Crimean-Congo hemorrhagic fever (CCHF) poses a significant global health threat as the most geographically widespread tick-borne viral disease. With mortality rates reaching up to 30%, accurate and timely diagnosis is crucial. Clinical diagnosis of CCHF is notoriously challenging due to its initial symptoms mimicking a range of other illnesses. This necessitates a strong reliance on laboratory diagnostics, especially for individuals residing in or traveling from CCHF-endemic regions where suspicion of the disease is raised. This article provides an in-depth guide to Cchf Diagnosis, detailing the essential laboratory tests and their application in effectively identifying CCHF in humans.
Understanding Crimean-Congo Hemorrhagic Fever
Crimean-Congo hemorrhagic fever virus (CCHFV) is an enveloped RNA virus belonging to the Orthonairovirus family within the Bunyavirales order. The primary mode of CCHF transmission to humans is through the bite of infected Hyalomma ticks. However, transmission can also occur via direct contact with bodily fluids from infected animals or humans, or through improperly sterilized medical equipment. A wide range of animals, including livestock and wildlife, can develop viremia after CCHFV exposure, acting as reservoirs for the virus. While most animals remain asymptomatic, certain species and experimental models can exhibit clinical signs. Detecting CCHFV in animals during the viremic stage is challenging due to its transient nature and the lack of symptoms in most animal hosts. In contrast, direct viral detection in Hyalomma ticks is feasible using various methods, including antigen testing and nucleic acid amplification techniques. Serological studies are the primary diagnostic tool for CCHFV surveillance in animal populations, revealing a significant seroprevalence globally, indicating widespread animal exposure to CCHFV.
CCHFV’s geographical reach extends across Africa, Asia, Eastern Europe, the Middle East, Russia, and even parts of Southern Europe. Annually, an estimated 10,000 to 15,000 CCHF cases occur, primarily in endemic countries. While travel-related cases are less frequent, they highlight the global implications of this disease. Seroprevalence studies in humans also indicate a notable level of exposure, particularly among high-risk groups such as those with frequent animal contact or exposure to tick habitats. The increasing seroprevalence trends in both humans and animals, coupled with the higher exposure rates in individuals with animal contact, underscore the potential for CCHFV to emerge as a significant zoonotic pathogen.
The CCHFV genome comprises three RNA segments (S, M, and L), each encoding crucial viral proteins. The virus exhibits substantial genetic diversity, categorized into seven clades based on S segment variations. These clades demonstrate geographical clustering, with distinct clades prevalent in Africa, Asia, and Europe. This genetic diversity presents challenges for diagnostic assay development, requiring tests to be robust enough to detect a broad spectrum of CCHFV strains for accurate CCHF diagnosis globally.
Clinical Presentation of CCHF: The Diagnostic Challenge
The majority of CCHFV infections are subclinical, meaning individuals are infected but do not develop noticeable symptoms. However, symptomatic CCHF progresses through distinct stages: incubation, prehemorrhagic, hemorrhagic, and convalescent. The incubation period can last from 1 to 13 days, during which the patient is asymptomatic but the virus is replicating. The prehemorrhagic stage marks the abrupt onset of nonspecific symptoms that are the major hurdle in clinical CCHF diagnosis. These early symptoms, including fever, chills, muscle aches, headache, dizziness, and gastrointestinal issues, closely mimic common bacterial, viral, and parasitic infections such as malaria and influenza. This lack of specific early indicators often leads to misdiagnosis and delayed treatment initiation. Studies have shown a high rate of initial misdiagnosis in CCHF patients, resulting in delayed hospitalization and poorer outcomes.
The hemorrhagic stage typically emerges 3 to 5 days after symptom onset, characterized by bleeding manifestations. These range from mild petechiae and ecchymosis to severe mucosal bleeding and internal hemorrhaging. Mortality in CCHF cases is significant, averaging around 30% and often occurring during the second week of illness. Survivors typically enter the convalescent stage after 9 to 10 days. Treatment for CCHF is primarily supportive, focusing on managing symptoms and complications. While the antiviral medication ribavirin is frequently used, its effectiveness remains debated. Other therapeutic interventions, such as steroids, convalescent plasma, and intravenous immunoglobulin, have been explored, but their efficacy is not yet definitively established due to limited evidence. Therefore, early and accurate CCHF diagnosis is paramount for effective patient management and improving outcomes.
Laboratory Diagnosis of CCHF: A Range of Essential Assays
Confirming CCHF diagnosis relies heavily on laboratory assays, which can be broadly categorized into viral detection and serological testing. The biosafety level (BSL) requirements for performing these tests vary depending on the assay type and national guidelines. Some countries mandate stringent BSL4 precautions for all CCHF diagnostic work, while others may allow BSL3 or BSL2 facilities, particularly when sample inactivation methods are employed. Inactivation procedures, such as heat treatment, irradiation, chemical fixatives, and specific buffer solutions, are critical for minimizing the risk of infection for laboratory personnel, especially when high containment facilities are not available. However, the chosen inactivation method must be compatible with the intended diagnostic assay to avoid compromising test accuracy.
The choice of diagnostic assay for CCHF diagnosis depends largely on the time elapsed since symptom onset. Direct viral detection methods are most effective in the early stages of the illness when viremia is present, while serological assays become more relevant later as the body mounts an antibody response. A crucial consideration in CCHF diagnosis is the genetic diversity of CCHFV. Assays must be designed or selected to ensure they can reliably detect the diverse strains circulating in the suspected region of exposure.
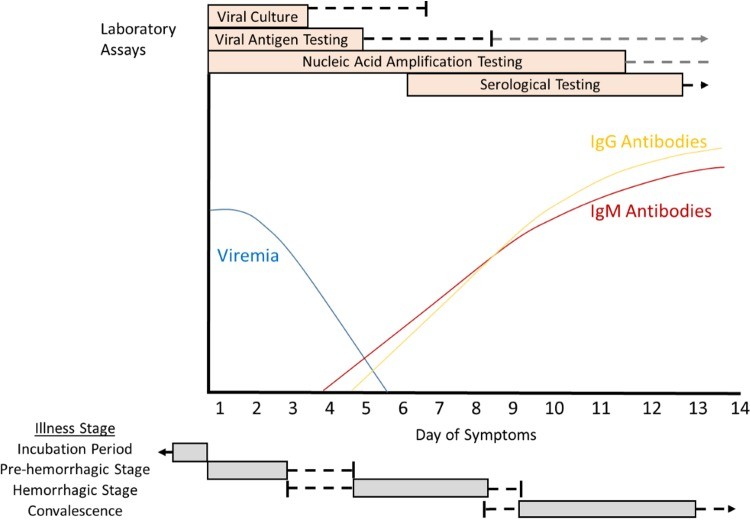 Cartoon overview of diagnostic testing for acute illness due to Crimean-Congo hemorrhagic fever virus in a non-fatal human infection
Cartoon overview of diagnostic testing for acute illness due to Crimean-Congo hemorrhagic fever virus in a non-fatal human infection
Figure 1: Diagnostic timeline for CCHF in a non-fatal case, showing viremia, antibody response, and optimal periods for different diagnostic assays. Direct viral detection is most effective early in the illness, while serological tests are more useful later.
Direct Viral Detection Assays for CCHF Diagnosis
Assays for direct viral detection, including viral culture, nucleic acid amplification tests (NAATs), and viral antigen detection, are most valuable in the first week following symptom onset, when viremia levels are typically highest. Viremia tends to decrease significantly after the first week, although it may persist longer in severe or fatal cases. Direct viral detection methods offer the advantage of confirming active infection by identifying the virus itself, rather than relying on the body’s immune response.
Viral Culture: Traditional but Specialized
Viral culture involves propagating the virus in susceptible cell lines, such as BHK-21, CER, LLC-MK2, and Vero cells, or through animal inoculation, particularly in mice. Animal inoculation is more sensitive but takes longer to yield results compared to cell culture. Viral culture is most effective when performed early in the symptomatic phase, when viral loads are high. A key advantage of viral culture is its potential to detect a broad spectrum of CCHFV strains. However, it has significant drawbacks: it is time-consuming, requires highly specialized BSL3 or BSL4 facilities, and samples cannot be inactivated prior to culture, posing a considerable biosafety risk. These limitations make viral culture less accessible as a routine CCHF diagnostic tool, especially in resource-limited endemic regions.
Nucleic Acid Amplification Tests (NAATs): Molecular Precision
NAATs, primarily reverse transcription-polymerase chain reaction (RT-PCR), are widely used for CCHF diagnosis, particularly within the first 10 to 12 days of symptom onset. RT-PCR is generally more accessible than viral culture and allows for sample inactivation before testing, enhancing biosafety. Numerous NAAT assays have been developed for CCHF diagnosis, targeting different segments of the CCHFV genome (S, M, or L). While many assays are specific for CCHFV, multiplex assays capable of detecting other viral hemorrhagic fever viruses are also available, which can be valuable in regions where multiple hemorrhagic fevers are endemic. NAATs primarily focus on detecting viral RNA in blood samples, but CCHFV RNA has also been detected in saliva and urine in some cases, potentially expanding the range of sample types for diagnosis.
| Authors | Assay type | Detects other viral hemorrhagic fever viruses? | Genomic target (segment) | No. of CCHFV strains and/or patient samples tested | Limit of detection | Yr published | Reference |
|---|---|---|---|---|---|---|---|
| Atkinson et al. | Real time RT-PCR | No | S | 18 strains | 5 RNA copies/reaction | 2012 | 41 |
| Bonney et al. | Isothermal recombinase polymerase amplification | No | S | 12 strains; human and tick samples from Tajikistan | 50–500 copies | 2017 | 7 |
| Brinkmann et al. | Multiplex amplification followed by next-generation sequencing | Yes | L | Patient samples from Turkey | Unknown | 2017 | 42 |
| Burt et al. | RT-PCR | No | S | Patient samples from southern Africa | Unknown | 1998 | 37 |
| Das et al. | Multiplex RT-PCR combined with universal array | Yes | L | 3 strains | 190 RNA copies/ml | 2015 | 43 |
| Drosten et al. | Real-time RT-PCR | Yes | S | Patient sample from Kosovo | 2,779 genome equivalent/ml | 2002 | 44 |
| Duh et al. | One-step real-time RT-PCR | No | S | Patient samples from Kosovo | 30 PFU/ml | 2006 | 9 |
| Fajfr et al. | Real-time RT-PCR | Yes | L | 1 strain | 33–100 fg/μl | 2014 | 45 |
| Filippone et al. | RT-PCR combined with a DNA microarray | Yes | L | 4 strains; patient samples from the Balkans and Middle East | 105–106 PFU/ml amplified cDNA | 2013 | 46 |
| Garrison et al. | Real-time RT-PCR | No | S | 18 strains; patient samples from Uzbekistan | 11.8 copies | 2007 | 11 |
| Ibrahim et al. | Real-time RT-PCR | No | S | 1 strain | 5 PFU | 2011 | 47 |
| Jaaskelainen et al. | Real-time RT-PCR | No | S | 8 strains; patient samples from Turkey | 11 genomes/reaction | 2014 | 48 |
| Kamboj et al. | Real-time RT-PCR | No | S | Animal and tick samples from India | 7.6 copies | 2014 | 49 |
| Koehler et al. | Real-time RT-PCR | No | S | 16 strains | 256 PFU/ml | 2018 | 50 |
| Osman et al. | RT-LAMPa | No | S | Patient samples from Sudan | 10 fg (naked eye turbidity), 0.1 fg (agarose gel electrophoresis) | 2013 | 51 |
| Papa et al. | Real-time RT-PCR | No | S | Patient samples from Albania | Unknown | 2007 | 52 |
| Sas et al. | One-step real-time RT-PCR | No | S | 4 strains | 2 copies/μl | 2018 | 53 |
| Schwarz et al. | Real-time RT-PCR | No | S | Patient samples from United Arab Emirates | Unknown | 1996 | 54 |
| Wölfel et al. | One-step RT-PCR combined with a DNA macroarray | No | S | 18 strains; patient samples from Iran, Namibia, Pakistan, and South Africa | 6.3 genome copies/reaction | 2009 | 55 |
| Wölfel et al. | Real-time RT-PCR | No | S | 12 strains; patient samples from Iran, Pakistan, and South Africa | 1,164 copies/ml | 2007 | 56 |
| Yapar et al. | One-step real-time RT-PCR | No | S | Patient samples from East Anatolia | 102 genome equivalents/ml | 2005 | 57 |
| Zahraei et al. | One-step real-time RT-PCR | No | S | Patient samples from Iran | 20 RNA copies/reaction | 2016 | 58 |
Table 1: Examples of Nucleic Acid Amplification Tests for CCHF diagnosis. These assays vary in their design, target regions, and detection limits, impacting their suitability for different diagnostic scenarios.
Quantitative RT-PCR (qRT-PCR) assays offer the advantage of viral load quantification, which has prognostic value in CCHF. Higher viral loads are associated with poorer outcomes and increased disease severity. A viral load threshold of ≥1 × 109 copies/ml has been identified as a strong predictor of fatal outcome. However, the genetic diversity of CCHFV can affect NAAT sensitivity. Assays validated against a limited number of strains or from specific geographic regions may not reliably detect all circulating strains globally. Therefore, selecting NAATs with broad strain coverage is crucial, especially when the origin of infection is uncertain. External quality control studies have highlighted variability in NAAT performance across laboratories, emphasizing the need for robust assay validation and quality assurance in CCHF diagnosis.
Viral Antigen Detection: Rapid Turnaround
Viral antigen detection, typically using enzyme-linked immunosorbent assays (ELISAs), offers a faster alternative to viral culture and requires less specialized equipment than NAATs. Antigen detection ELISAs can detect CCHFV antigens in serum as early as 2 to 3 days post-infection in animal models. In human cases, antigen detection is most sensitive in the early symptomatic phase, particularly within the first 5 days of illness and in patients with high viremia. However, antigen detection sensitivity may decrease as patients develop antibodies, potentially due to antibody interference with the assay. Like NAATs, antigen detection assays allow for sample inactivation, improving biosafety. Immunohistochemistry can also detect CCHFV antigens in tissue specimens, particularly liver tissue from fatal cases, but is less practical for acute CCHF diagnosis unless biopsies are part of the clinical evaluation.
Serological Testing for CCHF Diagnosis: Detecting the Antibody Response
Serological assays become increasingly important for CCHF diagnosis after the first week of illness, as the body’s antibody response develops. Serological tests detect anti-CCHFV IgM and IgG antibodies, indicating past or recent infection. Historically, various serological methods were used, but ELISA and immunofluorescence assays (IFA) have become the preferred methods due to their improved sensitivity and reliability.
ELISA and IFA: Modern Serological Standards
ELISAs for CCHF diagnosis commonly utilize recombinant CCHFV nucleocapsid protein as the target antigen. Numerous in-house ELISAs have been developed, and some commercial kits are available for research use, although clinical diagnostic approvals are still limited. Commercial ELISA kits have demonstrated varying sensitivities and specificities, and performance can be influenced by CCHFV genetic diversity, potentially affecting antibody detection for certain strains. Indirect IFAs using recombinant CCHFV nucleoproteins are also commercially available for research purposes. External validation of commercial IFAs has shown high sensitivity and specificity for both IgM and IgG antibody detection.
Alternative Serological Approaches
Lateral flow assays for CCHF antibody detection are under development, aiming to provide rapid, point-of-care testing. However, current lateral flow assays have shown limitations in sensitivity. Luminex technology-based assays offer multiplex detection capabilities for antibodies to various hemorrhagic fever viruses, including CCHFV, but require further validation against diverse CCHFV strains. Neutralizing antibody assays, such as plaque reduction neutralization tests, can measure functional antibodies but are not routinely used for CCHF diagnosis.
Conclusion: Optimizing CCHF Diagnosis for Improved Outcomes
Accurate CCHF diagnosis is complex due to the nonspecific early symptoms, the variable utility of diagnostic assays across disease stages, and the biosafety considerations for handling potentially infectious samples. Many current diagnostic tools require specialized equipment and facilities that are not readily available in resource-limited endemic regions. While some assays demonstrate good performance within specific geographic contexts, the development of pan-CCHFV assays capable of detecting the wide spectrum of CCHFV strains globally remains a priority.
In the first week of illness, direct viral detection assays are crucial for CCHF diagnosis, as antibodies may be absent during this early phase. NAATs and antigen detection assays offer quicker results than viral culture but may have limitations in broad strain detection. From the second week onwards, serological assays for IgM and IgG antibodies become increasingly valuable. However, it’s important to note that some fatal cases may not develop detectable antibodies, highlighting the need for a combined approach using both direct viral and serological testing to minimize the risk of missed CCHF diagnosis, particularly in the second week and beyond.
The ideal CCHF diagnostic assay would be rapid, simple to use, and accessible at the point of care, enabling early intervention and effective infection control measures to prevent nosocomial spread. Such assays should minimize sample handling, avoid sharps, and reduce aerosol generation to protect laboratory personnel. The World Health Organization has recognized the urgent need for improved CCHF diagnostics, setting a goal for rapid, reliable, and accessible diagnostic tools by 2023. Future research should focus on enhancing assay sensitivity across diverse CCHFV clades and improving diagnostic accuracy throughout all stages of CCHF illness, ultimately contributing to reduced morbidity and mortality from this significant viral hemorrhagic fever.
REFERENCES
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84