Chronic Lymphocytic Leukemia (CLL) diagnosis heavily relies on flow cytometry to identify the unique immunophenotype of CLL cells. As a critical tool in modern hematopathology, flow cytometry not only confirms CLL but also provides crucial prognostic insights and monitors treatment response. This article details the standard procedures for utilizing flow cytometry in CLL diagnosis, from sample preparation to data analysis, ensuring accurate and clinically relevant results.
1. Understanding CLL and the Power of Flow Cytometry
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL) is a prevalent malignancy affecting mature B-lymphocytes, impacting the blood, bone marrow, and lymphoid tissues. It stands as the most common type of leukemia in adults in Western countries, representing a significant portion of non-Hodgkin lymphomas. Flow cytometry (FC) has become indispensable in diagnosing CLL by immunophenotyping circulating B-lymphocytes, complementing traditional blood counts and morphology assessments [1].
Flow cytometry’s strength lies in its ability to detect the characteristic immunophenotype of CLL cells. These cells typically express a specific combination of markers, including CD5, CD19, dim CD20, dim CD22, CD23, bright CD43, dim CD45, dim-to-negative CD79b, dim CD81, CD200, and monoclonal surface immunoglobulin (Ig) with dim expression (Fig. 1) [2]. Notably, they are negative for CD10, CD103, CD123, and other T-cell and myeloid antigens [3, 4]. A diagnosis of CLL requires identifying ≥5 × 10^3/μl circulating monoclonal B-lymphocytes exhibiting this CLL immunophenotype in the peripheral blood. Small Lymphocytic Lymphoma (SLL) is diagnosed when patients with CLL-phenotype cells have a lower count in the blood (<5 × 10^3/μl) but present with lymphadenopathy, splenomegaly, or other extramedullary involvement.
Differentiating CLL/SLL from other conditions like monoclonal B-cell lymphocytosis (MBL) and mantle cell lymphoma (MCL) is crucial. MBL, characterized by <5 × 10^3/μl circulating monoclonal B-lymphocytes without other CLL symptoms, shares an immunophenotype with CLL in most cases (CLL-type MBL, 75% of MBL). Flow cytometry is key in distinguishing these conditions. The combined expression of CD200 and CD23, along with dim CD20, CD22, CD45, CD79b, and CD81, and bright CD43, helps differentiate CLL from MCL [4]. While typical, it is important to note that some CLL cases may present with atypical immunophenotypes, including variations in CD5 and CD23 expression, normal intensity of CD20, CD22, and CD79b, or aberrant expression of T-cell, myeloid, or other B-cell antigens [6–8].
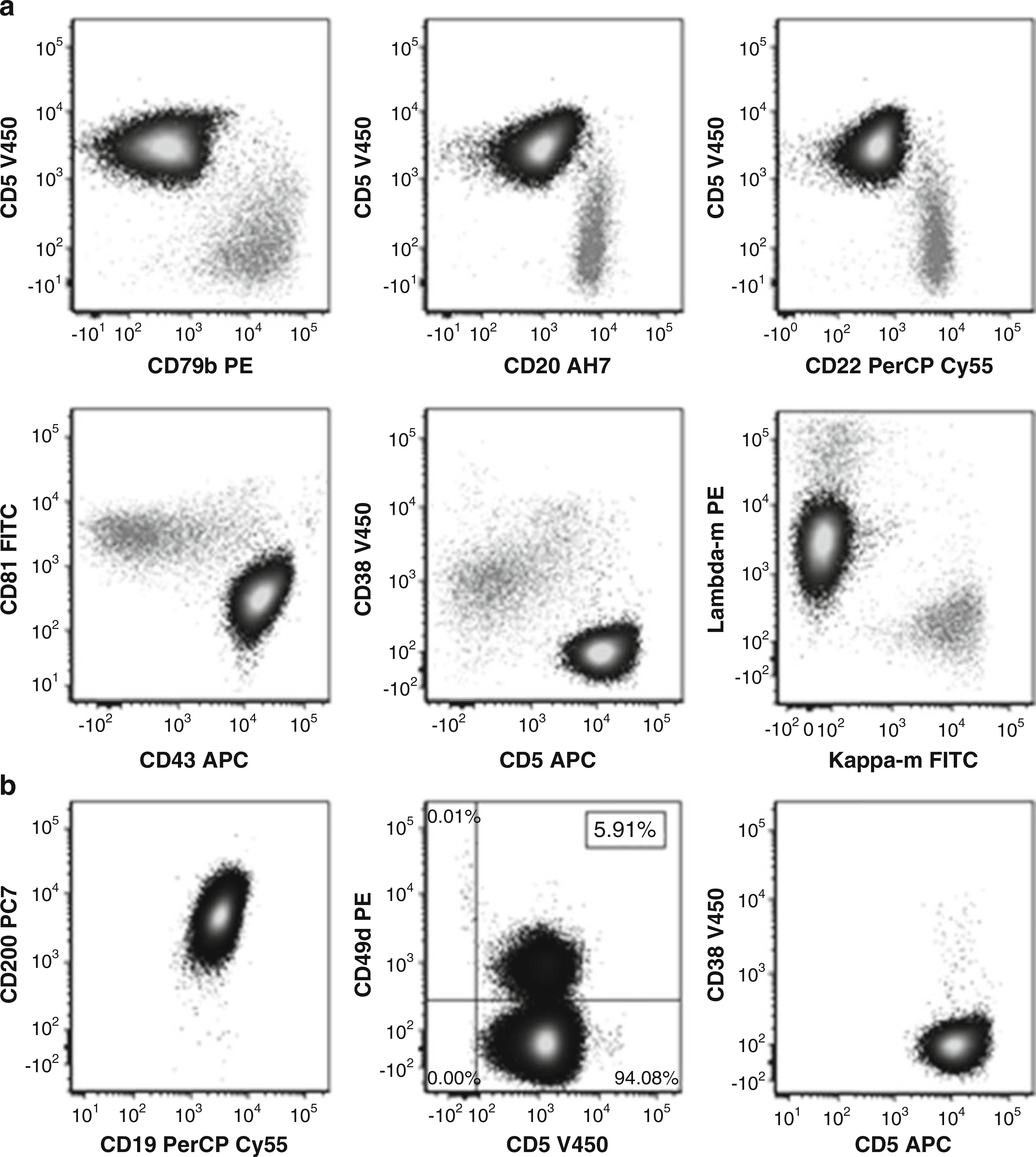
Figure 1: Typical Immunophenotype of CLL Cells by Flow Cytometry
(a) CLL cells (black) distinguished from normal B-cells (gray) based on CD19 positivity. CLL cells show positivity for CD5, bright CD43, and dim CD79b, CD20, CD22, and CD81. CD38 is negative. Monoclonality is indicated by dim lambda surface light chain positivity and kappa negativity. (b) CD200 positivity in CLL cells. Prognostic markers CD49d (<30% positive) and CD38 are negative.
Beyond diagnosis, flow cytometry plays a vital role in prognosis and monitoring. Expression of CD38 in ≥30% of CLL cells is associated with a more aggressive disease course and correlates with Ig heavy chain mutational status [9–11]. Similarly, CD49d expression in ≥30% of CLL cells is an independent indicator of poorer prognosis, potentially superior to CD38 in predicting clinical progression. CD49d is also linked to unfavorable cytogenetics, particularly trisomy 12 [12, 13]. The stable expression of CD49d makes its assessment reliable over time [14, 15]. Furthermore, flow cytometry is crucial for detecting minimal residual disease (MRD) during and after therapy, providing an independent predictor of progression-free and overall survival in CLL patients [16, 17].
2. Materials for CLL Flow Cytometry Processing
Successful flow cytometry for CLL diagnosis relies on meticulous preparation. The following materials are essential for lysing red blood cells, washing, and fixing samples.
2.1. Red Blood Cell Lysing Solution (1×)
This solution selectively lyses red blood cells, crucial for analyzing leukocyte populations in whole blood samples.
- Components: Ammonium chloride (NH4Cl) 8.29 g, potassium bicarbonate (KHCO3) 1 g, 4 ml 0.5 M EDTA solution, and distilled water (H2O) to 1000 ml.
- Preparation: Dissolve NH4Cl and KHCO3 in distilled water. Add EDTA solution and bring the volume to 1000 ml. Mix thoroughly.
- Storage: Stable at room temperature for up to 6 months.
2.2. Wash Solution: PBS with BSA and Sodium Azide
This solution is used to wash cells, removing unbound antibodies and maintaining cell viability.
- Components: 4 g Bovine Serum Albumin (BSA) in 4 liters of 1× Phosphate Buffered Saline (PBS) with 0.1% sodium azide.
- Preparation: Dissolve BSA in PBS with sodium azide. Mix well and allow to sit for 30 minutes to ensure BSA dissolution. Re-mix and verify dissolution. Adjust pH to 7.3–7.5 using concentrated HCl or NaOH if necessary (Notes 2 and 3).
- Storage: Store at 4 °C for up to 2 months.
2.3. Fixative Solution: 0.5% Formalin
Formalin fixation preserves cell morphology and stabilizes antibody staining for flow cytometry.
- Preparation: Mix 50 ml of 10% buffered formaldehyde with 950 ml of PBS. Mix well (Note 4).
- Protection: Wrap the bottle with foil and tape to protect from light. Adjust pH to 7.3–7.5.
- Storage: Stable at room temperature for up to 1 year.
3. Methodology: Step-by-Step Flow Cytometry for CLL
This section outlines the detailed procedure for performing flow cytometry in CLL diagnosis and monitoring.
3.1. Antibody Cocktails and Panel Design
The antibody panel is critical for identifying the CLL immunophenotype. Table 1 provides an example of an 8-color panel suitable for CLL diagnosis and follow-up.
Table 1: Example Antibody Panel for CLL Immunophenotyping
| FITC | PE | PerCP | PC7 | APC | AH7 | v450 | v500 |
|---|---|---|---|---|---|---|---|
| CD103 | CD25 | CD123 | CD19 | CD23 | CD20 | CD11c | CD45 |
| CD81 | CD79b | CD22 | CD19 | CD43 | CD20 | CD5 | CD3 |
| Kappa-m | Lambda-m | CD20 | CD19 | CD5 | CD14 | CD38 | CD45 |
Each cocktail is prepared by combining specific volumes of antibodies as recommended by manufacturers and through laboratory-specific titration. The total volume of cocktail used per staining tube is determined by the sum of individual antibody volumes. Cocktails should be stored at 4 °C and protected from light. Stability should be validated by each laboratory.
3.2. Specimen Collection and Handling
Optimal specimen collection is crucial for accurate flow cytometry results.
Peripheral blood (PB) and bone marrow (BM) are the primary sample types. Lymph node (LN) biopsies or fine needle aspirates (FNA) may also be used. Sodium heparin is the preferred anticoagulant for PB and BM, offering stability for up to 72 hours at room temperature for PB and 24 hours for BM. EDTA samples are less stable, with PB stable for 24 hours and BM for 12 hours. Delays or improper storage can compromise antigen expression [18]. Overnight shipping, especially with temperature fluctuations, can alter antigen expression. LN biopsies require mechanical disaggregation into a cell suspension. FNA samples usually do not need further disaggregation. FNA or cell suspensions can be stored in RPMI with 10% fetal bovine serum at 4 °C for up to 18 hours.
3.3. Pre-lysis, Cell Counting, and Viability Assessment
Minimizing pre-processing and ensuring timely processing is critical for maintaining cell viability and preventing loss of target cells, especially in MRD detection.
- Transfer ≤5 ml PB or BM into 50 ml conical tubes.
- Add ammonium chloride lysing solution to fill tubes. Mix gently and incubate for 10 minutes at room temperature, mixing occasionally.
- Centrifuge at 500 × g for 5 minutes. Aspirate supernatant (Note 5).
- Vortex gently to dislodge pellet. Fill tube with PBS and repeat step 3 (Note 6).
- Vortex gently to resuspend cell pellet. Add PBS based on pellet size (0.2–2 ml for small, 3–5 ml for large). Record final volume.
- Assess viability and count cells using a viability dye and automated counter or hemocytometer [20]. Reject samples with <75% viability unless irreplaceable.
3.4. Cell Concentration Adjustment
Adjust cell concentration to optimize staining. Refer to Note 7 for detailed guidance on concentration adjustments.
3.5. Antibody Staining Procedure
- Dispense pre-made antibody cocktails into 12 × 75 mm tubes.
- Add 100 μl of patient cell preparation to each tube. Vortex briefly and incubate for 30 minutes at room temperature, protected from light.
- Add 3–4 ml wash solution. Vortex, centrifuge at 500 × g for 5 minutes, and aspirate supernatant.
- Repeat wash step 3.
- Add 200–300 μl formalin fixative. Vortex and store at 4 °C until acquisition (Note 8).
3.6. Data Acquisition on the Flow Cytometer
Acquire data using a flow cytometer, ensuring low FSC events are included. Store ungated data as list mode data. Acquire 1,000,000 to 2,000,000 events for standard diagnostics, and more for MRD detection if cell counts allow. For low cell count samples (e.g., FNA), acquire as many events as possible. Essential parameters to record include all fluorescence channels, time, FSC-A, FSC-H, and SSC-A.
3.7. Flow Cytometry Data Analysis for CLL
Sequential gating is used to identify and analyze abnormal CLL cells, distinguishing them from normal cells based on light scatter and antigen expression. Figures 2-4 illustrate typical gating strategies for CLL diagnosis and MRD detection.
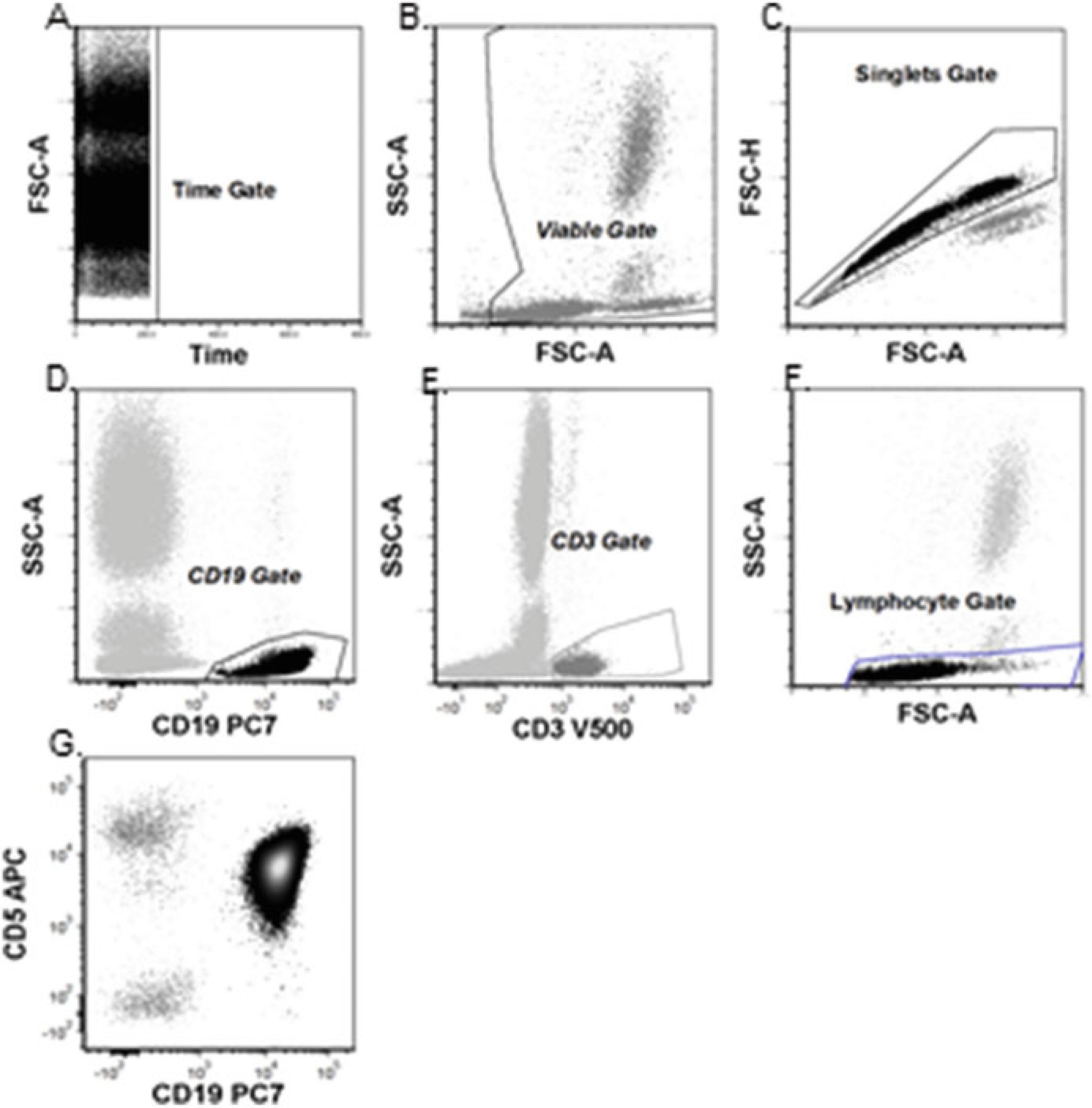
Figure 2: Basic Flow Cytometry Gating Strategy for CLL Diagnosis
(a) Time gate to ensure consistent data acquisition. (b) Viability gate to exclude debris. (c) Singlet gate to exclude cell aggregates. (d) T-cell (CD3+) identification. (e) B-cell identification. (f) Lymphocyte gate verification on FSC-A vs. SSC-A plot. (g) Final analysis gate encompassing time, viability, singlets, and lymphocytes.
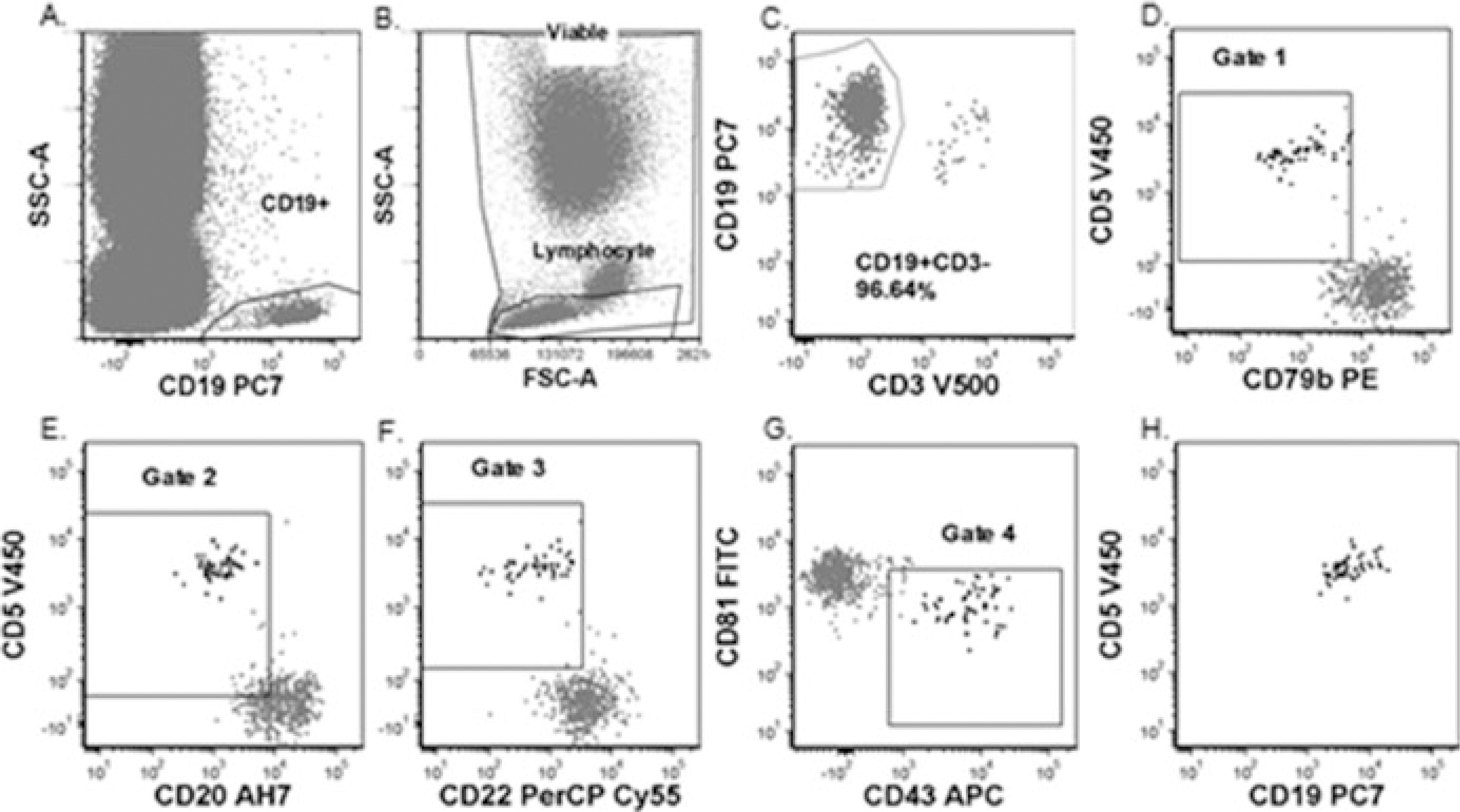
Figure 3: Flow Cytometry Gating for CLL MRD Detection – Method 1
(a) Viability and Lymphocyte gates. (b) CD19+ B-cell gate. (c) CD19+CD3− gate to exclude T-cells. (d–g) Sequential gating strategy for MRD detection. (h) Identification of minimal residual CLL cells using combined gating.
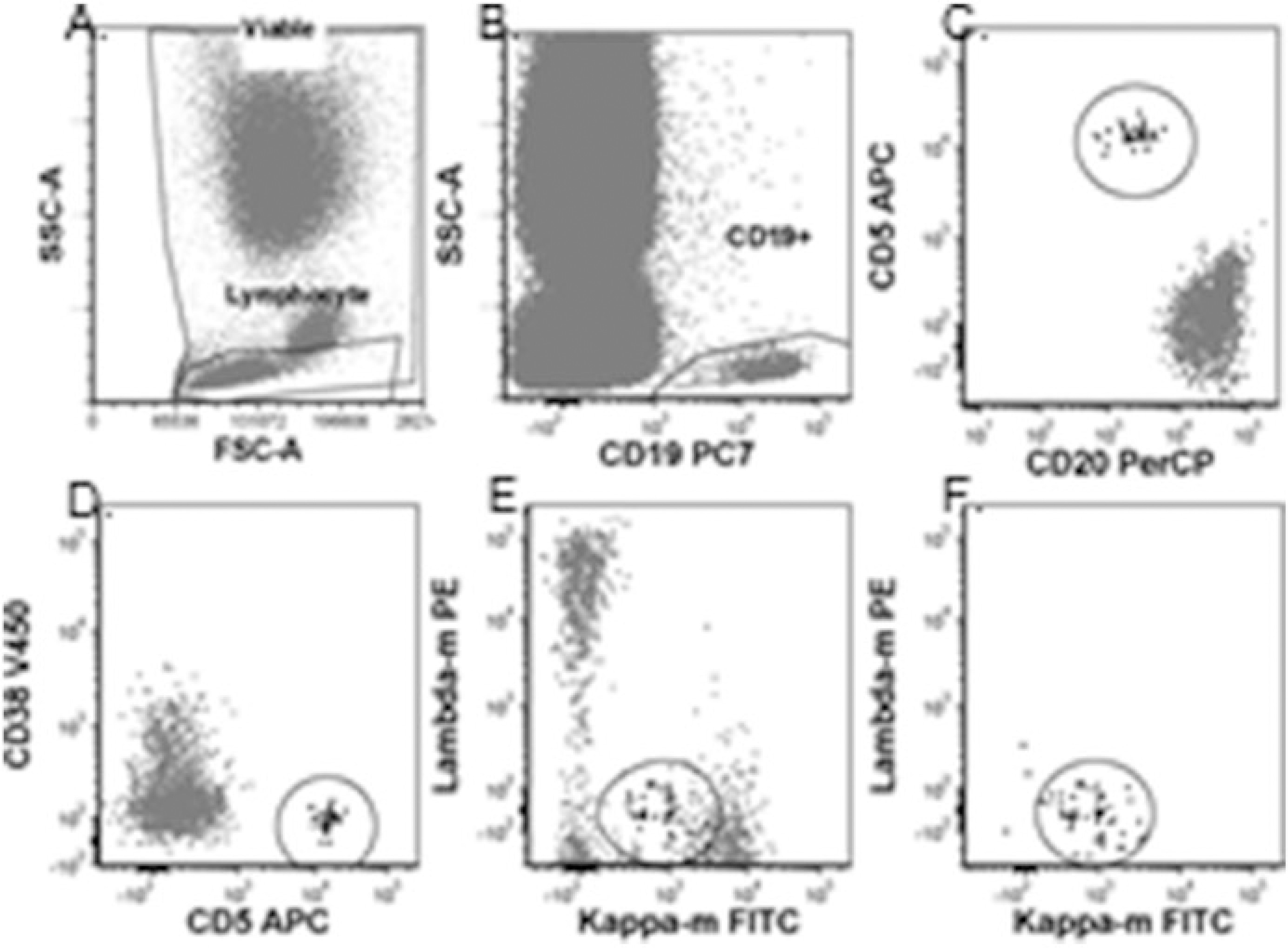
Figure 4: Flow Cytometry Gating for CLL MRD Detection – Method 2
(a) Viability and lymphocyte gating. (b) CD19+ B-cell gate. (c) CLL cell identification based on dim CD20 and CD5+ expression. (d) CLL cell identification based on CD5+ and homogenous CD38 negativity. (e) Polyclonal B-cells masking abnormal CLL cells. (f) Monoclonal CLL cell identification by kappa dim positivity and lambda negativity within the abnormal cell population.
4. Important Notes for Optimal Flow Cytometry Performance
This section highlights critical considerations and troubleshooting tips for flow cytometry in CLL diagnosis.
- CD23 expression can diminish in stored specimens. Process samples promptly.
- Always test wash solution with a fresh PB sample to ensure no hemolysis before patient use. Discard and remake if hemolysis is observed.
- Sodium azide in wash solution is hazardous. Avoid inhalation and sewer disposal due to explosion risk.
- Formaldehyde is carcinogenic and hazardous. Avoid inhalation and skin contact. Flush with water if contact occurs.
- When removing supernatant after lysis, carefully wipe debris from the tube interior without disturbing the cell pellet.
- Washing cells with room temperature PBS before staining removes cytophilic antibodies. Analyze kappa and lambda expression with B-cell markers (CD19, CD20, or CD22) and exclude CD3+ or CD14+ events to minimize interference from non-B cells.
- Cell concentration adjustment guidelines are detailed in the original text (Note 7 in the source article).
- Acquire data on fixed, stained cells within 24 hours for best results.
References
[1] Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-5456.
[2] Morilla R, Catovsky D. Immunophenotype of chronic lymphocytic leukaemia. Baillieres Clin Haematol. 1993;6(4):801-816.
[3] Flinn IW, Byrd JC. Chronic lymphocytic leukemia. In: Greer JP, Foerster J, Rodgers GM, Paraskevas F, Glader B, Arber DA, Means RT Jr, eds. Wintrobe’s Clinical Hematology. 12th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:2231-2272.
[4] Jain N, O’Brien S, Cortes J, et al. Chronic lymphocytic leukemia in first remission after fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy: analysis of prognostic factors and therapy. Cancer. 2011;117(12):2733-2742.
[5] Rawstron AC, Bennett FL, O’Connor SD, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575-583.
[6] Böttcher S, Ritgen M, Fischer K, et al. Minimal residual disease detection in chronic lymphocytic leukemia: comparison of flow cytometry and allele-specific polymerase chain reaction. Leukemia. 2003;17(2):371-378.
[7] D’Arena G, Musto P, Cascavilla N, et al. CD5- chronic lymphocytic leukemia: clinical and biologic features of a rare variant. Leukemia. 2007;21(1):30-34.
[8] Parreira A, Macedo A, Gonçalves F, et al. CD5-negative chronic lymphocytic leukemia. Leuk Lymphoma. 2000;39(3-4):297-305.
[9] Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig VH genes are associated with a worse prognosis in chronic lymphocytic leukemia. Blood. 1999;94(6):1848-1854.
[10] Krober A, Seiler T, Benner A, et al. V(H) mutation status, CD38 expression, and cytogenetics as independent prognosticators in chronic lymphocytic leukemia: results of a comprehensive analysis of 179 patients. Blood. 2002;100(4):1410-1416.
[11] Ibrahim SM, Keating M, Doehner H, et al. CD38 expression as an important prognostic factor in chronic lymphocytic leukemia. Blood. 2001;98(6):181-186.
[12] Ghia P, Guida G, Stella S, et al. CD49d is reliably assessed by flow cytometry in chronic lymphocytic leukemia and is associated with shorter time to treatment and survival. Blood. 2003;102(4):1427-1435.
[13] Bulian P, Nanni P, Negro R, et al. CD49d protein expression predicts unmutated IgV(H) status and shorter survival in chronic lymphocytic leukemia. Leukemia. 2004;18(6):1147-1148.
[14] Ritgen M, Doehner H, Stilgenbauer S. Relevance of minimal residual disease in chronic lymphocytic leukemia. Semin Oncol. 2003;30(3):343-349.
[15] Rawstron AC. Minimal residual disease in chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2007;20(3):515-527.
[16] Del Giudice I, Mauro FR, De Novi LA, et al. Minimal residual disease monitoring in chronic lymphocytic leukemia: a comparative analysis of flow cytometry and molecular methods. Haematologica. 2008;93(4):588-592.
[17] Bottcher S, Busch R, Ritgen M, et al. Minimal residual disease detection by flow cytometry in chronic lymphocytic leukemia: impact of assay sensitivity on outcome prediction. Haematologica. 2007;92(1):64-71.
[18] Sutherland DR, Anderson RE, Marti GE, et al. Flow cytometry and cell sorting. In: Rose NR, Hamilton RG, Detrick B, eds. Manual of Clinical Laboratory Immunology. 6th ed. Washington, DC: ASM Press; 2002:122-153.
[19] Davis BH, Olsen SH, Ahmad I, et al. Flow cytometric methods for clinical immunophenotyping. In: Detrick B, Hamilton RG, Folds JD, Rose NR, eds. Manual of Molecular and Clinical Laboratory Immunology. 8th ed. Washington, DC: ASM Press; 2016:892-918.
[20] Keeney M, Gratama JW, Sutherland DR, et al. Validation of cell-based fluorescence assays: practice guidelines from the ICSH and ISAC; part 1–method validation. Cytometry B Clin Cytom. 2011;79(6):386-404.