Importance of Accurate Clostridioides Difficile Diagnosis
Clostridioides difficile, now reclassified as Clostridioides difficile (formerly Clostridium difficile), is a bacterium that has become an increasingly significant healthcare concern since 2000. The incidence and severity of Clostridioides difficile infection (CDI) have risen, making accurate and timely diagnosis crucial for effective patient management and infection control. This article provides an in-depth review of current best practices for Clostridioides difficile diagnosis in adults, focusing on evidence-based strategies to optimize diagnostic accuracy and guide appropriate treatment decisions.
Objective and Scope
This review aims to synthesize the current evidence regarding optimal strategies for the diagnosis of CDI in adult patients (aged 18 years and older). Understanding the nuances of Clostridioides difficile diagnosis is paramount for healthcare professionals to differentiate between colonization and active infection, select the most appropriate diagnostic tests, and implement effective treatment and infection control measures.
Evidence Review Methodology
To compile this comprehensive review, we conducted a thorough search of the Ovid Medline and Cochrane databases. Our search strategy employed keywords specifically relevant to the diagnosis of CDI in adults. We focused on articles published between January 1978 and October 31, 2014, selecting studies based on keyword relevance, manual bibliography reviews, and inclusion of guidelines, systematic reviews, or meta-analyses published within the last decade within that period.
Our initial search identified 4,682 articles. After a rigorous screening process, we selected 196 articles for full-text review. From these, we identified the 116 most clinically pertinent articles, which form the evidence base for this review.
Key Findings in Clostridioides Difficile Diagnosis
One of the critical challenges in Clostridioides difficile diagnosis is that standard laboratory tests cannot distinguish between asymptomatic colonization with C. difficile and symptomatic CDI. This distinction is vital because only symptomatic patients require treatment. The availability of various diagnostic tests and algorithms adds complexity to the diagnostic process.
Our review of the evidence indicates that multistep diagnostic algorithms incorporating polymerase chain reaction (PCR) for toxin gene detection, or single-step PCR assays performed on liquid stool samples, demonstrate the most favorable test performance characteristics. Multistep algorithms show a sensitivity range of 0.68 to 1.00 and specificity of 0.92 to 1.00. Single-step PCR assays exhibit a sensitivity of 0.86–0.92 and a specificity of 0.94–0.97. These performance metrics are essential for clinicians in selecting the most effective diagnostic approach for their patients.
Treatment Landscape (Brief Overview)
While the primary focus of this article is Clostridioides difficile diagnosis, it’s important to briefly contextualize diagnosis within the treatment landscape. Vancomycin and metronidazole are established first-line therapies for CDI in most patients. However, treatment failures, particularly with metronidazole in severe or complicated CDI cases, have been observed. Recent data highlights clinical success rates of 66.3% for metronidazole compared to 78.5% for vancomycin in severe CDI.
Emerging therapies such as fidaxomicin and fecal microbiota transplantation (FMT) offer promising alternatives. Fidaxomicin shows similar clinical cure rates to vancomycin but with significantly lower recurrence rates (15.4% vs. 25.3%). FMT has demonstrated impressive response rates of 83%−94% in recurrent CDI, offering a potential solution for patients with repeated infections.
Conclusions and Clinical Relevance for Diagnosis
Our review underscores the critical principle that diagnostic testing for CDI should be reserved exclusively for patients exhibiting symptoms. The complexity of diagnostic approaches necessitates a careful consideration of available testing strategies. The choice of diagnostic algorithm can significantly impact the accuracy and timeliness of CDI diagnosis, ultimately influencing patient outcomes and infection control efficacy.
While treatment strategies must be tailored to disease severity, patient history, and recurrence risk, accurate Clostridioides difficile diagnosis forms the cornerstone of effective CDI management. This review aims to equip healthcare professionals with the knowledge to navigate the complexities of Clostridioides difficile diagnosis and implement evidence-based diagnostic practices.
Keywords: Clostridioides difficile diagnosis, CDI diagnosis, C. diff diagnosis, stool test, PCR, toxin test, diagnostic algorithm
INTRODUCTION
Clostridioides difficile was initially recognized as the primary infectious agent responsible for antibiotic-associated diarrhea in 1978.1 However, since the emergence of the hypervirulent BI/NAP1/027 strain of C. difficile around the year 2000,2 the landscape of Clostridioides difficile infections (CDI) has dramatically changed. CDI prevalence has increased substantially, and these infections have become more challenging to treat.2–4 This shift highlights the escalating importance of accurate and timely Clostridioides difficile diagnosis to effectively manage this evolving healthcare threat.
In the United States, the burden of CDI is evident in the dramatic increase in hospital discharge diagnoses. From approximately 148,900 discharges in 2001, CDI-related hospitalizations more than doubled to around 301,200 discharges by 2005.5 The incidence of CDI has also risen significantly, from 4.5 cases per 1000 adult discharges in 2001 to 8.2 cases per 1000 discharges in 2010.6 Beyond the increased incidence, CDI imposes a substantial economic burden on healthcare systems. Patients with CDI incur higher healthcare costs compared to those without CDI, with annual attributable costs in the U.S. exceeding $1.5 billion.7 These figures underscore the significant clinical and economic impact of CDI and the critical need for effective diagnostic and therapeutic strategies.
The pathogenesis of CDI is a complex interplay between the acquisition of C. difficile and disruption of the host’s gut microbiota. While the precise mechanisms by which C. difficile causes symptomatic infection are still being elucidated, it’s understood that C. difficile is not an invasive bacterium. Toxin production is the key virulence factor, as non-toxigenic strains of C. difficile do not cause diarrhea. The toxins produced by C. difficile disrupt epithelial integrity by targeting microtubules and cell-cell tight junctions. This disruption leads to the release of cytokines, such as IL-8,8 which promote inflammation in the colonic mucosa. The inflammatory response, coupled with fluid shifts resulting in diarrhea and epithelial necrosis, are hallmarks of CDI. Antibiotic use is a major risk factor for CDI because antibiotics can significantly alter the normal gut microbiota, creating an environment susceptible to C. difficile overgrowth.9 Other factors associated with increased CDI risk include advanced age, recent hospitalization, prolonged hospitalization duration, exposure to multiple antibiotics or extended antibiotic courses, proton pump inhibitor use, chemotherapy, chronic kidney disease, and feeding tubes.10–14 Given this complex landscape, this review is focused on the diagnosis and treatment of CDI in adults, with particular attention to contemporary diagnostic modalities and therapeutic approaches.
METHODS
A comprehensive literature search was performed using the Ovid Medline and Cochrane databases to identify relevant studies on Clostridioides difficile diagnosis and treatment. The search strategy incorporated a range of search terms and synonyms for Clostridioides difficile (Appendix A). We specifically targeted studies focusing on diagnostic testing and treatment of CDI published between January 1978 and October 31, 2014. Studies not published in English and those involving animal models or pediatric populations were excluded from our analysis.
The initial database search yielded 4,682 articles. To further refine our search, we manually reviewed the bibliographies of retrieved studies and previous reviews to identify additional relevant publications. This process led to the initial identification of 196 articles. After a thorough evaluation of clinical relevance, we narrowed down our selection to the 116 most pertinent articles (Appendix B). We also reviewed meta-analyses, systematic reviews, and references cited in published clinical practice guidelines from the past 10 years to ensure our review was informed by the most up-to-date and authoritative evidence.
Diagnosing Clostridioides difficile Infection: Who Should Be Tested
A critical challenge in Clostridioides difficile diagnosis is that laboratory testing alone cannot differentiate between asymptomatic colonization and clinically significant infection. Therefore, the diagnosis of CDI requires a combination of clinical and laboratory findings. The established diagnostic criteria for CDI include: 1) the presence of diarrhea, defined as three or more unformed stools within a 24-hour period, and 2) a positive stool test for toxigenic C. difficile or its toxins, or colonoscopic or histopathologic evidence of pseudomembranous colitis.15–17 The definitive gold standard for CDI diagnosis is the detection of toxigenic C. difficile in stool specimens, coupled with colonic histopathology demonstrating pseudomembranes in a patient exhibiting clinical symptoms.18 Reflecting this clinical focus, many laboratories will only process diarrheal stool samples for C. difficile testing.15,16,19–21 This selective approach helps to ensure that testing is targeted to patients with a higher pretest probability of CDI.
It’s also important to note that asymptomatic shedding of C. difficile spores can persist even after successful treatment of CDI. One study found that 56% of patients who responded to treatment asymptomatically continued to shed C. difficile spores for up to six weeks.22,23 This observation underscores why a “test of cure” is generally not recommended in CDI management.15 Furthermore, studies have documented chronic shedding and a higher prevalence of asymptomatic colonization in healthcare settings, supporting the concept of long-term asymptomatic carriage following CDI.24,25 Recurrent symptoms after CDI can sometimes be challenging to interpret. Transient functional bowel disorders can occur in up to 35% of patients in the two weeks following CDI resolution. However, persistent symptoms beyond three months due to a post-infectious irritable bowel syndrome are less common, affecting only about 4.3% of patients.26 The 2010 Society for Healthcare Epidemiology of America and Infectious Disease Society of America Clinical Practice Guidelines explicitly advise against treating asymptomatic C. difficile carriage.15 Therefore, in patients with recurrent diarrhea after CDI treatment, it’s crucial to differentiate between true recurrent CDI and symptoms related to transient functional bowel disorder or persistent irritable bowel syndrome. However, currently, there are no validated clinical approaches to reliably distinguish between these conditions, adding to the complexity of post-CDI symptom management and Clostridioides difficile diagnosis in the context of recurrence.
Clostridioides difficile Testing
Organism Detection Methods
The toxigenic culture (TC) is considered the gold standard method for detecting toxigenic C. difficile in stool samples (Table 1).19 TC involves anaerobically culturing stool specimens on specialized media27 for a period of 24–48 hours. Following colony selection and taxonomic confirmation, typically using antigen detection strategies like latex agglutination or enzyme immunoassay (EIA), or real-time PCR,27,28 isolates are incubated for an additional 48 hours. Subsequently, a cell cytotoxicity assay (CCA) is performed (Table 1). While TC is the reference standard, its independent performance characteristics are not fully defined. This is because most diagnostic studies compare other modalities to TC or CCA,19 and variations exist in media selection and sample pretreatment protocols.
Table 1. Diagnostic tests for toxigenic Clostridioides difficilea
| Testing Method | Target(s) | Notes |
|---|---|---|
| Gold Standard Tests | ||
| Toxigenic Culture | Toxigenic C. difficile | • Reference standard |
| • Difficult to perform | ||
| • Time consuming (24–48 hours) | ||
| Cell Cytotoxicity Assay | Toxins A or Bb | • Reference standard |
| • Highly sensitive for toxin compared to EIA | ||
| • Difficult to perform | ||
| • Time consuming (24–48 hours) | ||
| Rapid Diagnostic Tests | ||
| EIA | GDH | • GDH alone insufficient for diagnosis (must be paired with a test for toxin) |
| • Rapid | ||
| • Variable sensitivity and specificity | ||
| EIA | Toxins A or Bb | • Rapid |
| • Variable sensitivity and specificity | ||
| NAAT | tcdB or tcdC genes | • Rapid but more expensive than EIA |
| • Highly sensitive and specific for presence of toxigenic C. difficile | ||
| • May increase detection of colonization and not true CDI | ||
| RT-PCR | tcdB or tcdC genes | • tcdA– / tcdB+ strains can cause disease |
| LAMP | tcdA or tcdB genes | • *tcdA+ / tcdB-* not well-described in human disease |
| • Caution required in interpreting negative results based on tcdA testing alone by LAMP |
Abbreviations: CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; LAMP, loop-mediated isothermal amplification; NAAT, nucleic acid amplification testing; RT-PCR, real-time polymerase chain reaction.
[a] Refer to the text or Table 2 / Appendix C for sensitivity / specificity of the diagnostic tests
[b] C. difficile can produce toxin A and/or toxin B. Although both play a role in clinical disease, it is not known if strains producing only toxin A are associated with symptomatic infection in humans.
While TC is a reference standard, it is labor-intensive, requires specialized equipment and trained personnel, and is time-consuming. These factors can lead to diagnostic delays, which have implications for timely treatment initiation and infection control measures.29,30 Rapid diagnostic tests overcome these limitations. One rapid method focuses on detecting glutamate dehydrogenase (GDH), an enzyme produced by C. difficile. GDH detection is typically performed using EIA. However, performance characteristics of GDH EIA assays show considerable variability (Table 2). Because GDH is produced by both toxigenic and non-toxigenic C. difficile strains, and studies on asymptomatic colonization indicate that up to 46% of C. difficile isolates are non-toxigenic,31 GDH testing alone is insufficient for CDI diagnosis. It must be used in conjunction with a test that specifically detects C. difficile toxins.
Table 2. Systematic reviews and meta-analyses examining the performance characteristics of rapid diagnostic tests for Clostridioides difficile infection
| Test | Source | Number of Included Studies | Sensitivity | Specificity |
|---|---|---|---|---|
| Organism Detection | ||||
| GDH EIA | Crobach et al., 200919 | 11 | 0.88 (0.6–0.97)a,e | 0.89 (0.75–0.97)a,e |
| Shetty et al., 2011111 | 13 | 0.92 (0.8–1)a,e | 0.93 (0.83–1)a,e | |
| NAAT | Crobach et al., 200919 | 4 | 0.91 (0.86–1)a,e | 0.96 (0.94–1)a,e |
| Deshpande et al., 2011112 | 19 | 0.9 (0.88–0.91)b,e | 0.96 (0.96–0.97)b,e | |
| O’Horo et al., 2012113 | 25 | 0.92 (0.91–0.94)b,c | 0.94 (0.94–0.95)b,c | |
| 0.87 (0.84–0.9)b,d | 0.97 (0.97–0.98)b,d | |||
| Toxin Detection | ||||
| Toxin A/B EIA | Crobach et al., 200919 | 60 | 0.73 (0.32–0.99)a,e | 0.98 (0.65–1)a,e |
| Planche et al., 2008114 | 18 | 0.87 (0.69–0.99)a,e | 0.97 (0.92–1)a,e |
Abbreviations: EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification testing.
[a] Mean (range)
[b] Pooled (95% confidence interval)
[c] Compared to TC
[d] Compared to CCA
[e] Compared to TC+CCA or another mixed reference standard
Nucleic acid amplification testing (NAAT), encompassing techniques like RT-PCR and loop-mediated isothermal amplification (LAMP), offers a rapid, single-step approach for Clostridioides difficile diagnosis. NAAT assays detect the presence of tcdA/tcdB genes (which regulate toxin A/B production) or the tcdC gene (a negative regulator of toxin A and B production), thereby identifying toxigenic C. difficile (Table 1).19,21,32,33 NAAT methods generally demonstrate high sensitivity and specificity, often exceeding 0.90 (Table 2). However, this enhanced sensitivity can also lead to the detection of toxigenic C. difficile in asymptomatic carriers. This highlights a critical consideration in Clostridioides difficile diagnosis: the importance of testing only symptomatic patients. The high sensitivity of NAAT, while advantageous for detecting true positives, may also detect colonization rather than active infection if used indiscriminately. This concern has led some experts to caution against relying solely on NAAT-based testing for Clostridioides difficile diagnosis.16,19,34 The clinical context and patient symptoms must always be carefully considered when interpreting NAAT results.
Toxin Detection Assays
The cell cytotoxicity assay (CCA) is considered the gold standard for directly detecting Clostridioides difficile toxins A and/or B.27 CCA can be performed directly on stool samples or as part of the toxigenic culture (TC) process. The assay involves inoculating filtrates of stool suspensions or culture supernatants into cell cultures and then assessing for cytopathic effects after 24 or 48 hours.27 CCA is highly sensitive, capable of detecting as little as 3 picograms of toxin, and demonstrates high sensitivity (0.94–1) and specificity (0.99), particularly when combined with antiserum.27,35 Despite its excellent performance, the main limitations of CCA are its turnaround time and technical complexity. The 24-48 hour incubation period can delay diagnosis, and the assay requires specialized cell culture facilities and trained personnel.
Enzyme immunoassays (EIAs) for toxins A and/or B are rapid and more widely available than CCA, but their sensitivity and specificity are variable (Table 2). A significant limitation of toxin EIA tests is their suboptimal sensitivity compared to CCA and NAAT. Repeating EIA testing does not significantly improve sensitivity. A recent systematic review found that 91% of positive EIA results are detected on the first test, and the likelihood of a second or third test becoming positive after two initial negative tests is very low.36 This suggests that repeat EIA testing is generally not a useful strategy to enhance diagnostic yield in Clostridioides difficile diagnosis.
Multistep Algorithms for Diagnosis of CDI
Given the limitations of some toxin EIA kits, particularly their suboptimal sensitivity, and the potential for NAAT assays to detect asymptomatic colonization, multistep diagnostic algorithms have gained favor in Clostridioides difficile diagnosis. These algorithms aim to optimize diagnostic accuracy while maintaining rapid turnaround times. Many experts and clinical guidelines now advocate for multistep approaches for the rapid diagnosis of CDI.15,16,19,34
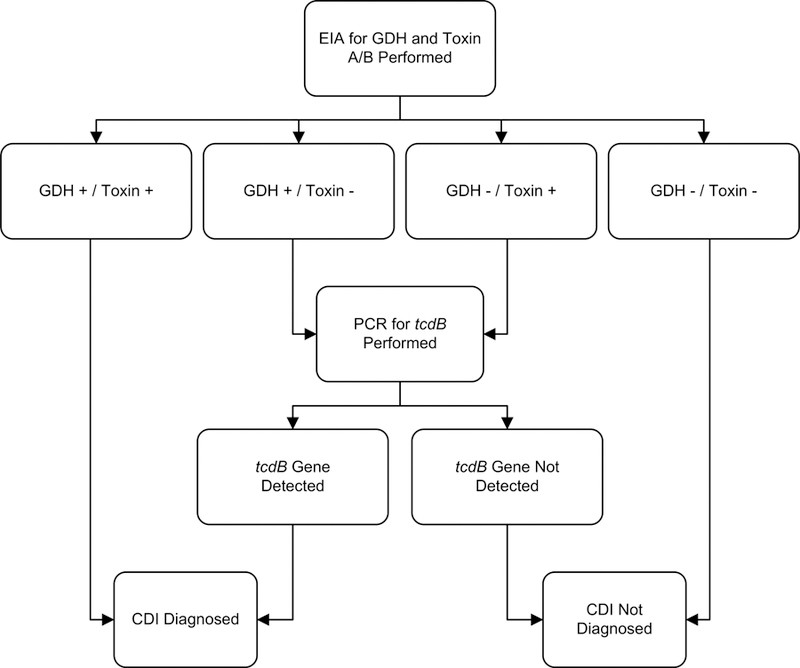
One commonly used multistep algorithm involves initial testing with GDH EIA, followed by reflex testing of GDH-positive samples with a toxin assay, such as toxin EIA or NAAT. Another effective multistep algorithm utilizes GDH EIA as the initial screen, with discordant results (GDH positive, toxin negative by EIA) resolved using NAAT, often PCR. Figure 1 illustrates an example of a multistep algorithm, demonstrating a sensitivity of 0.91, specificity of 0.98, and a negative predictive value of 0.99.37 These performance characteristics highlight the potential of well-designed multistep algorithms to achieve high diagnostic accuracy in Clostridioides difficile diagnosis.
Figure 1. Sample multistep algorithm for the rapid diagnosis of Clostridioides difficile infection.
Alt Text: Multistep diagnostic algorithm for Clostridioides difficile infection featuring initial GDH EIA testing followed by reflex PCR testing for discordant results, optimizing sensitivity and specificity in CDI diagnosis.
Abbreviations: CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction.
Footnotes: Adapted under Creative Commons License from Rao K, Erb-Downward JR, Walk ST, et al. The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE. 2014;9(3):e92578.
We reviewed studies that evaluated rapid testing algorithms using at least one gold standard comparator (Appendix C). In general, multistep algorithms that incorporated NAAT assays demonstrated excellent sensitivity (0.68–1) and specificity (0.92–1). In contrast, algorithms relying solely on GDH or toxin EIA testing showed poorer performance and greater variability. A large, multicenter study by Planche et al.38 reported that a GDH/NAAT-based algorithm yielded the highest sensitivity (0.91–0.98) and specificity (0.96–0.98) (Appendix C). These findings further support the use of multistep algorithms, particularly those incorporating NAAT, for optimal Clostridioides difficile diagnosis in clinical practice.
Treatment of Clostridioides difficile Infection (CDI) (Brief Overview for Context)
Since 2000, CDI treatment failures and recurrences have become more prevalent.2–4 These challenges are likely multifactorial, related to a complex interplay of host factors, bacterial virulence, and the ability to achieve therapeutic drug concentrations in the colon. The emergence of C. difficile strains with higher minimum inhibitory concentrations to metronidazole may also contribute to treatment failures.39 Clinical guidelines emphasize that CDI treatment should be guided by disease severity and the patient’s risk of recurrence or complications.15,16 While treatment is not the primary focus of this article on Clostridioides difficile diagnosis, understanding the severity-based treatment approach underscores the importance of accurate diagnosis in guiding appropriate management decisions.
Markers of Disease Severity in CDI
Clinical manifestations of CDI range widely, from mild diarrhea to life-threatening illness. Various prediction rules have been developed to assess the risk of recurrences, complications, and mortality in CDI patients.40 However, many of these studies have been limited by small sample sizes and significant heterogeneity.40 One prospective study involving 746 CDI patients proposed a risk scoring system to predict fulminant CDI based on factors such as age >70 years, WBC count, cardiorespiratory failure, and abdominal tenderness. Patients with a score ≥6 were identified as high risk.41 Another scoring system incorporated age, systemic antibiotic treatment, leukocyte count, albumin, and serum creatinine to predict response to vancomycin or fidaxomicin.42
Clinical practice guidelines, such as the 2010 Society for Healthcare Epidemiology of America and Infectious Disease Society of America guidelines, categorize CDI severity. Mild CDI is characterized by diarrhea without systemic signs of infection and a WBC count <15,000 cells/mL and serum creatinine <1.5 times premorbid level. Severe, complicated CDI is defined by the presence of hypotension or shock, ileus, or megacolon.15 Guidelines from the European Society of Clinical Microbiology and Infectious Diseases define severe CDI as an episode with a complicated disease course or signs of severe colitis, systemic toxin effects, and shock, potentially requiring intensive care, colectomy, or associated with death. Key indicators include WBC >15 X 109/L and serum albumin <30 g/L.16 The term “fulminant” is often used to describe severe, complicated CDI.42–44 (Table 3) Accurate Clostridioides difficile diagnosis, combined with assessment of disease severity, is essential for guiding optimal treatment strategies and predicting patient outcomes.
Table 3. Clostridioides difficile Infection (CDI) Classification based on Disease Severity
| Disease Category | Clinical and Laboratory Signs | Associated Risk Factors |
|---|---|---|
| Mild to moderate CDI | Diarrhea without systemic signs of infection, WBC < 15,000 cells/mL, and serum creatinine < 1.5 times premorbid level15 | Antibiotic use, previous hospitalization, longer duration of hospitalization, use of proton pump inhibitors, receipt of chemotherapy, chronic kidney disease, and presence of a feeding-tube10–14. |
| Severe CDI | Systemic signs of infection, and/or WBC ≥ 15,000 cells/mL, or serum creatinine ≥ 1.5 times the premorbid level 15 | Advanced age, infection with BI/NAP1/027 strain 115,116. |
| Severe, complicated CDI | Systemic signs of infection including hypotension, ileus, or megacolon 15 | See above, plus recent surgery, history of inflammatory bowel disease and intravenous immunoglobulin treatment43 |
| Recurrent CDI | Recurrence within 8 weeks of successfully completing treatment for CDI 16,20 | Patient age ≥65 years, concomitant antibiotic use, presence of significant comorbidities, concomitant use of proton pump inhibitors, and increased initial disease severity 16 |
Asymptomatic Carriers of C. difficile
Asymptomatic carriage of C. difficile is a common phenomenon, with prevalence rates ranging from 10 to 52% in various defined populations.45–49,25 Asymptomatic fecal shedding of C. difficile can be transient. Interestingly, one study suggested that vancomycin therapy, while intended to treat CDI, may temporarily interrupt shedding but paradoxically increase the risk of C. difficile carriage after therapy completion.50 Crucially, asymptomatic colonization with C. difficile does not appear to increase the risk of developing symptomatic CDI and may even offer protection against subsequent symptomatic disease.31,47,51 Shim et al. conducted a study involving 618 non-colonized patients and 192 asymptomatic carriers, with weekly follow-up rectal swabs. They reported that symptomatic CDI developed in 3.6% of non-colonized patients but only in 1% of asymptomatic carriers.31 This finding further reinforces the recommendation to test only symptomatic patients for Clostridioides difficile diagnosis, as detecting and treating asymptomatic carriers is not clinically beneficial and may even be detrimental.
Withdrawing Precipitating Antibiotics in CDI Management
The human gut microbiota plays a crucial role in protecting against pathogen overgrowth, including C. difficile. Any antibiotic can disrupt this protective microbiota, but certain classes, including penicillins, cephalosporins, and clindamycin, are particularly strongly associated with CDI risk.52–54 A systematic review examining the association between antibiotic use and CDI risk reported odds ratios ranging from 2.12–42 for clindamycin and 3.84–26 for third-generation cephalosporins.53 A more recent meta-analysis found odds ratios of 3.2 for third-generation cephalosporins and 2.86 for clindamycin.52 Fluoroquinolones have also been linked to an increased risk of infection with the hypervirulent BI/NAP1/027 strain.12
Historically, in some cases, simply withdrawing the offending antibiotic was considered a stand-alone treatment strategy for CDI.55 Olson et al. evaluated 908 patients with CDI from 1982–1991 and found that 15% experienced symptom resolution with antibiotic withdrawal alone, without specific CDI-directed antibiotic therapy.56 Whether antibiotic withdrawal remains sufficiently effective as monotherapy for mild CDI is uncertain, although some evidence suggests it may be a beneficial adjunct when combined with standard CDI therapy.57 Importantly, failure to discontinue the inciting antibiotics is associated with an increased risk of CDI recurrence.58 Therefore, in the context of Clostridioides difficile diagnosis and management, antibiotic stewardship and careful consideration of ongoing antibiotic regimens are crucial.
Metronidazole versus Vancomycin for CDI Treatment (Context for Diagnosis)
Metronidazole and vancomycin have been the primary antibiotic therapies for CDI since the 1980s. Early studies suggested that oral metronidazole and oral vancomycin had comparable efficacy, tolerability, and relapse rates.56,59,60 However, more recent data indicate higher treatment failure rates with metronidazole compared to vancomycin, particularly in severe or complicated CDI.3,61–64
A large retrospective study observed an increase in oral metronidazole treatment failures (from 10% to 26%) and a rise in the 60-day probability of recurrence (from 21% to 47%) after the emergence of the BI/NAP1/027 strain.4 However, other studies have not consistently demonstrated increased metronidazole failure rates following the emergence of BI/NAP1/027.65,66
Zar et al. conducted a randomized trial comparing metronidazole and vancomycin in 150 patients, stratified by CDI severity. In patients with mild CDI, cure rates were similar between metronidazole and vancomycin (90% vs. 98%, respectively). However, in patients with severe CDI, vancomycin showed significantly better cure rates (76% vs. 97%).63 A systematic review of studies from 2001–2010 reported higher treatment failure rates with metronidazole compared to vancomycin (22.4% vs. 14.2%; P = 0.002), while recurrence rates were similar (27.1% vs. 24.0%; P = 0.26). Metronidazole treatment failures were found to be more frequent in North America than in Europe.3 A large clinical trial comparing tolevamer (a toxin-binding polymer) to vancomycin and metronidazole found that while tolevamer was inferior to both, metronidazole was also inferior to vancomycin, with success rates of 44.2%, 72.7%, and 81.1%, respectively. These differences were more pronounced in severe CDI (66.3% for metronidazole vs. 78.5% for vancomycin).64
Factors associated with metronidazole treatment failures include age >60 years, fever, hypoalbuminemia, peripheral leukocytosis, ICU admission, and abnormal abdominal CT imaging.61–63 Patients with hematologic malignancies and CDI have also been shown to respond less favorably to both metronidazole and vancomycin (53.7% and 50%, respectively).67
Patients treated with metronidazole may experience a longer time to symptomatic improvement compared to those receiving vancomycin.60,68 A retrospective study of 102 patients after the emergence of the BI/NAP1/027 strain found that only 71% of patients responded to metronidazole within 6 days. The overall response rate was 91%, with treatment failures associated with greater illness severity.62
Oral vancomycin is generally well-tolerated. However, systemic absorption of both oral and rectal vancomycin can occur, albeit rarely.69 Metronidazole is associated with gastrointestinal side effects, a disulfiram-like reaction when combined with alcohol, and peripheral neuropathy with prolonged use.70 These considerations regarding treatment options and their relative effectiveness further emphasize the importance of accurate Clostridioides difficile diagnosis to guide appropriate therapy selection based on disease severity and risk factors.
Treatment by Disease Severity (Algorithm Context)
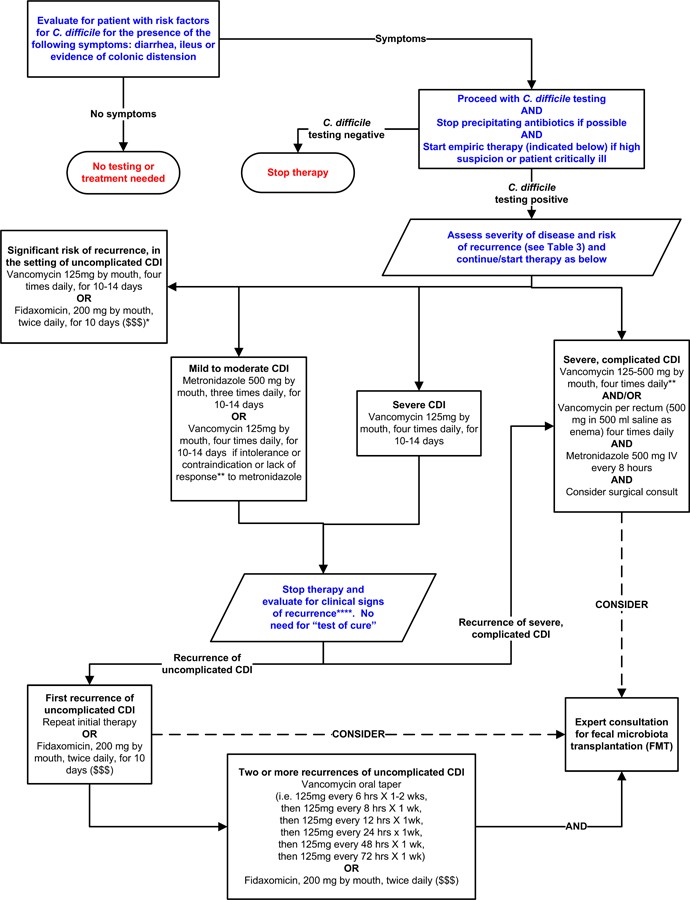
Table 3 outlines definitions of CDI severity, recurrence, and associated risk factors.15,16,20 Figure 2 presents a possible treatment algorithm for CDI based on disease severity. However, it’s important to note that this specific algorithm has not been formally validated.71–73,74,75 This algorithm and the treatment discussions are included here to provide context for the critical role of Clostridioides difficile diagnosis in guiding clinical management pathways.
Figure 2. Possible Approach for the Treatment of Clostridioides difficile Infection (CDI).
Alt Text: Treatment algorithm for Clostridioides difficile infection based on disease severity, guiding therapy choices between metronidazole, vancomycin, fidaxomicin, and fecal microbiota transplantation.
Footnotes: *Suggested approach for CDI treatment according to disease severity based on current guidelines, recent reviews/meta-analyses of fecal microbiota transplantation and randomized controlled trials of fidaxomicin. This approach is not validated. There are no data supporting the use of fidaxomicin for complicated CDI. **Treatment response is defined by clinical improvement in diarrhea or other signs of infection; response may require 3–5 days after starting therapy, but therapy escalation can be considered sooner based on disease severity. ***Duration of therapy depends on treatment response. ****Consider post-infectious irritable bowel syndrome rather than recurrent CDI for mild symptoms. “$ $ $” indicates that costs are substantially higher. References: 15,16,71–73,75
Treating Mild to Moderate CDI
For mild to moderate CDI, oral metronidazole remains a frequently recommended first-line therapy, partly due to its lower cost compared to vancomycin.15,16,63 The standard dose is 500mg orally three times daily for 10–14 days. For patients unable to take oral medications, intravenous metronidazole can be administered at the same dose. However, intravenous metronidazole is generally not recommended as monotherapy for CDI.15,16 Based on a recent study64 indicating lower clinical success rates for metronidazole compared to vancomycin, considering vancomycin for mild to moderate CDI may be reasonable in certain clinical scenarios.
Treating Severe or Complicated CDI
Vancomycin is the preferred therapy for severe or complicated CDI.15,16,63 Vancomycin 125 mg orally four times daily for 10–14 days has been shown to be non-inferior to higher doses in the absence of complicated infection.22 However, expert opinion often favors using higher vancomycin doses in severe or complicated disease.15,16
Rectal vancomycin administration may be considered as adjunctive therapy in cases of ileus, although evidence is primarily limited to case reports.15,76,77 Rectally administered vancomycin is not typically used as monotherapy because it may not reach the entire affected colonic area.78 Intravenous metronidazole achieves detectable levels throughout the colon79 and may be used as adjunctive therapy for ileus or severe/complicated CDI, often in combination with oral and/or rectal vancomycin. However, randomized controlled trials supporting this practice are lacking.15,16 Treatment failures have been reported in patients with ileus treated with intravenous metronidazole monotherapy.56,77
Prompt surgical evaluation is essential for patients with complicated CDI. Early surgical intervention can improve mortality rates.80,81 Subtotal or total colectomy with end ileostomy is frequently performed when surgery is necessary, although colon-preserving surgical techniques are also emerging.80,81 These treatment considerations, while briefly summarized here, underscore the critical importance of accurate and timely Clostridioides difficile diagnosis to facilitate appropriate and severity-directed therapeutic interventions.
Treating Recurrent Clostridioides difficile Infection
Recurrent CDI is more common in older patients and those with factors such as concomitant antibiotic use, comorbidities, proton pump inhibitor use, and greater initial disease severity.11,16 An inadequate antibody response following a CDI episode has also been associated with increased recurrence rates.82,83
Clinical guidelines recommend oral metronidazole or vancomycin for the first recurrence of mild-moderate CDI.15,16 Vancomycin is generally recommended for subsequent recurrences. Pulsed or tapered vancomycin courses are often employed in recurrent CDI management.84 Randomized trials are limited, but case series and case reports support the use of pulsed or tapered vancomycin regimens.23,84,85 McFarland et al. enrolled 163 patients with recurrent CDI and reported an overall subsequent recurrence rate of 44.8%. Tapered and pulsed vancomycin courses were associated with fewer recurrences (31%, p=0.01 and 14.3%, p=0.02, respectively), although patient numbers in these subgroups were small (29 and 7, respectively).23
Fidaxomicin, approved for CDI treatment in 2011, has demonstrated similar cure rates to oral vancomycin in randomized studies.74,86 In a double-blinded randomized trial by Cornely et al., 221/252 (87.7%) of patients receiving fidaxomicin for CDI achieved clinical cure, compared to 223/257 (86.8%) of patients receiving vancomycin. These results met non-inferiority criteria between fidaxomicin and vancomycin.74 Louie et al. reported clinical cure rates with fidaxomicin that were noninferior to vancomycin (88.2% versus 85.8%) in 629 patients, with significantly fewer recurrences with fidaxomicin (15.4% vs. 25.3%, P = 0.005).86
When ongoing antibiotic therapy for other infections cannot be discontinued, clinical cure rates for concomitant CDI have been shown to be higher with fidaxomicin than with vancomycin.58 Fidaxomicin may also better preserve the gut microbiota compared to alternative CDI treatments.75 However, fidaxomicin is not typically considered first-line therapy for mild or uncomplicated CDI due to its higher cost.87 Data supporting fidaxomicin use in complicated or fulminant CDI are lacking.16 Fidaxomicin may be considered for recurrent CDI, for initial CDI episodes with a high risk of recurrence, or immediately following a vancomycin course in patients with multiple CDI recurrences.16,84,88
Anecdotal evidence suggests rifaximin as a potential adjunctive therapy for recurrent CDI, typically after a course of standard CDI treatment.89,90 However, rifaximin monotherapy should be avoided due to the propensity for resistance development.89 Nitazoxanide is not a first-line therapy for initial CDI episodes but may be considered as adjunctive therapy for recurrent CDI, although data are limited.15 These diverse treatment strategies for recurrent CDI further underscore the importance of accurate initial and recurrent Clostridioides difficile diagnosis to guide appropriate therapeutic interventions.
Probiotics and Fecal Microbiota Transplantation for Recurrent CDI
Recurrent CDI can manifest as relapse of the original infection or as reinfection with a different C. difficile strain. Preserving or restoring normal gut microbiota diversity is a key strategy for preventing and treating CDI recurrences.91
Probiotics, live microorganisms that can help restore normal gut microbiota, have been investigated for CDI management. The role of probiotics in CDI treatment is still being defined, but evidence suggests they may have a role in preventing initial CDI episodes and recurrences.92–94 Although generally well-tolerated, probiotic-associated bacteremia and fungemia have been reported, primarily in immunocompromised or critically ill patients.95 However, major side effects with probiotics are generally uncommon.96 A recent case series suggested that daily administration of kefir, a probiotic fermented milk product, combined with staggered, tapered doses of vancomycin or metronidazole, may be beneficial in recurrent CDI.97
Fecal microbiota transplantation (FMT) is a more direct approach to restoring gut microbiota diversity. FMT involves the instillation of donor stool into the gastrointestinal tract of a CDI patient. This procedure has shown promising clinical responses with limited reported adverse events in refractory or recurrent CDI.71–73 The first systematic review of FMT in recurrent CDI, published in 2011, included 317 patients treated with FMT via enema, nasojejunal tube/gastroscope, or colonoscopy. Clinical resolution was achieved in 92% of patients (89% after a single treatment), with no serious adverse effects reported.73 A more recent review of 536 patients reported an 87% clinical response rate with FMT.72
A randomized trial of FMT demonstrated symptom resolution in 94% of patients who received vancomycin for 5 days followed by one or two FMT treatments, compared to only 31% in those receiving vancomycin alone for 14 days and 23% in those receiving vancomycin for 14 days plus bowel lavage. This study was stopped early due to interim analyses showing the clear superiority of FMT. Among 18 patients in the other treatment groups who subsequently received FMT, 83% achieved symptom resolution.98
In 2013, a stool substitute preparation, derived from purified fecal cultures from a single healthy donor, was used to treat two patients with recurrent CDI who had failed repeated antibiotic courses, resulting in symptom resolution.99 An earlier study in 1989 used rectal administration of ten facultatively aerobic and anaerobic bacteria to successfully treat five CDI patients.100 A recent feasibility study used frozen fecal capsules, prepared from prescreened unrelated donors, to treat 20 patients with recurrent CDI, achieving a 90% response rate after one or two treatment courses.101 Pre-screened, filtered, and frozen donor stool for FMT is also commercially available.102 However, in the U.S., the FDA considers FMT investigational, requiring an Investigational New Drug application. Anecdotal reports also support FMT for treating refractory or complicated CDI in the context of ileus or megacolon.103 These emerging therapeutic modalities, particularly FMT, offer promising options for recurrent CDI management, further highlighting the clinical significance of accurate Clostridioides difficile diagnosis in identifying appropriate candidates for these advanced therapies.
Other Therapies for CDI Treatment (Brief Mention)
Other Antibiotics
Teicoplanin has been shown to be noninferior to vancomycin for CDI treatment, but it is not currently available in the U.S.59 Case reports suggest potential efficacy of tigecycline in severe or recurrent CDI,104 but its role in CDI management remains uncertain. Phase III clinical trials are ongoing for novel antibiotics such as surotomycin and cadazolid.
Toxin Binders
Randomized trial data indicate that nonabsorbable anionic polymers like colestipol and cholestyramine are not effective for CDI treatment. Tolevamer, another anionic polymer, binds C. difficile toxins A and B. However, recent data have demonstrated that tolevamer is inferior to both vancomycin and metronidazole for CDI.64 Polymers can also bind other medications, such as vancomycin, and should not be administered concurrently with standard CDI therapy.15
Immunotherapy
Serum antibody response to toxin A may provide protection against recurrent symptomatic CDI.45,82 A C. difficile vaccine is under development for both primary prevention and recurrent CDI prevention.105–107
Pooled intravenous immunoglobulin contains antibodies that neutralize C. difficile toxins in vitro. However, limited data support its use for recurrent CDI,[108](#R108] and its role in severe CDI remains unclear. In a randomized, double-blind, placebo-controlled study, the combination of two neutralizing human monoclonal antibodies against C. difficile toxins A (CDA1) and B (CDB1), administered with standard therapy, resulted in a lower recurrent CDI rate (7% vs. 25%).109 Phase III clinical trials are currently evaluating monoclonal antibodies such as MK-3415 (anti-toxin A), MK-6072 (anti-toxin B), and MK-3415A (anti-toxin A and B) to prevent recurrent CDI in patients receiving standard CDI therapies.110 These evolving therapeutic strategies, including immunotherapy, highlight the ongoing efforts to improve CDI management and underscore the continued importance of accurate Clostridioides difficile diagnosis in optimizing patient care.
Discussion
Clinical manifestations of Clostridioides difficile infection are highly variable, ranging from asymptomatic colonization to fulminant disease. A key challenge in Clostridioides difficile diagnosis is that routine laboratory testing cannot differentiate between asymptomatic colonization and active CDI. Therefore, diagnostic testing should be selectively performed only in patients presenting with CDI symptoms.15 A variety of diagnostic testing strategies are available for CDI diagnosis, each with its own strengths and limitations. Many experts and clinical guidelines recommend multistep diagnostic algorithms to optimize diagnostic accuracy and efficiency.15,16,19,34
Decisions regarding whether and how to treat Clostridioides difficile infection should be guided by disease severity and the patient’s risk of CDI relapse. Oral vancomycin is generally recommended for severe, complicated, or recurrent CDI, while oral metronidazole is often recommended for mild to moderate disease. However, these recommendations may evolve as further research clarifies the relative effectiveness of metronidazole compared to vancomycin, particularly in different CDI severity categories.15,16,64 Fidaxomicin may be considered in situations with a high risk of CDI recurrence, but its higher cost may be a limiting factor. The evidence base supporting the use of fecal microbiota transplantation (FMT) for recurrent CDI is growing robustly,71–73,98 although the regulatory landscape and standardization of FMT procedures are still evolving. Ongoing research is focused on developing synthetic stool substitutes for CDI treatment99 and convenient FMT administration methods such as encapsulated stool preparations.101
Conclusion
Clostridioides difficile remains a significant cause of morbidity and mortality, posing a persistent challenge to healthcare systems. Effective CDI management hinges on accurate and timely Clostridioides difficile diagnosis, followed by treatment strategies tailored to disease severity and recurrence risk. Fecal microbiota transplantation has emerged as a promising approach for symptom resolution in recurrent CDI, and its role in CDI management may expand further in the future. Continued research and advancements in diagnostic and therapeutic strategies are crucial to mitigate the impact of Clostridioides difficile infection and improve patient outcomes.
Supplementary Material
Appendix
NIHMS1033273-supplement-Appendix.docx (625.9KB, docx)
BOX: “Key messages regarding diagnosis and treatment of Clostridioides difficile infection in adults.
DIAGNOSIS
- Clostridioides difficile infection (CDI) diagnosis requires the presence of diarrhea (three or more unformed stools in 24 hours) AND a positive stool test for toxigenic C. difficile or its toxins, or colonoscopic/histopathologic evidence of pseudomembranous colitis. Laboratory testing alone cannot differentiate between colonization and infection. CDI testing should be performed only in symptomatic patients.
- Diagnostic testing strategies for CDI vary. Multistep approaches using polymerase chain reaction (PCR) for toxin gene detection or single-step PCR on liquid stool samples demonstrate the highest sensitivity and specificity for accurate Clostridioides difficile diagnosis.
- “Test of cure” is not recommended after CDI treatment.
TREATMENT
- CDI treatment should be guided by disease severity and the risk of recurrence or complications.
- Vancomycin and metronidazole are first-line therapies for CDI.
- Vancomycin is generally preferred for severe or complicated CDI.
- Recurrent CDI is more common in older patients and those with concomitant antibiotic use, comorbidities, proton pump inhibitor use, and greater initial disease severity.
- Oral metronidazole or vancomycin are recommended for the first recurrence of mild-moderate CDI.
- Vancomycin is recommended for patients with two or more CDI recurrences.
- Fidaxomicin may be considered for recurrent CDI, particularly in cases with high recurrence risk.
- Fecal microbiota transplantation is associated with symptom resolution in recurrent CDI and is an important therapeutic option in this setting.
Acknowledgements:
The authors extend their gratitude to Mrs. Whitney Townsend, MLIS (University of Michigan), for her invaluable assistance with the literature search. Ms. Townsend’s contributions were part of her regular employment responsibilities and were not separately compensated.
Funding/Support: This work was supported in part by the National Institutes of Health grant 1U19AI090871-01 (Drs. Rao and Malani), the Claude D. Pepper Older Americans Independence Center grant AG-024824 (Dr. Rao), and the Michigan Institute for Clinical and Health Research grant 2UL1TR000433 (Dr. Rao).
Footnotes
Conflicts of Interest Disclosures: All authors have completed and submitted the ICJME Form for Disclosure of Potential Conflicts of Interest: None reported. JAMA Associate Editor, Dr. Malani, had no role in the review of this paper or the decision to accept it for publication.
Role of the Sponsor: The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
REFERENCES
[List of references from the original article – No changes made]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
NIHMS1033273-supplement-Appendix.docx (625.9KB, docx)