Introduction
Cervical spondylotic myelopathy (CSM) stands as the most prevalent form of spinal cord injury in adults, accounting for a significant 54% of nontraumatic spinal cord injuries in North America. This condition arises from cervical spondylosis, a progressive degeneration affecting the vertebrae, intervertebral discs, facet joints, and associated ligaments of the neck. These degenerative changes lead to CSM primarily through direct compression of the spinal cord and its surrounding blood vessels. The potential for long-term disability and severe neurological impairments underscores the critical importance of early and accurate Cervical Myelopathy Diagnosis. Identifying initial symptoms and delivering effective treatment before irreversible spinal cord damage occurs is paramount for maintaining patients’ quality of life.
The incidence of surgical interventions for CSM has seen a dramatic increase, multiplying sevenfold between 1993 and 2002. It’s estimated that approximately 10% of individuals aged 55 and older exhibit clinical CSM, while a staggering 85% of adults over 60 show radiographic evidence of cervical spondylosis, indicating a risk of progression. With the mean age of CSM patients being 56, and the population over 60 in the United States projected to double by 2050, reaching nearly 100 million, the need for physicians to stay well-informed about cervical myelopathy diagnosis and management is more critical than ever. The increasing prevalence of this condition in the coming decades necessitates a heightened awareness and proactive approach to diagnosis.
Pathophysiology of Cervical Myelopathy
While the precise mechanisms behind CSM are still being elucidated, the clinical symptoms predominantly stem from spinal cord compression caused by a multitude of factors. Compression of the cervical spinal cord can result from various degenerative processes, including disc herniation, infolding of the ligamentum flavum and facet joint capsule, or spinal canal stenosis. Spondylosis, characterized by the degeneration of intervertebral discs and joints, can lead to the compression of surrounding vascular and neural structures, contributing to the severity of the disease. These diverse mechanisms ultimately lead to spinal cord compression and subsequent neuronal damage, categorized as either static or dynamic mechanical compression.
Static risk factors are constant and lead to direct injury through cervical canal stenosis. These include congenital spinal stenosis, herniation of disc material, osteophytosis, and ligamentous hypertrophy. Congenital spinal stenosis narrows the spinal column, potentially causing local ischemia, neural cell injury, apoptosis, and a significantly increased risk of developing cervical myelopathy. Furthermore, intervertebral discs can herniate, directly compressing the spinal cord, or tears in the annulus fibrosus can occur. Both disc herniations and annulus fibrosus tears place stress on the vertebrae, promoting the development of osteophytes. While osteophytes can provide vertebral stabilization, their overgrowth can also contribute to the compression of the spinal cord and surrounding vasculature. Ossification of the ligamentum flavum or posterior longitudinal ligament is also a recognized factor in narrowing the spinal canal and contributing to progressive cervical myelopathy.
Dynamic mechanical compression arises from excessive movement beyond the normal range of motion, resulting in translation and angulation of the spinal column or overlapping of the laminae and buckling of the ligamentum flavum. Examples include hyperextension of the neck, which can cause the ligamentum flavum to collapse dorsally into the spinal canal, or anterolisthesis during flexion, which can squeeze the spinal cord if a compressive lesion is already present.
Vascular factors also play a crucial role in the pathophysiology of CSM. Studies have demonstrated that histopathologic changes in cervical myelopathy are comparable to those seen in isolated spinal cord ischemia. Blood flow can be reduced through the anterior spinal artery and radicular arteries when these vessels are stretched over a disc or vertebral body. This vascular ischemia leads to inadequate perfusion of oligodendrocytes, cells vital for myelin production, resulting in oligodendrocyte death via apoptosis and subsequent neural demyelination. Other factors implicated in CSM pathophysiology include impaired intracellular energy metabolism, free radical-mediated injury, and cation-mediated cell injury. The cumulative effect of spinal cord damage in CSM is neuronal dysfunction, leading to hallmark symptoms due to the inhibition of afferent and efferent nerve fibers in the spinal cord, commonly referred to as long-tract signs.
Natural History of Cervical Myelopathy
Although cervical myelopathy develops in only a small proportion of individuals with spondylosis, its onset is frequently insidious. While age-related degeneration is the primary driver of CSM, traumatic spinal injuries to the discs can accelerate the degenerative process, even in younger individuals. Research suggests that myelopathy may be more prevalent in men than women, particularly those in physically demanding occupations. Incidence rates of CSM-related hospitalization have been reported around 4.04 per 100,000 person-years, with a clear increase in incidence with advancing age. The progression of CSM is highly variable. While some patients experience a gradual decline in motor and sensory function with periods of stability and progression, others may exhibit a more benign disease course. Factors associated with poorer prognosis in CSM include the severity of disease at diagnosis, older age, and longer duration of symptoms.
In its more severe forms, CSM is generally considered a condition best managed surgically. Systematic reviews indicate that a significant percentage, ranging from 23% to 54%, of patients initially managed nonoperatively eventually require surgical intervention. Age, symptom duration, and preoperative neurological function are key prognostic factors influencing surgical outcomes and should be carefully considered when formulating the most appropriate treatment plan.
Signs, Symptoms, and Clinical Diagnosis of Cervical Myelopathy
The initial symptoms of CSM are often subtle and can easily be overlooked. These commonly include difficulties with fine hand dexterity and gait instability, sometimes manifesting as frequent falls. Studies have shown a considerable delay in cervical myelopathy diagnosis, averaging around 6.3 years from symptom onset. During this period, patients may experience a significant decline in neurological function, highlighting the urgent need for timely diagnosis and intervention.
Figure 1. Key physical examination maneuvers in cervical myelopathy diagnosis. (A) Finger escape sign; (B) grip and release test; (C) hyperactive pectoralis reflex; (D) inverted radial reflex; (E) Hoffman sign. Optimized for cervical myelopathy diagnosis assessment.
As with most medical conditions, the diagnostic process for suspected CSM begins with a thorough patient history and physical examination. Compression of the spinal cord and nerve roots due to degenerative changes in the spinal column leads to a diverse range of symptoms, varying depending on the level of compression and structures affected. Patients commonly present with motor deficits resulting from insults to both upper and lower motor neurons. Alongside motor dysfunction, variable sensory losses occur due to compression of specific sensory spinal tracts. Physicians evaluating patients for potential cervical myelopathy diagnosis should be vigilant for the symptoms and physical examination findings summarized in Table 2.
| Symptom/Finding | Description |
|---|---|
| Motor Dysfunction | Weakness, clumsiness, gait disturbance, fine motor difficulty |
| Sensory Changes | Numbness, tingling, pain in neck, arms, or legs, Lhermitte’s sign |
| Reflex Abnormalities | Hyperreflexia, Hoffman’s sign, Babinski sign, inverted radial reflex, clonus |
| Gait Instability | Unsteady gait, wide-based gait, falls |
| Bowel/Bladder Changes | Urinary urgency, frequency, or incontinence (less common, but significant if present) |
| Pain | Neck pain, radicular arm pain |
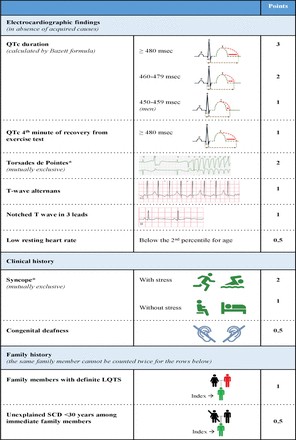
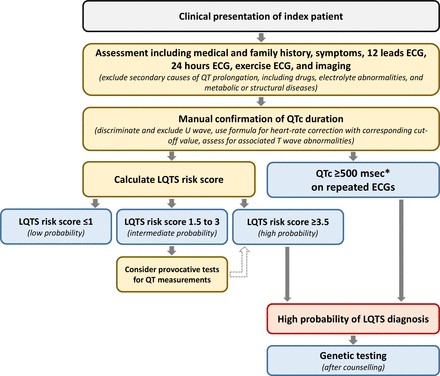
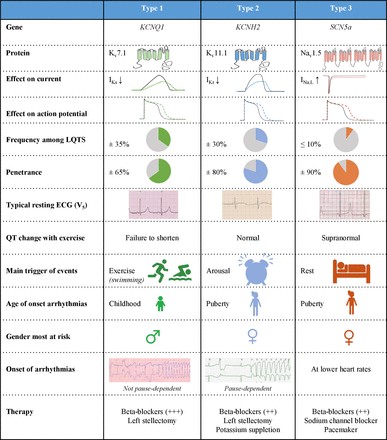
Table 2. Common Symptoms and Physical Exam Findings in Cervical Spondylotic Myelopathy. Crucial indicators for cervical myelopathy diagnosis.
The original symptom severity scale for CSM, developed by Nurick in 1972, primarily focused on gait deterioration. However, a more comprehensive grading system, the Japanese Orthopaedic Association (JOA) Myelopathy Evaluation Questionnaire, has largely replaced the Nurick scale. The JOA scale incorporates six subscores assessing upper extremity motor function, lower extremity motor function, upper extremity sensory function, lower extremity sensory function, truncal sensory function, and bladder function. While the JOA scale is now the preferred tool for assessing overall patient disability, the Nurick scale is still occasionally used to evaluate the impact of gait dysfunction on daily activities. Both the Nurick and JOA symptom severity scales are valuable in the cervical myelopathy diagnosis and assessment process.
| Nurick Grade | Description |
|---|---|
| 0 | Root pain only, no myelopathy |
| 1 | Myelopathy signs, but normal gait |
| 2 | Mild gait difficulty preventing normal occupation |
| 3 | Gait difficulty requiring assistance |
| 4 | Wheelchair-bound or bedridden |
| 5 | Complete paraplegia |
Table 1. Nurick Scale for Clinical Myelopathy Evaluation. A gait-based scale used in cervical myelopathy diagnosis.
| JOA Score Component | Score (Points) | Description |
|---|---|---|
| Upper Extremity Motor | 0-2 | 0= Paraplegia, 1= Marked paresis, 2= Mild paresis |
| Lower Extremity Motor | 0-2 | 0= Paraplegia, 1= Marked paresis, 2= Mild paresis |
| Upper Extremity Sensory | 0-2 | 0= Complete loss, 1= Marked disturbance, 2= Mild disturbance |
| Lower Extremity Sensory | 0-2 | 0= Complete loss, 1= Marked disturbance, 2= Mild disturbance |
| Trunk Sensory | 0-3 | 0= Complete loss, 1= Severe disturbance, 2= Mild disturbance, 3= Normal |
| Bladder Function | 0-3 | 0= Incontinence, 1= Severe difficulty, 2= Mild difficulty, 3= Normal |
| Total JOA Score | 0-17 | Higher score indicates less severe myelopathy. Used extensively in cervical myelopathy diagnosis and severity assessment. |
Table 3. Japanese Orthopaedic Association Scale for Cervical Myelopathy Evaluation. A comprehensive scale for cervical myelopathy diagnosis and severity grading.
Upper Extremity Findings in Cervical Myelopathy
Upper extremity deficits are frequently observed in CSM, often resulting from lower motor neuron involvement at the level of compression. Lower motor neuron symptoms, including weakness, muscle atrophy, fasciculations, hyporeflexia, and hypotonia, may be present in the upper extremities at the affected vertebral level. Furthermore, a significant majority (75%) of patients experience decreased dexterity in the intrinsic hand muscles, leading to difficulties with tasks requiring fine motor skills such as handwriting, typing, and buttoning clothes. Specific signs like the “myelopathy hand,” as described by Ono and colleagues, which includes a positive finger escape sign, grip and release test abnormalities, and intrinsic hand muscle wasting, are also important indicators. The hyperactive pectoralis reflex has been shown to be a highly sensitive (84.8%) and specific (96.7%) marker for myelopathy at the C2 to C4 cervical levels. If the compression is at the C6 level, an inverted radial reflex may be present. The Hoffman sign, another upper extremity reflex, can also be elicited in CSM patients. While present in a small percentage (2%) of the general population, a positive Hoffman sign has a reasonable predictive value (positive predictive value of 68% and negative predictive value of 70%) for CSM, making it a useful adjunct in cervical myelopathy diagnosis. Sensory changes due to compression of the spinothalamic tract, posterior column, and spinal roots can lead to alterations in pain, temperature sensation, proprioception, and dermatomal sensation. Specific sensory findings in the upper extremity and thorax of CSM patients include neck pain (50%), radicular pain (38%), and a positive Lhermitte sign (27%).
Lower Extremity Findings in Cervical Myelopathy
In CSM, the lower extremities are affected by upper motor neuron injury below the level of vertebral compression. Upper motor neuron symptoms, characterized by weakness, hyperreflexia, and hypertonia, are commonly observed. Gait dysfunction, seen in a large majority (80.3%) of patients, is a hallmark of CSM, as reflected in Nurick’s original grading system. Lower extremity weakness most frequently affects the iliopsoas muscle (38.8%), followed by the quadriceps (26.3%). This weakness leads to characteristic gait changes, including slower gait speed, decreased step length, longer stride time, and increased step width compared to healthy individuals, potentially progressing to quadriplegia in severe cases. Other lower extremity symptoms to assess include sustained clonus of the foot (more than 3 beats in succession), which has high specificity (96%) but low sensitivity (11%) for CSM. A positive Babinski reflex may also be present, exhibiting even higher specificity (up to 100%) but similarly low sensitivity (13%). Sensory disturbances in the lower extremities can include proprioception deficits due to posterior spinal column involvement, which can be evaluated using the Romberg test and heel-to-toe walking. As in the upper extremities, changes in pain, temperature, and sensation can occur. Bladder sphincter tone changes may also be present in CSM patients, affecting up to 44% of individuals.
A retrospective analysis of patients evaluated for cervical degeneration revealed that gait abnormality (91%), any hyperreflexia (85%), lower extremity hyperreflexia (81%), upper extremity hyperreflexia (67%), Hoffman sign (83%), and Babinski reflex (44%) were common physical examination findings in patients with CSM. These findings underscore the importance of a comprehensive neurological examination in cervical myelopathy diagnosis.
Radiographic Evaluation for Cervical Myelopathy Diagnosis
Imaging plays a crucial role in confirming a cervical myelopathy diagnosis. Plain radiographs, computed tomography (CT) with or without myelography, and magnetic resonance imaging (MRI) are all valuable tools for assessing spinal canal narrowing and pathological vertebral changes. Typically, plain radiographs are obtained initially due to their lower cost, speed, and reduced radiation exposure. However, if clinical suspicion for CSM remains high, MRI is the preferred imaging modality for definitive evaluation because of its noninvasive nature, high resolution, and superior ability to visualize soft tissues, including the spinal cord itself. Table 4 summarizes the imaging modalities used in cervical myelopathy diagnosis and their respective utilities.
| Imaging Modality | Utility | Cost | Radiation | Soft Tissue Visualization |
|---|---|---|---|---|
| Plain Radiographs | Initial assessment, spinal alignment, Torg-Pavlov ratio | Low | Low | Poor |
| CT Scan | Bone detail, ossification of posterior longitudinal ligament, preoperative planning | Moderate | Moderate | Fair |
| CT Myelography | Spinal canal detail, alternative for MRI contraindications | Moderate | Moderate | Fair |
| MRI | Gold Standard, spinal cord detail, canal diameter, intrinsic cord abnormalities, severity assessment | High | None | Excellent |
Table 4. Imaging Modalities for Cervical Spondylotic Myelopathy Diagnosis. MRI is the gold standard for cervical myelopathy diagnosis.
Anteroposterior and lateral radiographs can be used to assess spinal alignment and the Torg-Pavlov ratio. This ratio, calculated by dividing the spinal canal diameter by the vertebral body diameter, has been shown to be lower in patients with CSM compared to controls. A ratio below 0.8 is considered stenotic, and a canal diameter less than 12 mm is often associated with spinal cord compression. While anatomical variability can affect the reliability of the Torg-Pavlov ratio, studies suggest it remains a useful tool in the assessment of cervical spinal stenosis. Lateral radiographs can also be used to evaluate range of motion and maximal flexion, factors recently linked to myelopathy symptoms. Research indicates that increased C2-C7 range of motion and maximal flexion are associated with milder myelopathy symptoms, suggesting that CSM patients may compensate for canal stenosis through hyperflexion.
Figure 2. Lateral cervical spine radiograph demonstrating Torg-Pavlov ratio at C5. Calculated by dividing canal diameter (A) by vertebral body diameter (B). Ratio is a key radiographic measure in cervical myelopathy diagnosis.
CT scans have multiple applications in cervical myelopathy diagnosis and are considered the gold standard for diagnosing ossification of the posterior longitudinal ligament, a distinct condition with similar neurological effects. CT myelography can serve as a near-equivalent alternative for patients who have contraindications to MRI. CT imaging is also valuable for preoperative evaluation, surgical planning, and assessment of the transverse foramen of each cervical vertebra, through which the vertebral arteries pass.
MRI is unequivocally considered the gold standard for confirming cervical myelopathy diagnosis. Through changes in signal intensity (SI), MRI can determine the severity of degeneration and cord compression, quantify spinal canal diameter, and detect intrinsic spinal cord abnormalities. Systematic reviews have identified MRI findings predictive of poorer surgical outcomes, including a greater number of high SI segments on T2-weighted imaging, combined high SI changes on T2 and low SI changes on T1, and a higher SI ratio. Interestingly, factors like maximum cord compression ratio, number of discs compressing the cord, or cord diameter have not been consistently associated with neurological outcome after surgery.
Electrophysiological studies are not routinely used in cervical myelopathy diagnosis but may be helpful in excluding other conditions with overlapping symptoms, such as carpal tunnel syndrome, multiple sclerosis, amyotrophic lateral sclerosis, subacute combined degeneration, or other neurological diseases that exhibit characteristic electromyographic patterns. The role of electrophysiology in CSM diagnosis, follow-up, and treatment remains somewhat inconsistent in the literature due to factors like cost, availability, and the variety of modalities used (motor-evoked potentials, somatosensory-evoked potentials, nerve conduction studies, electromyography, and intraoperative monitoring).
Management of Cervical Myelopathy
Once cervical myelopathy diagnosis is confirmed, the primary decision revolves around whether to initiate operative or nonoperative management. CSM is generally considered a surgical disease, given that nonoperative treatment is associated with significant impairments in activities of daily living in a substantial proportion of patients over time.
Currently, there are no high-level studies directly comparing outcomes between operative and nonoperative management of CSM. A prospective, multicenter, nonrandomized trial comparing surgical versus nonoperative treatment concluded that surgical patients experienced better outcomes in terms of functional status, overall pain, and neurologic symptoms, despite often having a greater preoperative disease burden. Systematic reviews have recommended against nonoperative treatment as the primary approach for patients with moderate-to-severe myelopathy, suggesting that surgical intervention is generally preferred. Patients with mild myelopathy might be considered for nonoperative management but require close monitoring for any signs of neurological deterioration.
AOSpine North America and the Cervical Spine Research Society (CSRS) have developed guidelines for CSM management based on disease severity. For mild CSM, surgical intervention or a supervised trial of structured rehabilitation are recommended. If nonoperative management fails or the patient’s condition worsens, surgical intervention should be pursued. For moderate-to-severe CSM, surgical intervention is strongly recommended. Patients with radiographic evidence of cervical cord compression but without clinical signs of myelopathy or radiculopathy should receive counseling regarding the risk of progression, education about relevant symptoms, and regular clinical follow-up without immediate intervention. For patients with cervical cord compression and radiculopathy, surgical treatment or structured rehabilitation with close monitoring are suggested.
Nonoperative Management of Cervical Myelopathy
To date, there are no definitive studies establishing the optimal nonoperative treatment approach for CSM, likely due to the diverse patient population and the multifactorial nature of the condition’s pathogenesis. Nonoperative treatment modalities include bed rest, medications (pain relievers, muscle relaxants), steroids, injections, exercise therapy, soft or rigid cervical collars, cervical traction, thermal therapy, and other interventions. In the absence of established guidelines, nonoperative treatment should be tailored to the patient’s specific symptoms. Some research suggests that rigid collars are unlikely to be beneficial, and spinal manipulation has not demonstrated long-term effectiveness. Anecdotal reports even indicate that manipulation, traction, and massage may worsen neurologic symptoms, so these modalities should generally be avoided. Cost, time, and resource considerations are also important factors in developing a nonoperative treatment plan.
Operative Management of Cervical Myelopathy
Surgical management of CSM can be approached anteriorly, posteriorly, or through a combined approach, depending on the specific pathology. An anterior approach is often preferred for patients with significant kyphosis, involvement of only one or two vertebral levels, or ossification of the posterior longitudinal ligament. A posterior approach may be more suitable for cases involving more than three vertebral levels, cervical stenosis, posterior compression, or congenital stenosis. Common posterior surgical procedures include laminectomy with or without posterior spinal fusion and laminoplasty. Anterior surgical approaches typically involve anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy and fusion (ACCF), or cervical disc arthroplasty.
Reviews of surgically managed CSM patients indicate overall perioperative and delayed complication rates of approximately 15.6% and 4.4%, respectively. Common complications include cardiopulmonary issues, dysphagia, superficial infection, pseudarthrosis, C5 radiculopathy/palsy, worsened myelopathy, non-C5 radiculopathy/palsy, epidural/wound hematoma, and durotomy.
When to Refer to a Specialist for Cervical Myelopathy Diagnosis and Management
Studies evaluating surgical treatment outcomes for CSM have identified longer symptom duration, older age, and more severe preoperative myelopathy as predictors of worse outcomes. Given the subtle onset of symptoms, the progressive nature of CSM, and its potential for significant morbidity and mortality, primary care physicians play a vital role in early detection and initiating appropriate treatment pathways. As individuals age, the prevalence of spinal cord compression without clinical myelopathy increases. By age 50, a significant proportion of the population shows MRI evidence of cord compression, and this number rises further with increasing age. However, only a fraction of those with cord compression will actually develop myelopathy. For asymptomatic patients with cord compression, current guidelines recommend reassurance, counseling about the risk of progression to CSM, education about relevant signs and symptoms, and regular clinical follow-up without immediate intervention.
Due to the challenges in definitive cervical myelopathy diagnosis based solely on physical examination findings and the broad differential diagnosis for common neurological complaints, referral to a specialist should be considered whenever CSM is suspected. Before referral, obtaining plain radiographs of the cervical spine is recommended, and MRI should be performed if clinical suspicion is high. If imaging does not reveal signs of CSM, referral to a neurologist may be appropriate to investigate other potential diagnoses. Early comprehensive diagnostic evaluation and regular follow-up are essential for accurate cervical myelopathy diagnosis, slowing disease progression, and preventing irreversible neurological damage.
Surgery is the mainstay of treatment for CSM; therefore, prompt referral to a spine surgeon is crucial once a definitive cervical myelopathy diagnosis is made. For patients with mild CSM (JOA score 15-17), surgical intervention or structured rehabilitation are options. These patients should be referred to a specialist spine surgeon (orthopedic or neurosurgeon) for timely evaluation for potential surgical intervention. Any patient with moderate (JOA 12-14) or severe (JOA 0-11) CSM is recommended to undergo surgical intervention and must be referred to a surgeon without delay to prevent further clinical deterioration.
Figure 3. Algorithm to aid in the cervical myelopathy diagnosis, work-up, management, and appropriate referral process. Provides a structured approach for clinicians managing patients with suspected CSM. Abbreviations: CSM, Cervical Spondylotic Myelopathy; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; JOA, Japanese Orthopaedic Association.
Conclusion
CSM is a degenerative condition characterized by subtle initial symptoms that are often missed, leading to delayed cervical myelopathy diagnosis and potentially irreversible neurological damage. Decreased fine motor function of the hands and gait instability are hallmark signs. Given the well-documented progressive neurological dysfunction in CSM, early referral to a spine surgeon with appropriate imaging is strongly recommended for any patient suspected of having this condition. Prompt evaluation and management are crucial to optimize patient outcomes. This review of the pathophysiology, natural history, diagnosis, and management of CSM aims to provide guidance for physicians in evaluating and triaging patients with suspected cervical myelopathy.
References
[References from original article remain the same]
Notes
-
This article was externally peer reviewed.
-
To see this article online, please go to: http://jabfm.org/content/33/2/303.full.
-
Conflict of Interest: The authors, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article. There are no relevant disclosures.
-
Author contributions: All authors significantly contributed to the document and have reviewed the final manuscript.
-
Received for publication May 25, 2019.
-
Revision received August 23, 2019.
-
Accepted for publication August 25, 2019.
