Transthoracic echocardiography
UEA: Ultrasound enhancement agents
2D: Two-dimensional
3D: Three-dimensional
1. Introduction
The occurrence of cardiac tumors is rare, with secondary tumors being more frequent than primary ones. Primary cardiac tumors are exceedingly rare. Malignant cardiac tumors historically portend a poor prognosis. However, advancements in surgical and medical treatments have led to improved outcomes for certain primary malignant tumors in recent years.
Secondary cardiac tumors commonly manifest as pericardial metastases, often originating from carcinomas such as lung or breast cancer, or melanoma. Myocardial metastases can arise from solid tumors or hematological malignancies. Intracavitary metastases or direct extensions may originate from gynecologic, urological, or lung cancers. Primary cardiac tumors are classified as benign or malignant, with some histotypes capable of presenting as either benign masses or malignant transformations (Table 1). Certain tumors, including inflammatory myofibroblastic tumors, paragangliomas, and some germ cell tumors, exhibit intermediate or unpredictable clinical behavior. The complexity of cardiac tumor classification is further compounded by the potential for diverse histological components within a single mass.
TABLE 1. Simplified Classification of Common Benign and Malignant Cardiac Tumors
| Benign | Malignant | |
|---|---|---|
| Myxoma | >>>>> | Myxosarcoma |
| Rhabdomyoma | >>>>> | Rhabdomyosarcoma |
| Angiomas | >>>>> | Angiosarcomas |
| Fibromas | >>>>> | Fibrosarcoma |
| Lipoma | >>>>> | Liposarcoma |
| Schwannoma | >>>>> | Malignant peripheral nerve sheath tumor (MPNST) |
| Cystic tumor of the AV node | Other sarcomas (myxofibro, leiomyo, undifferentiated pleomorphic, synovial) | |
| Papillary fibroelastoma | Lymphomas | |
| Metastatic tumors | ||
| Mesothelioma | ||
| Intermediate/Uncertain Behavior | ||
| Paraganglioma | ||
| Inflammatory myofibroblastic tumor | ||
| Germ cell tumors | ||
| Mature teratoma | >>>>> | Immature teratoma |
| Yolk sac tumor |
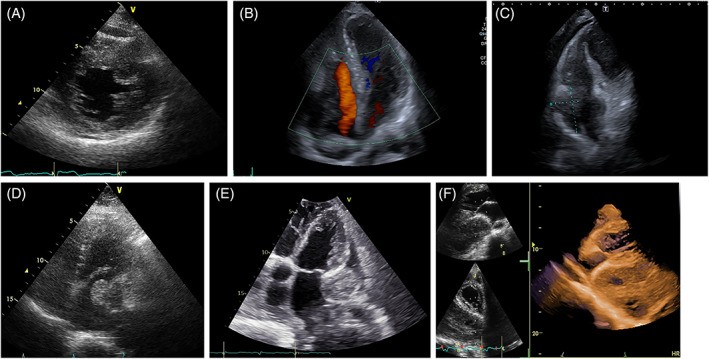
Benign cardiac tumors may be surgically removed or managed conservatively with follow-up if they are hemodynamically stable and pose no embolic risk. Notably, some tumors, like rhabdomyomas, can spontaneously regress, often justifying a watchful waiting approach. However, even benign tumors can induce clinical symptoms due to hemodynamic compromise (valvular obstruction, vessel or chamber compression), arrhythmias, or embolization, necessitating medical or surgical intervention. Rhabdomyomas have been associated with atrial or ventricular arrhythmias in a significant proportion of cases (16%–47%). Interestingly, rhabdomyoma cells share structural similarities with Purkinje cells and can function as accessory pathways, potentially causing pre-excitation syndromes that resolve with tumor regression. Hematological neoplasms, whether primary (like some lymphomas) or metastatic, are primarily treated with chemotherapy, with surgery rarely indicated. Similarly, targeted therapy and/or immunotherapy are the standard approaches for metastatic melanomas. In contrast, primary cardiac sarcomas are typically managed surgically, often in conjunction with chemotherapy or radiotherapy. Aggressive surgical resection and multimodality therapy are crucial for improving survival in these cases.
In patients with known systemic malignancies presenting with a cardiac mass, differentiating between a thrombus and metastasis is critical for therapeutic planning and prognostic assessment.
Therefore, accurate differential diagnosis is paramount for guiding optimal therapy, often requiring a multimodality imaging strategy.
2. General Diagnostic Approach to Cardiac Masses
The initial step in diagnosing a cardiac mass involves distinguishing it from other intracardiac entities such as thrombi or vegetations. A systematic four-step approach is recommended to guide the diagnostic process.
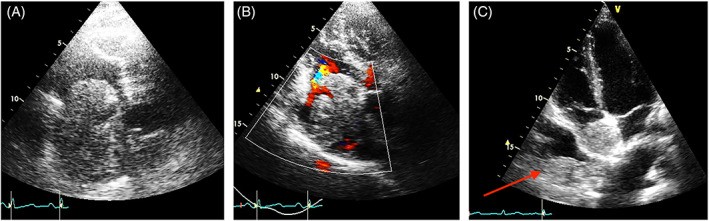
FIGURE 1. Differentiating Tumor Thrombus from Metastatic Cardiac Mass
Alt Text: Echocardiogram comparison illustrating the differential diagnosis of cardiac masses. Panel A and B show a tumor thrombus extending from the inferior vena cava into the right atrium, visualized using 2D and color Doppler TTE. Panel C demonstrates a metastatic sarcoma in the right atrium, distinct from the vena cava, also seen on 2D TTE, highlighting the similar echogenicity between the cardiac mass and an intrapleural metastasis.
TABLE 2. Clinicopathologic Features of Primary Heart Tumors
| Histologic Type | Age | Site in Heart | Multiplicity | Syndromic Association |
|---|---|---|---|---|
| Fetuses/Infants | Children/Adults | Layer | Location | |
| Benign Congenital Tumors | ||||
| Rhabdomyoma | ++ | + | Myocardium | Ventricles |
| Fibroma | + | ++ | Myocardium | Ventricle, Septum |
| Histiocytoid Cardiomyopathy | ++ | +/1 | Endocardium, Myocardium | Ventricles, Atria, Nodes |
| Benign Acquired Tumors | ||||
| Myxoma | +/− | ++ | Endocardium | LA, Septum |
| Papillary Fibroelastoma | ++ | Endocardium | Valves > Atria > Ventricles | |
| Hemangioma | + | + | Myocardium, Endocardium | Atria > Ventricles |
| Lipomatous Hypertrophy | ++ | Myocardium of Atrial Septum | ||
| Lipoma | ++ | Myocardium, Epicardium, Endocardium | All Sites | |
| Inflammatory Myofibroblastic Tumor | ++ | + | Endocardium | Valves > Atria |
| Germ Cell Tumors | ||||
| Teratoma | ++ | + | Pericardial Cavity | No |
| Yolk Sac Tumor | ++ | + | Pericardial Cavity | No |
| Malignant Tumors | ||||
| Angiosarcoma | +/− | ++ | All Layers | RA, Pericardium |
| UPS/Myxofibrosarcoma | +/− | +/− | Endocardium | LA, Other Sites |
| Rhabdomyosarcoma | +/− | ++ | Myocardium | Ventricles |
| Leiomyosarcoma | +/− | ++ | Endocardium | LA |
| Lymphoma | +/− | ++ | Myocardium | RA, Others |
Abbreviations: AV, atrioventricular; LA, left atrium; RA, right atrium; UPS, undifferentiated pleomorphic sarcoma; SA, sinoatrial.
The diagnostic approach involves:
- Clinical Context: Evaluate patient history, including pre-existing systemic malignancies or conditions predisposing to thrombus formation (e.g., atrial fibrillation).
- Location and Site of Attachment: Determine the cardiac chamber involved and the mass’s attachment point (e.g., atrial septum, ventricular wall, valve).
- Morphological and Functional Characteristics: Assess size, shape, mobility, tissue characterization, vascularity, and metabolic activity.
- Hemodynamic Impact: Evaluate the mass’s effect on cardiac function, such as valve obstruction or regurgitation.
Points 3 and 4 are investigated using various imaging modalities: echocardiography, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT). Each technique offers unique advantages and limitations regarding cost, availability, and diagnostic accuracy. Often, a combination of modalities is necessary for a comprehensive evaluation.
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TOE), due to their accessibility and affordability, are typically the initial imaging tests for suspected cardiac tumors.
This article focuses on the echocardiographic features relevant to the differential diagnosis of cardiac masses.
3. Echocardiographic Approach to Cardiac Mass Evaluation
A comprehensive echocardiographic examination, utilizing TTE and TOE modalities, is crucial for characterizing cardiac masses. This includes employing two-dimensional (2D), three-dimensional (3D), Color Doppler imaging (CDI), Spectral Doppler imaging (SDI), and advanced techniques like agitated saline and ultrasound enhancement agents (UEAs). TOE-2D, TOE-3D, and intracardiac ultrasound also play a role in guiding biopsies for histological confirmation.
The inherent contrast between the cardiac mass and the blood within the cardiac chambers or vessels facilitates visualization of intracavitary tumors. Color Doppler imaging can enhance the delineation of tumor borders, particularly for masses with low intrinsic echogenicity (Figure 5).
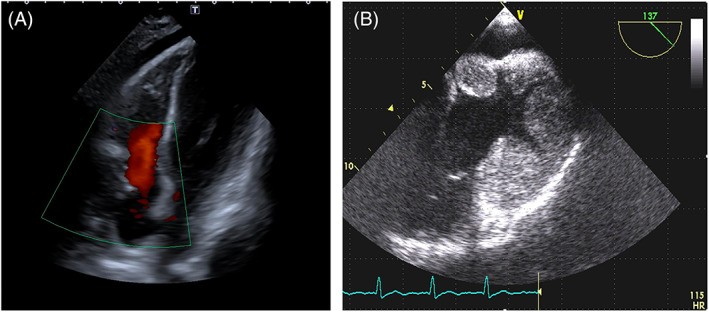
FIGURE 2. Papillary Fibroelastoma vs. Angiosarcoma: Echocardiographic Differentiation
Papillary fibroelastoma of the aortic valve vs. Right atrium angiosarcoma
Alt Text: Echocardiographic comparison of benign and malignant cardiac tumors. Panel A shows a papillary fibroelastoma on the aortic valve with a thin stalk, imaged by 2D TTE in diastole. Panel B displays a right atrial angiosarcoma with broad attachment to the atrial wall and infiltration of the tricuspid annulus, visualized by 2D TTE, illustrating the lobulated surface of the malignant mass.
FIGURE 3. Angiosarcoma: Echocardiographic Views of Right Heart Infiltration
Alt Text: Echocardiographic images of angiosarcoma cases demonstrating right heart involvement. Panel A is a 2D TTE apical four-chamber view of a large mass infiltrating the right atrium, right ventricle, and pericardium. Panel B is a 2D TOE view focused on the right heart chambers, showing the angiosarcoma infiltrating the interatrial septum and right atrial walls.
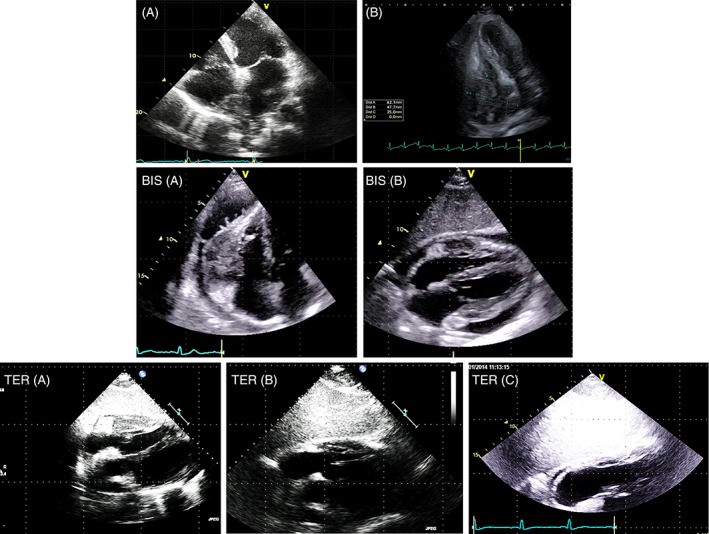
FIGURE 4. Cardiac Lymphoma: Echocardiographic Presentation and Chemotherapy Response
Alt Text: Echocardiographic series illustrating cardiac lymphoma and treatment response. Panels A and B show 2D TTE apical four-chamber views of lymphoma infiltrating the interatrial septum, right atrium, superior vena cava, and right ventricular walls before treatment. Panels BIS A and B are 2D TTE subcostal views showing pericardial lymphoma. Panels TER A, B, and C demonstrate the lymphoma regression over two months of chemotherapy, with panel C showing no recognizable tumor after treatment.
Cardiac lymphomas can affect any cardiac chamber or the pericardial space and are characterized by rapid growth (Figure 4). Myxomas, while typically located in the left atrium attached to the fossa ovalis, can arise from atypical locations, including the inferior atrial septum, lateral left atrial wall, or even within the ventricles.
The systematic echocardiographic assessment of cardiac tumors, regardless of etiology (primary/metastatic, benign/malignant), aims to define morphology, dynamics, hemodynamic consequences, and guide therapeutic interventions.
4. Tumor Size Assessment by Echocardiography
Cardiac tumors exhibit a wide range of sizes, from millimeters to centimeters. Transthoracic echocardiography often provides accurate size estimation.
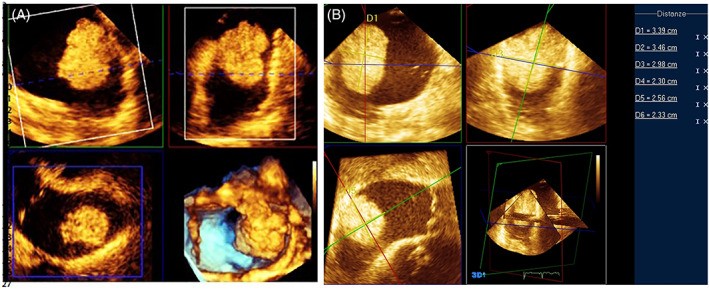
Studies, such as Goswami’s series of 70 patients with 73 cardiac myxomas, reported sizes ranging from 2.0 to 9.5 cm in maximum diameter measured by 2D echocardiography, with good correlation to surgical measurements. However, myxomas and other soft, mobile tumors can deform and elongate during prolapse, potentially altering size assessment. Irregularly shaped tumors may also lead to inaccurate diameter measurements with 2D echocardiography. Three-dimensional echocardiography, particularly full-volume imaging, offers improved visualization of the entire tumor volume, enabling linear measurements along orthogonal planes and volumetric assessments (Figure 7).
FIGURE 5. Color Doppler Enhancement of Low Echogenicity Left Atrial Sarcoma
Alt Text: Echocardiographic images demonstrating color Doppler’s role in defining low-echogenicity cardiac masses. The images show a left atrial sarcoma in a patient with a mitral bioprosthetic valve, pre- and post-chemotherapy. Color Doppler in the pre-chemotherapy images (left panels) helps delineate the tumor contour, while post-chemotherapy images (right panels) show reduced tumor size and increased blood flow within the left atrium.
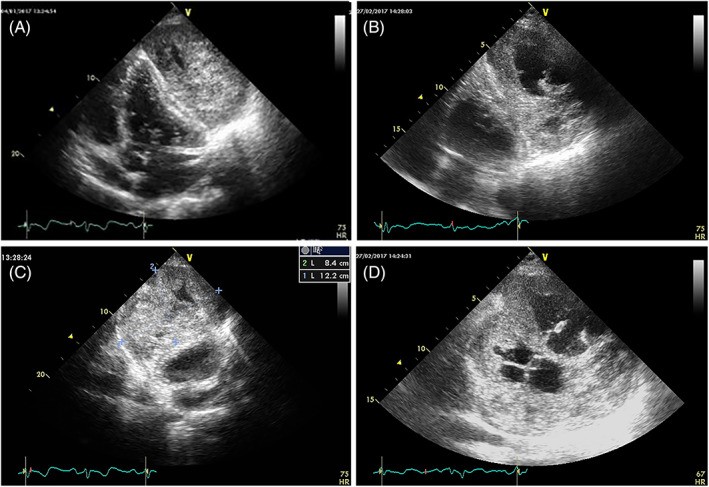
FIGURE 6. Size Variability in Left Atrial Myxomas: Echocardiographic Examples
Echocardiographic examples showing varying sizes of left atrial myxomas
Alt Text: Echocardiographic comparison illustrating size variability in left atrial myxomas. The 2D TTE apical four-chamber views display left atrial myxomas of different sizes in six patients, highlighting the range in tumor dimensions.
FIGURE 7. 3D Echocardiography for Accurate Tumor Measurement
Alt Text: 3D TOE imaging for precise cardiac tumor assessment. Panel A shows a multiplanar reconstruction of a cardiac mass using 3D TOE. Panel B demonstrates linear measurements taken along orthogonal planes using 3D TOE, enabling accurate tumor size determination.
5. Location and Attachment Site in Differential Diagnosis
The site of attachment provides valuable clues for Cardiac Mass Differential Diagnosis. Left atrial myxomas typically attach to the fossa ovalis region of the atrial septum (Figure 8). Right atrial angiosarcomas can arise from any part of the right atrial wall (Figures 2B, 3, and 9). While myxomas are commonly found at the fossa ovalis, atypical locations such as the inferior atrial septum or lateral left atrial wall are also possible (Figure 10).
Tumor attachment can be pedunculated (stalk-like) or sessile (broad-based). Papillary fibroelastomas typically have a narrow stalk (Figure 11), whereas myxoma stalk width varies (Figures 6, 7, 8, and 10). Determining the attachment site can be challenging with TTE alone, and TOE is often necessary for clearer visualization, especially of the stalk. 3D TOE further enhances localization of stalk attachment and its relationship to surrounding structures (Figure 7, Videos 2 and 3). Sessile tumors, particularly malignant ones, have a broad base and may infiltrate adjacent cardiac structures (Figures 2B, 3, 4, 5, and 9).
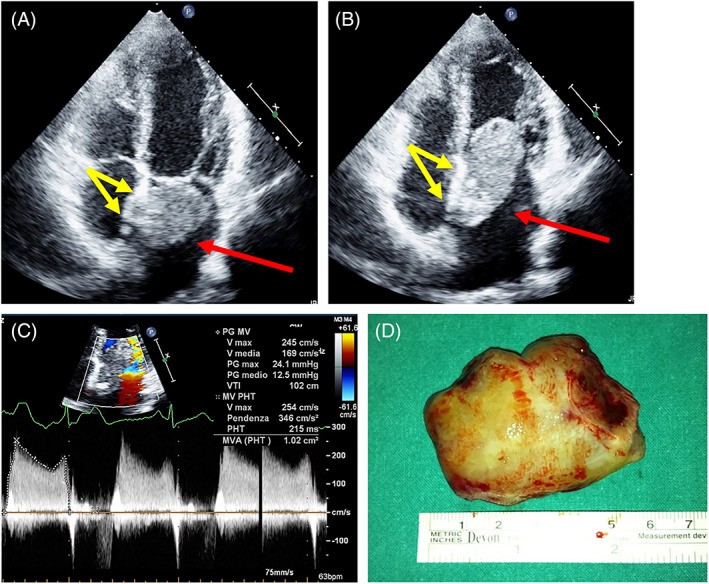
FIGURE 8. Typical Myxoma Attachment at Fossa Ovalis
Alt Text: Echocardiographic and surgical correlation of a typical left atrial myxoma. The top panels show 2D TTE apical four-chamber views in systole (A) and diastole (B) with red arrows indicating the myxoma and yellow arrows highlighting the fossa ovalis attachment site. The bottom panel (C) shows the surgical specimen of the myxoma.
FIGURE 9. Right Atrial Angiosarcoma: TOE Imaging
TOE images of right atrial angiosarcoma
Alt Text: TOE imaging of right atrial angiosarcoma. 2D TOE views focused on the right heart chambers in systole reveal an irregular, heterogeneous giant mass infiltrating the right atrial free wall and tricuspid annulus, protruding into the right atrium.
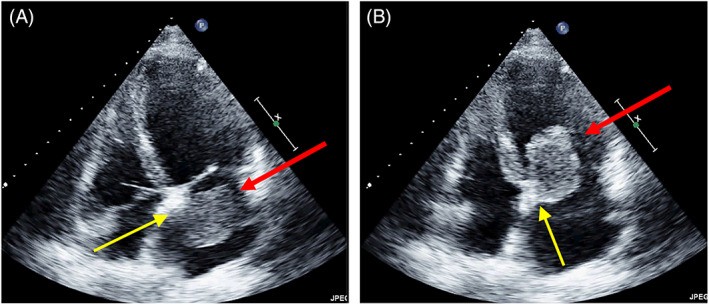
FIGURE 10. Atypical Myxoma Location: Below Fossa Ovalis
Alt Text: Echocardiographic visualization of an atypical myxoma attachment site. 2D TOE apical four-chamber views in systole (A) and diastole (B) show a left atrial myxoma attached to the atrial septum below the fossa ovalis (unusual site), with red arrows indicating the mass and yellow arrows pointing to the stalk.
FIGURE 11. Papillary Fibroelastoma: Stalk and Surgical Specimen
TOE and surgical specimen of aortic valve papillary fibroelastoma
Alt Text: TOE and surgical correlation of aortic papillary fibroelastoma. The top panel is a TOE long-axis view showing the fibroelastoma with a thin stalk on the aortic valve. The bottom panel displays the surgical specimen, confirming the tumor’s morphology.
6. Shape and Surface Morphology of Cardiac Masses
Tumor shape, as visualized by 2D and 3D echocardiography, is influenced by size, consistency, mobility, and the size of the cardiac chamber. Benign primary tumors typically exhibit regular, rounded shapes. Atrial myxomas show the greatest shape variability. Encapsulated myxomas are round or ovoid with smooth surfaces. Papillary and gelatinous myxomas, lacking encapsulation, have irregular, multilobulated surfaces, associated with a higher risk of embolization due to surface fragmentation. Myxoma shape can elongate when prolapsing into a ventricle.
Papillary fibroelastomas are club-shaped with well-defined heads and peduncles, rarely strand-like, and typically have smooth surfaces (Figure 11). Intramural tumors within ventricular walls are generally ovoid. Primary malignant tumors are usually broad-based, arising within a cardiac chamber with multilobulated or polypoid shapes. Metastatic tumor shapes vary depending on invasion mechanism and site (pericardial, intramyocardial, intracavitary).
7. Mobility as a Diagnostic Feature
Tumor mobility depends on location, attachment site, stalk characteristics (if present), size, and tissue properties. Intramyocardial or intrapericardial tumors move with the heart but lack intrinsic mobility, potentially restricting cardiac motion (Figure 12, Video 4).
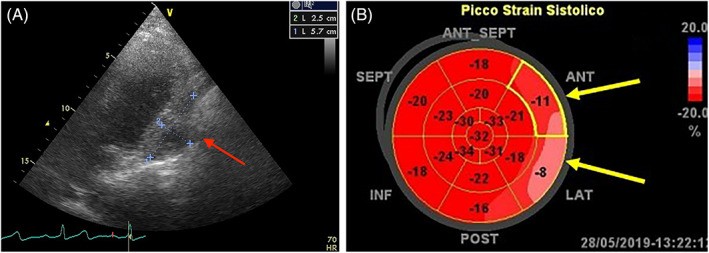
FIGURE 12. Sarcoma Infiltration and Reduced Myocardial Strain
Alt Text: Echocardiographic and strain imaging of pericardial sarcoma. Panel A shows a sarcoma infiltrating the pericardium and left ventricular lateral wall. Panel B demonstrates global longitudinal strain imaging, revealing reduced strain values at the infiltrated walls.
Intracavitary tumors may exhibit high mobility if pedunculated or with prolapsing extensions (Video 5). Papillary fibroelastomas are highly mobile, often fluttering as they prolapse. Myxoma mobility varies significantly, from immobile (broad-based, encapsulated, solid) to hypermobile (narrow-stalked, gelatinous, polypoid) during prolapse (Video 6).
Distinguishing the origin of atrial or valvular masses can be challenging with TTE. 2D and 3D TOE aid in identifying attachment location, insertion type (stalked vs. sessile), surrounding relationships, and mass characteristics, including mobility and surface vibration. These factors are crucial for therapeutic decisions, as tumors with thin stalks and irregular/vibrating surfaces have a higher embolic risk.
8. Tissue Characterization by Echocardiography
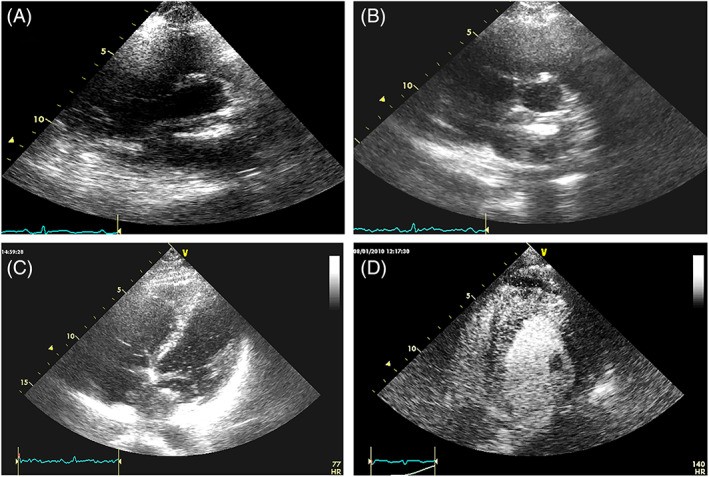
Echocardiographic tissue characterization, based on 2D-3D intra-mass appearance and echogenicity, provides preliminary information about tumor composition. Rhabdomyomas, rhabdomyosarcomas, and leiomyosarcomas typically show homogeneous echogenicity, with hyperechoic or hypoechoic central areas potentially indicating necrosis or calcification. Lipomas can appear hypo- to hyperechoic but are usually homogeneous. Fibromas generally exhibit increased echogenicity compared to normal myocardium. Hemangiomas, angiosarcomas, and lymphomas often show inhomogeneous echogenicity with scattered echolucent areas (Figure 13).
FIGURE 13. Echogenicity Variations in Different Cardiac Tumors
Alt Text: Echocardiographic examples showing echogenicity variations in different cardiac tumors. Panel A: Neuroendocrine tumor infiltrating the interventricular septum with granular echogenicity. Panel B: Non-Hodgkin lymphoma infiltrating right chambers with mixed hyper- and hypoechoic areas. Panel C: Angiosarcoma with irregular echogenicity. Panel D: Myxosarcoma with hyperechoic chondroid areas. Panel E: Extraskeletal osteosarcoma with hyperechoic calcifications. Panel F: Pericardial sarcoma with hypoechoic necrotic areas.
Tumor echogenicity can change over time, particularly after chemotherapy or radiotherapy. Chemotherapy may induce necrosis, appearing as anechoic areas on echocardiography (Figure 14). Radiotherapy-induced necrosis can lead to inflammation and fibrosis, increasing echogenicity (Figures 14 and 15).
FIGURE 14. Echogenicity Changes Post-Chemotherapy in Leiomyosarcoma
Alt Text: Echocardiographic changes in pericardial leiomyosarcoma following chemotherapy. Pre-chemotherapy image (left) shows the tumor. Post-chemotherapy image (right) reveals large anechoic areas indicative of necrosis.
FIGURE 15. Echogenicity Changes During and Post-Radiotherapy in Leiomyosarcoma
Echocardiographic changes in leiomyosarcoma during and post-radiotherapy
Alt Text: Echocardiographic changes in left ventricular leiomyosarcoma during and after radiotherapy. The series shows pre-radiotherapy homogeneous low echogenicity (left), mixed anechoic and echoic areas during radiotherapy (middle), and uniform high echogenicity post-radiotherapy (right).
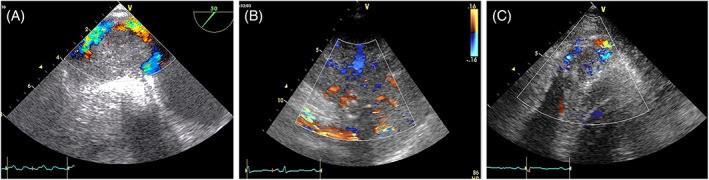
Vascularity, particularly in angiomas and angiosarcomas, can be assessed with color Doppler imaging, optimized for venous flow detection (Figure 16, Video 7). Ultrasound-enhancing agents (UEAs) are increasingly used in tumor detection and characterization, extending beyond liver tumors to various tumor types and lymph nodes.
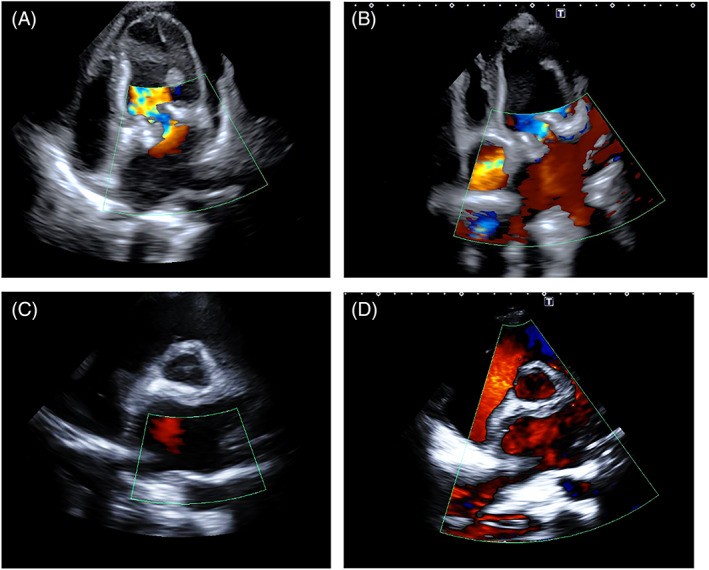
FIGURE 16. Hypervascularity of Angiosarcomas on Color Doppler
Alt Text: Color Doppler imaging illustrating hypervascularity in angiosarcoma. Panel A: TOE of a mass originating from the right atrium and inferior vena cava. Panel B: Parasternal long-axis view of a mass extending from the right atrium to the right ventricle. Panel C: Apical view of a mass infiltrating the right ventricular apex, all showing increased vascularity within the tumors.
Echocardiographic perfusion imaging with UEAs, using low mechanical index and intermittent high-mechanical index flashes, aids in characterizing tumor vascularity, differentiating hypervascular malignant tumors from hypovascular benign tumors and avascular thrombi. UEAs can improve tumor visualization and enhance CDI of abnormal tumor vessels (Video 7). Tissue characterization with UEAs involves qualitative and quantitative analysis of tumor enhancement compared to adjacent myocardium. Hyperenhancement, indicative of abnormal neovascularization in rapidly growing malignant tumors, is a hallmark of malignancy (Figure 17, Video 8). However, benign, highly vascular tumors like hemangiomas can also enhance. Stromal tumors like myxomas show partial enhancement due to poor vascularity, while avascular thrombi and fibroelastomas show no enhancement.
FIGURE 17. UEA Enhancement in Left Atrial Sarcoma and Lymphoma
Alt Text: UEA enhancement demonstrating tumor vascularity. Panel A: Standard TTE image of a poorly defined, low-echogenicity left atrial sarcoma. Panels B and C: Enhanced echogenicity of the sarcoma 5 minutes post-SonoVue injection. Panel D: Myocardial infiltration by non-Hodgkin lymphoma showing granular echogenicity shortly after SonoVue injection, indicating tumor vascularity.
9. Hemodynamic Impact of Cardiac Masses
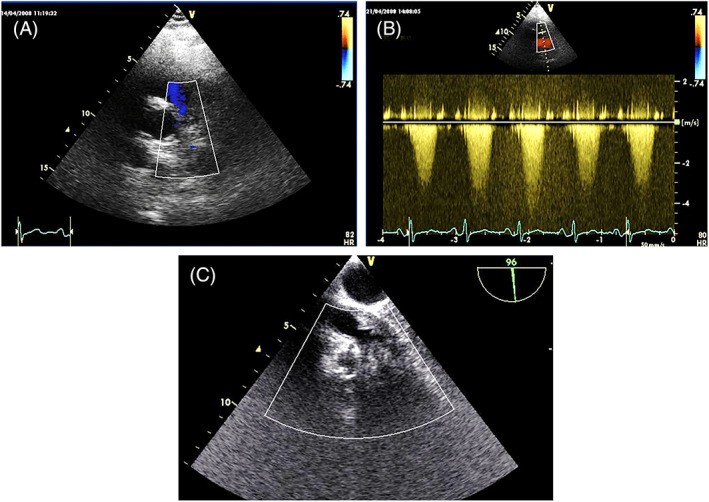
Atrial tumors are considered obstructive if the mitral or tricuspid valve area is reduced to less than 2 cm², calculated by pressure half-time (Figure 8). Obstruction occurs due to prolapsing masses reducing the atrioventricular orifice (Video 5). Rapidly growing sarcomas and lymphomas of the atria can also cause obstruction by infiltrating the mitral or tricuspid annulus. Pulmonary artery sarcomas frequently induce significant stenosis (Figure 18). Sarcomas extending from the atria can obstruct the vena cava or pulmonary veins (Figures 5 and 16). Valvular regurgitation is a rare complication of valvular fibroelastomas, caused by cusp traction. Extracardiac tumors, particularly large solid masses, can compress vessels or cardiac chambers.
FIGURE 18. Pulmonary Artery Sarcoma: Echocardiographic Views
Alt Text: Echocardiographic visualization of pulmonary artery sarcoma. Panel A shows a poorly echogenic mass causing pulmonary artery stenosis. Panel B demonstrates severe pulmonary artery stenosis due to the mass. Panel C shows improved mass definition using the TOE approach.
10. Integrating Echocardiography in the Clinical Framework
Echocardiography provides essential information for cardiac mass evaluation, including location, attachment, size, shape, tissue characteristics, mobility, spatial relationships, hemodynamic impact, and pericardial effusion. Its high temporal resolution is ideal for detecting small, mobile masses, especially valvular lesions. TTE is the primary diagnostic tool for suspected cardiac masses. However, advanced echocardiographic techniques are often needed for better characterization, particularly for intra-atrial masses. Misdiagnosis of malignant tumors as myxomas has been reported, highlighting the need for careful evaluation.
TOE-2D, TOE-3D, and UEAs are used based on clinical context and diagnostic needs. TOE-2D is crucial for precise assessment of atrial masses, pulmonary vein and superior vena cava relationships, pulmonary artery tumors, and valvular masses. TOE-3D offers incremental value by providing more accurate assessments of location, attachment, size, shape, tissue characterization, and adjacent structure relationships. Table 3 summarizes the utility of different echocardiographic modalities for cardiac tumor diagnosis.
TABLE 3. Comparison of Echocardiographic Modalities for Cardiac Tumor Diagnosis
| Feature | TTE-2D | TTE-3D | TOE-2D | TOE-3D | UEAs |
|---|---|---|---|---|---|
| Detection | +++ | + | ++++ | ++ | − |
| Location | +++ | ++ | ++++ | ++++ | + |
| Attachment | ++ | ++ | +++ | ++++ | − |
| Size | ++ | +++ | +++ | ++++ | − |
| Shape/Surface | ++ | ++ | +++ | ++++ | − |
| Mobility | +++ | +++ | ++++ | ++++ | |
| Tissue Characterization | ++ | ++ | +++ | ++ | ++++ |
| Hemodynamic Impact | +++ | ++ | +++ | ++ | − |
Note: Utility: – no, + fair, ++ good, +++ very good, ++++ very very good.
Echocardiography has limitations in determining mass origin (especially extra-cardiac origins or large masses), extent, adjacent structure relationships, and precise tissue characterization. Benign and malignant tumors can appear morphologically similar, particularly early on, requiring pathology for definitive diagnosis. These limitations can be partly overcome by other imaging techniques. PET/CT is valuable for differentiating benign from malignant masses (myxoma vs. myxosarcoma, etc.) based on metabolic activity using 18-Fluorodeoxyglucose (18FDG). High SUVmax and intratumoral glucose metabolic heterogeneity factor (HF) can distinguish malignant from benign masses and tumor thrombus from simple thrombus. Optimizing 18FDG PET/CT sensitivity and specificity for cardiac tumors requires minimizing myocardial glucose uptake through dietary modifications and heparin administration. Other tracers like 68Ga-DOTA-peptide are needed for neuroendocrine tumors and paragangliomas.
For ventricular tumors, especially those near coronary arteries, coronary angiography or CT angiography with cardiac synchronization and 3D reconstruction can better define mass size and coronary relationships. CT and MRI also aid in tissue characterization. However, extensive multimodality imaging can sometimes be avoided based on a diagnostic algorithm considering histology likelihood, patient age, tumor location, and echocardiographic features. Subsequent diagnostic steps should be tailored to the clinical setting (Tables 2 and 4).
TABLE 4. Preliminary Diagnostic Hypothesis Based on Clinical Presentation and Echocardiographic Features
| Intracavitary |
|---|
| (a) Left Atrium: Myxoma, Sarcoma, Lung Cancer Metastasis |
| – Interatrial septum, thin stalk: Likely Myxoma |
| – Other sites: Consider Sarcoma |
| – Broad insertion: Likely Sarcoma (Differential diagnosis: Thrombus) |
| – Continuity with pulmonary vein(s): Lung Carcinoma, Sarcoma, Intrathoracic Tumor |
| (b) Right Atrium, Right Ventricle: Angiosarcoma, Liver/Ovarian Metastasis, Lymphoma |
| – Atrial wall infiltration, intrapericardial extension: Angiosarcoma, Lymphoma |
| – Continuity with inferior vena cava: Liver/Gynecological Cancer (Differential diagnosis: Thrombus, Tumor Thrombosis) |
| – Continuity with superior vena cava: Likely Lymphoma |
| – Thin stalk, single mass: Consider Myxoma |
| (c) Left Ventricle: Leiomyosarcoma, Myxoma |
| (d) Valve: Papillary Fibroelastoma (Differential diagnosis: Endocarditis) |
| (e) Pulmonary Artery: Intimal Sarcoma |
| Intramural |
| 1. Fetuses, Newborns, Children, Teenagers: Rhabdomyoma, Fibroma, Angioma |
| – Adults with known systemic malignancy: Metastasis |
| – Adults without other diseases: Sarcoma, Fibroma, Angioma |
| Intrapericardial |
| 1. Adults with known systemic malignancy: Metastasis |
| – Signs of infiltration of right chambers: Angiosarcoma |
| – Diffuse mass encasing the heart: Angiosarcoma, Lung Carcinoma, Lymphoma |
11. Conclusions
Echocardiography is the cornerstone imaging modality for cardiac tumor evaluation. When integrated with clinical context, it guides diagnosis and helps identify patients requiring urgent surgery, non-neoplastic treatment, or watchful follow-up. However, other imaging techniques like CT, MRI, and PET/CT are often necessary to differentiate tumors from other masses and to plan therapy. Selecting the most appropriate imaging strategy should be based on clinical presentation and, in suspected malignancy, guided by a multidisciplinary team including cardiologists, oncologists, radiologists, nuclear medicine experts, and cardio-oncologists.
Supporting Information
Video S1 Videoclips
Click here for additional data file. (30.5MB, zip)
Pino PG, Moreo A, Lestuzzi C. Differential diagnosis of cardiac tumors: General consideration and echocardiographic approach. J Clin Ultrasound. 2022;50(8):1177‐1193. doi: 10.1002/jcu.23309
Data Availability Statement
This is a review, without experimental data to share.
References
[References will be listed here if extracted and formatted]
Associated Data
Supplementary Materials
Video S1 Videoclips
Click here for additional data file. (30.5MB, zip)
Data Availability Statement
This is a review, without experimental data to share.