Clinically Isolated Syndrome (CIS) marks the initial neurological episode that could be the harbinger of multiple sclerosis (MS). This syndrome is characterized by acute or subacute neurological symptoms resulting from inflammation and demyelination in the central nervous system (CNS). Typically, CIS manifests in young adults, with symptoms peaking within a few weeks. In a significant majority of individuals who go on to develop MS, CIS is the first clinical sign, often affecting the optic nerves, brainstem, or spinal cord. The presence of clinically silent brain lesions, detected through Magnetic Resonance Imaging (MRI), significantly elevates the likelihood of progressing to MS. Diagnosing CIS is intricate, lacking a single definitive clinical feature or test. Instead, it relies on a combination of clinical evaluation and paraclinical investigations. The internationally recognized McDonald criteria play a crucial role, incorporating MRI evidence to assess the dissemination of lesions in both time and space, which can lead to a diagnosis of definite MS even after a CIS event. Given that CIS is frequently the earliest clinical expression of MS, research focused on CIS patients is vital for understanding the initial pathological changes and disease mechanisms that influence the subsequent course of MS. Advances in diagnostic techniques and the emergence of disease-modifying therapies for MS have spurred increased interest and research activity in the field of CIS.
Keywords: Clinically isolated syndrome, multiple sclerosis, CIS diagnosis, McDonald criteria, demyelination, early MS detection
Understanding Clinically Isolated Syndrome and Its Connection to Multiple Sclerosis
Clinically Isolated Syndrome (CIS) is defined as the first episode of neurological symptoms caused by inflammation and demyelination within the central nervous system (CNS), raising suspicion for multiple sclerosis (MS). CIS presentations are varied, ranging from monofocal, affecting a single area, to multifocal, involving multiple areas. Commonly affected sites include the optic nerve, brainstem, cerebellum, spinal cord, and cerebral hemispheres. While CIS can be the initial presentation of definite multiple sclerosis (DMS), it is important to note that not all individuals with CIS will experience subsequent relapses indicative of MS. For an event to be classified as CIS, the symptoms must persist for at least 24 hours and occur in the absence of fever, infection, or encephalopathy [1, 2, 3, 4].
The progression of MS following a CIS event is highly variable. Long-term studies over 15 to 20 years reveal that approximately one-third of patients experience a benign disease course with minimal or no lasting disability. Conversely, about half of CIS patients will develop secondary progressive MS, characterized by a gradual increase in disability. Predicting the long-term trajectory of the disease at the CIS onset remains challenging [1]. In young adults (typically aged 20–45 years), MS often begins with a subacute CIS affecting the optic nerves, brainstem, or spinal cord in approximately 85% of cases. Although MRI scans frequently reveal multifocal brain lesions in CIS patients, quantitative MRI studies have also identified subtle abnormalities in seemingly normal white and gray matter, suggesting a broader underlying pathological process [5]. While patients often recover from the initial CIS episode, it is frequently the first clinical manifestation of MS. Key indicators that increase the risk of developing MS post-CIS are the presence of clinically silent MRI lesions and cerebrospinal fluid (CSF) oligoclonal bands (OCBs). Other factors with less definitive but potential associations include vitamin D deficiency, Epstein–Barr virus infection, smoking, specific HLA genes, and various immunological abnormalities [1].
Analysis of a large CIS patient database indicated that common presentations include optic neuritis (21%), long-tract signs and symptoms (46%), brainstem syndrome (10%), and multifocal abnormalities (23%). This data highlights that while all CIS presentations are isolated in time (a single episode), only 77% are isolated in space (affecting a single location). The nature of the initial CIS presentation can influence the subsequent disease course and prognosis, as summarized in Table 1 [4].
Table 1. Prognostic Features of CIS and Early Multiple Sclerosis [4]
| Good Prognosis | Poor Prognosis |
|---|---|
| Optic Neuritis | “Multifocal” CIS |
| Isolated Sensory Symptoms | Efferent Systems Affected |
| Long Interval to Second Relapse | High Relapse Rate in the First 2–5 Years |
| No Disability After 5 Years | Substantial Disability After 5 Years |
| Normal MRI | Abnormal MRI with a Large Lesion Load |




CIS: clinically isolated syndrome; MRI: magnetic resonance imaging
Diagnostic Approaches for Clinically Isolated Syndrome
When a patient presents with CIS, clinicians face crucial questions regarding diagnosis and management. Key considerations include distinguishing CIS from other neurological disorders, assessing the probability of MS development, evaluating the risk of future disability, and determining the necessity of investigations like MRI or CSF analysis. Furthermore, decisions must be made about patient counseling and whether initiating treatment is appropriate to aid recovery from CIS or delay the onset of MS [4].
Differential Diagnosis of Clinically Isolated Syndrome
Accurate and early diagnosis of CIS is paramount for effective patient management, counseling, and timely initiation of optimal therapy [6]. Differentiating CIS that will convert to MS (CIS-MS) from other neurological conditions or isolated CIS events requires careful clinical judgment by a neurologist experienced in differential diagnosis. Certain inflammatory and infectious disorders, such as systemic lupus erythematosus and neuroborreliosis, are commonly considered in the differential diagnosis and can be investigated through blood and CSF analyses [4].
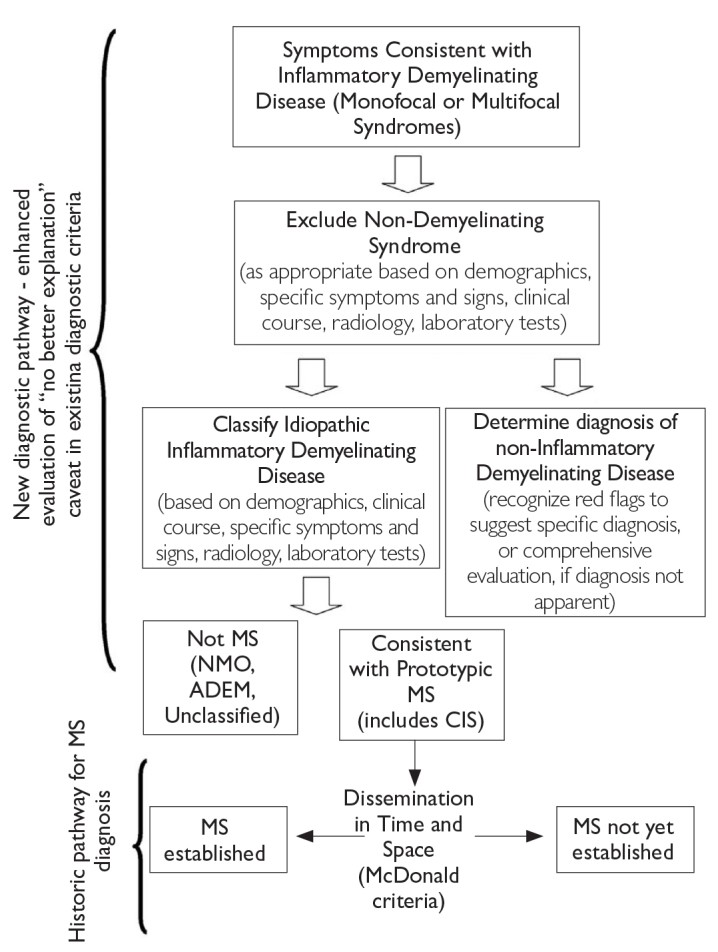
Figure 1. Steps in MS Differential Diagnosis [6]
MS: multiple sclerosis
Table 2 categorizes CIS presentations based on their likelihood of signaling a future MS diagnosis. It distinguishes between typical CIS features seen in MS, less common features that might still be associated with MS or other diseases, and atypical features that strongly suggest alternative diagnoses [6].
Table 2. Clinical Features of CIS and Likelihood of Signaling an MS Diagnosis [6]
| CIS Features Typically Seen in MS | Less Common CIS Features that May Be Seen in MS | Atypical CIS Features Not Expected in MS |
|---|---|---|
| Optic Nerve | ||
| Unilateral Optic Neuritis | Bilateral Simultaneous Optic Neuritis | Progressive Optic Neuropathy |
| Pain on Eye Movement | No Pain | Severe, Continuous Orbital Pain |
| Partial, Mainly Central Visual Blurring | No Light Perception | Persistent Complete Loss of Vision |
| Normal Disc or Mild Disc Swelling | Moderate to Severe Disc Swelling with No Hemorrhages, Uveitis (mild, posterior) | Neuroretinitis (Optic Disc Swelling with Macular Star), Uveitis (severe, anterior) |
| Brain Stem/Cerebellum | ||
| Bilateral Internuclear Ophthalmoplegia | Unilateral Internuclear Ophthalmoplegia, Facial Palsy, Facial Myokmia | Complete External Ophthalmoplegia; Vertical Gaze Palsies |
| Ataxia and Multidirectional Nystagmus | Deafness | Vascular Territory Syndrome, e.g., Lateral Medullary |
| Sixth Nerve Palsy | One-and-a-Half Syndrome | Third Nerve Palsy |
| Facial Numbness | Trigeminal Neuralgia, Paroxysmal Tonic Spasms | Progressive Trigeminal Sensory Neuropathy, Focal Dystonia, Torticollis |
| Spinal Cord | ||
| Partial Myelopathy | Complete Transverse Myelitis | Anterior Spinal Artery Territory Lesion (Sparing Posterior Columns Only) |
| Lhermitte’s Symptom, Deafferented Hand | Radiculopathy, Areflexia, Segmental Loss of Pain and Temperature Sensation | Cauda Equina Syndrome |
| Numbness | Partial Brown–Sequard Syndrome (Sparing Posterior Columns) | Sharp Sensory Level to All Modalities and Localized Spinal Pain |
| Urinary Urgency, Incontinence, Erectile Dysfunction | Fecal Incontinence | Complete Brown–Sequard Syndrome |
| Progressive Spastic Paraplegia (Asymmetrical) | Progressive Spastic Paraplegia (Symmetrical) | Acute Urinary Retention, Progressive Sensory Ataxia (Posterior Columns) |
| Cerebral Hemispheres | ||
| Mild Subcortical Cognitive Impairment | Epilepsy | Encephalopathy (Obtundation, Confusion, Drowsiness)[a] |
| Hemiparesis | Hemianopia | Cortical Blindness |
[a] Although encephalopathy is required for ADEM, it may also be seen at presentation and/or during the course of MS.
CIS: clinically isolated syndrome; MS: multiple sclerosis
Magnetic resonance imaging (MRI) is an indispensable tool in differentiating brainstem and spinal cord CIS caused by inflammatory demyelination from other structural lesions such as brainstem vascular malformations or spinal cord compression [6]. MRI also helps determine whether the presentation is due to monofocal or multifocal lesions and assess the likelihood of an eventual MS diagnosis, as detailed in Table 3 [6].
Table 3. Clinically Isolated Syndromes in the Differential Diagnosis of MS [6]
| Type 1 CIS: Clinically Monofocal, At Least One Asymptomatic MRI Lesion |
|---|
| Type 2 CIS: Clinically Multifocal, At Least One Asymptomatic MRI Lesion |
| Type 3 CIS: Clinically Monofocal, May Appear Normal; No Symptomatic MRI Lesion |
| Type 4 CIS: Clinically Multifocal, May Appear Normal; No Symptomatic MRI Lesion |
| Type 5 CIS: No Clinical Presentation to Suggest Demyelinating Disease, but MRI is Suggestive |
Note: Symptomatic lesion should appear typical for demyelination; they may be located in the brain or cord, although they more often occur in the brain; current evidence on the prognostic value of asymptomatic lesion mainly comes from brain imaging. CIS: clinically isolated syndrome; MS: multiple sclerosis; MRI: magnetic resonance imaging
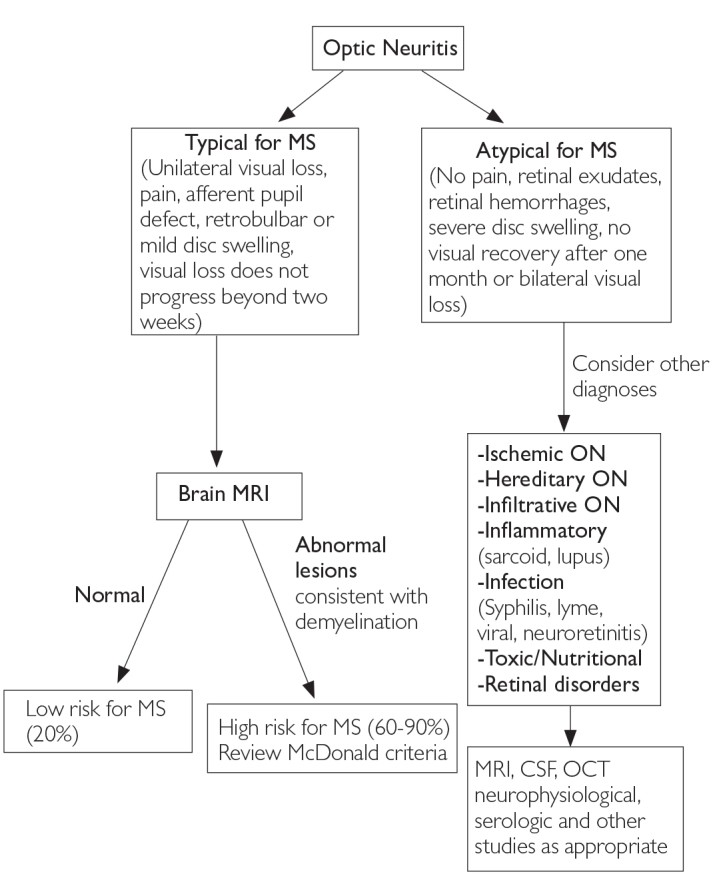
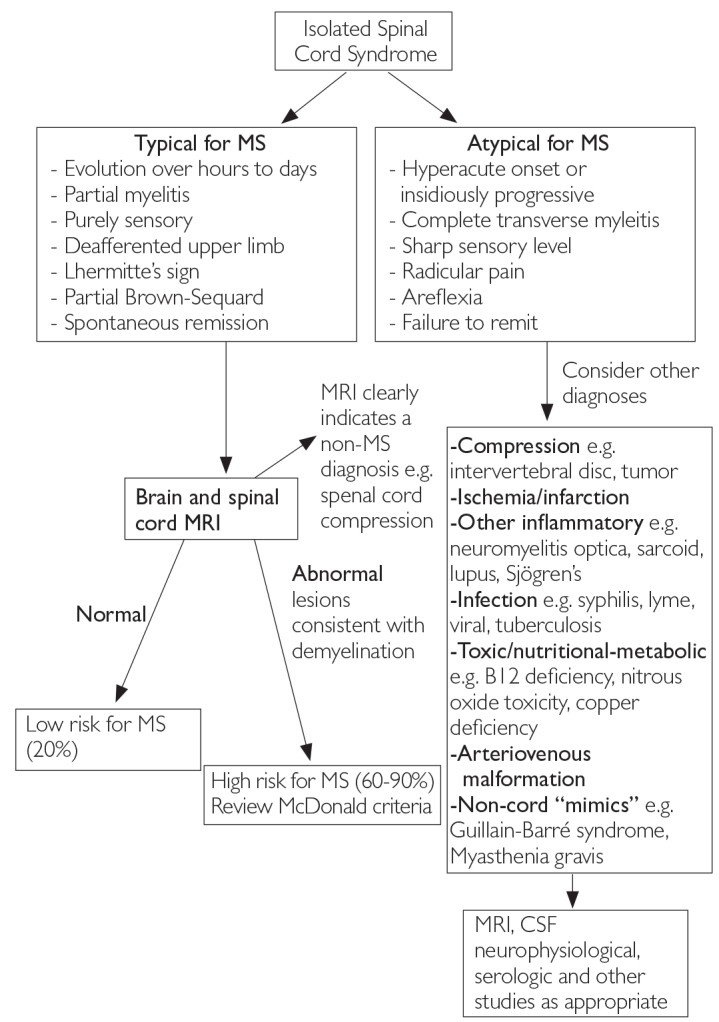
The most frequent CIS presentations in MS diagnosis involve the optic nerve, brain stem, and spinal cord [6]. In cases of suspected optic neuritis, certain clinical features may point to causes other than inflammatory demyelination [4]. Figures 2, 3, and 4 illustrate typical and atypical clinical aspects of demyelination seen in MS, aiding in differential diagnosis [1, 6].
Figure 2. Differential Diagnosis Upon Presentation with Demyelinating Optic Neuritis [6]
MRI: magnetic resonance imaging; MS: multiple sclerosis; CSF: cerebrospinal fluid
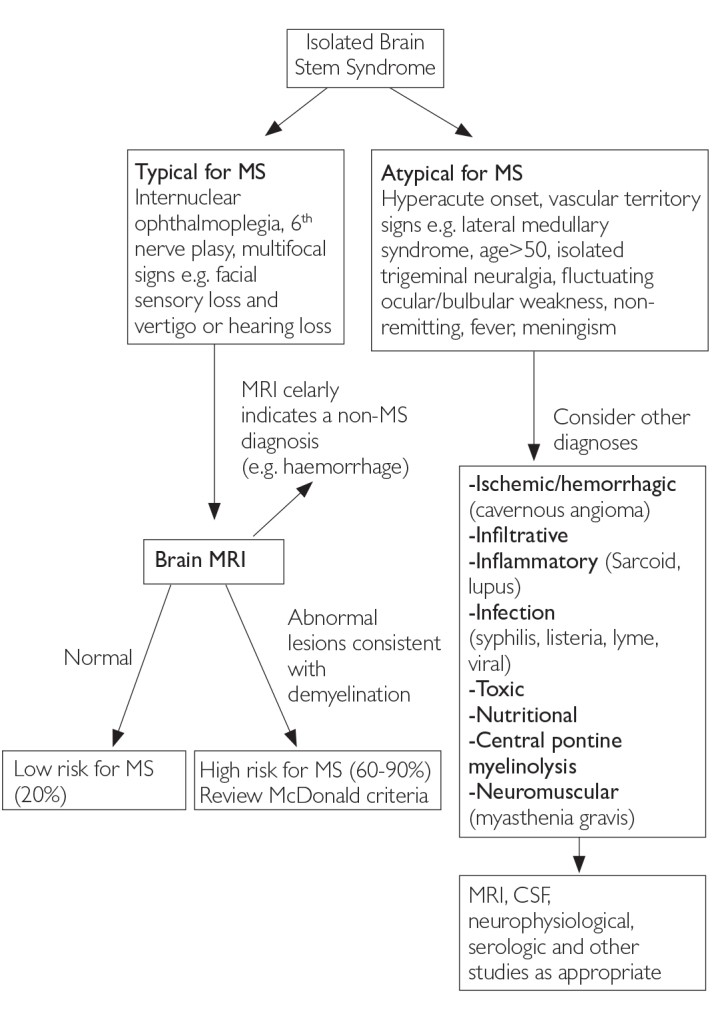
Figure 3. Differential Diagnosis Upon Presentation with Demyelinating Brain Stem Syndrome [6]
Figure 3: Diagnostic algorithm for differential diagnosis in patients presenting with demyelinating Brain Stem Syndrome.
MRI: magnetic resonance imaging; MS: multiple sclerosis; CSF: cerebrospinal fluid
Figure 4. Differential Diagnosis Upon Presentation with Demyelinating Spinal Cord Syndrome [6]
MRI: magnetic resonance imaging; MS: multiple sclerosis; CSF: cerebrospinal fluid
Cerebrospinal Fluid (CSF) Analysis in CIS Diagnosis
While the presence of CSF oligoclonal bands (OCBs) elevates the risk of CIS progressing to MS, its contribution is considered marginal when MRI findings are significant. A study by Tintore et al. [7] reported MS conversion in 59% of CIS patients with over 10 brain lesions on MRI and in 64% of patients with similar MRI findings and CSF OCBs. Many neurologists consider MRI alone sufficient for MS diagnosis or risk prediction. However, CSF examination remains valuable in predicting MS conversion, particularly in patients with negative MRI or those showing few lesions that do not meet McDonald criteria for dissemination in space. In MRI-negative patients, OCB presence increases the MS risk from 4% to 23%. Conversely, MS development is less likely in CIS patients with few or no MRI lesions and absent CSF OCBs [1].
Typical CSF findings in MS include a slightly elevated leukocyte count (5–50 cells per μL, compared to the normal range in Table 4). CSF albumin levels and the CSF albumin:serum ratio, indicative of blood-CSF barrier integrity, are usually normal but may be elevated in some MS patients. Notably, normal CSF findings can be observed in up to 10% of individuals with MS [8].
Table 4. Diagnostic CSF Markers in Clinically Definite MS [8]
| Marker | Detection Method | Normal Range | MS Range | Prevalence in Patients with MS (%) |
|---|---|---|---|---|
| Oligoclonal lgG Bands | Isoelectric Focusing and Immunoblot | ≤1 band | Unique in CSF >1 band | 90–100 |
| Albumin CSF:Serum Ratio | Nephelometry | Age dependent −3 | −3 | 85–90 |
| Activated B Lymphocytes or Plasma Cells | Cytochemical Staining | lgG type predominant | 80 | |
| lgG Index (Linear Formula) | Nephelometry and Calculation | ≤0.7 | >.7 | 50–75 |
| lgG Synthesis Rate (Linear Formula) | >6.0 mg per day | |||
| lgG Local Synthesis (Linear Formula) | 0 g/L | >.0 g/L | ||
| Leukocyte Count | Funchs–Rosenthal Chamber, Panoptical Staining and Light Microscopy | 5.0–50.0 cells per μL | 50–60 | 50–60 |
| MRZ Reaction (Optional) | Quantitative Immunoassay and Antibody Index | Antibody index | Antibody index >1.4 for two or more virus-specific antibodies | 70–90 |
CSF: cerebrospinal fluid; MRZ: measles rubella zoster; MS: multiple sclerosis
While typical CSF findings can support an MS diagnosis, they are not exclusively indicative of MS. However, deviations from typical CSF results can be valuable in refining the diagnosis and guiding patient management [8].
Evolution of Diagnostic Criteria for Clinically Isolated Syndrome and Multiple Sclerosis
Diagnostic criteria for MS have undergone significant evolution over the past half-century. Successive iterations have consistently emphasized the need to demonstrate dissemination of disease in both space and time, using clinical, paraclinical, or laboratory evidence. Crucially, MS diagnostic criteria necessitate ruling out alternative explanations for the clinical presentation before confirming an MS diagnosis [6].
Poser Criteria
The primary rationale behind establishing diagnostic criteria is to ensure therapeutic trials and research protocols are focused on patients with definite MS (DMS). The category of “probable” MS was designed for evaluating novel diagnostic methods prospectively [9]. In 1983, Poser et al. [9] published diagnostic criteria that, while initially intended for research protocols, became widely adopted in clinical practice. These criteria defined two main categories: “definite” and “probable” MS, each further divided into “clinically definite” and “laboratory supported” subgroups. The classification incorporated paraclinical evidence of CNS lesions, such as evoked potentials and tissue imaging, with laboratory support defined by OCBs or increased IgG production in CSF. According to Poser criteria, a diagnosis of “clinically definite” MS requires at least two attacks and clinical evidence of two separate lesions, or two attacks with clinical evidence of one lesion and paraclinical evidence of another distinct lesion Table 5 [10].
Table 5. Poser Criteria for Multiple Sclerosis [8]
| New Diagnostic Criteria for Multiple Sclerosis | ||||
|---|---|---|---|---|
| Category | Attacks | Clinical Evidence | Paraclinical Evidence | CSF OB/lgG |
| A. Clinically Definite | ||||
| CDMS A1 | 2 | 2 | ||
| CDMS A2 | 2 | 1 | and | 1 |
| B. Laboratory-Supported Definite | ||||
| LSDMS B1 | 2 | 1 | or | 1 |
| LSDMS B2 | 1 | 2 | + | |
| LSDMS B3 | 1 | 1 | and | 1 |
| C. Clinically Probable | ||||
| CPMS C1 | 2 | 1 | ||
| CPMS C2 | 1 | 2 | ||
| CPMS C3 | 1 | 1 | and | 1 |
| D. Laboratory Supported Probable | ||||
| Probable | ||||
| LSPMS D1 | 2 | + |
OB/lgG=oligoconal bands or increased lgG. CDMS: clinically definite MS. Needs two attacks and some clinical or paraclinical evidences; LSDMS: laboratory-supported definite MS, showing oligoclonal bands and clinical or paraclinical evidences; CPMS: clinically probable MS, with less restrict combinations; LSPMS: laboratory-supported probable MS. Only two attacks are enough to enter this category; CSF: cerebrospinal fluid
McDonald Criteria: 2001 and 2005 Revisions
In 2001, the International Panel of McDonald and colleagues introduced diagnostic criteria that integrated MRI evidence of dissemination in time and space, enabling MS diagnosis in patients presenting with CIS. Prospective clinical and MRI evaluations at baseline and at 3 months, 1 year, and 3 years were conducted to ascertain the frequency of MS development using both the new McDonald criteria and the Poser criteria for clinically definite MS (CDMS) [8]. The primary distinction of the revised McDonald criteria was the ability to diagnose MS earlier, potentially after just a single suggestive clinical attack. This advancement facilitated earlier diagnosis and treatment initiation [10].
Dalton et al. [2] demonstrated that McDonald criteria more than doubled the rate of MS diagnosis within a year of CIS presentation. The high specificity, positive predictive value, and accuracy of these criteria for CDMS underscore their clinical significance [2].
Subsequent publications have affirmed the utility of the McDonald criteria. Retrospective analyses of extensive datasets confirmed their ability to reliably signal CDMS development earlier than previous criteria, with comparable or improved specificity and sensitivity. Further studies explored potential refinements, focusing on defining lesion dissemination in time and space (core concepts in MS diagnosis), incorporating diverse imaging criteria, and evaluating the role of CSF analysis, particularly for primary progressive MS (PPMS) diagnosis [11].
While Dalton et al. [12] established that a new T2 lesion at 3 months is a robust marker of dissemination in time, baseline MRI scans in their studies were performed a median of 5 weeks post-symptom onset. The International Panel on Diagnosis of MS (the Panel) recognized the value of T2 lesions for demonstrating quicker dissemination in time than the 3-month interval in the original McDonald criteria. However, they agreed that T2 lesions appearing in the initial weeks after the first clinical episode should not be considered separate new events. The revised criteria specified that any new T2 lesion detected after a reference scan, performed at least 30 days after the initial clinical event onset, could fulfill imaging criteria for dissemination in time. This revision aimed to simplify and clarify the criteria, expedite diagnosis, and enhance imaging criteria flexibility while maintaining unequivocal evidence of dissemination in time Table 6 [11].
Table 6. MRI Criteria to Demonstrate Dissemination of Lesions in Time [11]
| Original McDonald Criteria