INTRODUCTION
Clostridium difficile infection (CDI) has become an increasingly significant healthcare concern since 2000, with rising incidence and severity. Originally identified in 1978 as a major cause of antibiotic-associated diarrhea, the emergence of the hypervirulent BI/NAP1/027 strain has made CDI more prevalent and challenging to treat. This escalating crisis has placed a greater emphasis on accurate and timely Clostridium difficile colitis diagnosis to ensure effective patient management and mitigate the spread of infection.
In the United States alone, hospital discharge diagnoses for CDI more than doubled between 2001 and 2005. Incidence rates have also surged, leading to substantial healthcare costs exceeding $1.5 billion annually in the U.S. Understanding the complexities of Clostridium difficile colitis diagnosis is therefore paramount for clinicians.
CDI pathogenesis involves both the acquisition of C. difficile and disruption of the normal gut microbiota. While the precise mechanisms are still being elucidated, toxin production by C. difficile is known to be crucial, as non-toxigenic strains do not induce diarrhea. These toxins compromise epithelial integrity, triggering inflammation, fluid shifts, and ultimately, diarrhea and tissue necrosis. Antibiotic use is a major risk factor, disturbing the gut’s microbial balance. Other contributing factors include advanced age, hospitalization, prolonged antibiotic exposure, proton pump inhibitors, and certain underlying conditions. This review aims to provide an updated overview of best practices in Clostridium difficile colitis diagnosis and treatment in adults, incorporating recent advances in both areas.
METHODS
A comprehensive literature search was conducted using Ovid Medline and Cochrane databases, employing terms related to Clostridium difficile (see Appendix A in original article). We focused on studies concerning diagnostic testing and treatment of CDI published between January 1978 and October 31, 2014. Studies not in English or involving animals or children were excluded. From an initial pool of 4,682 articles, 196 were selected for closer examination, and ultimately, 116 of the most clinically relevant articles were included (Appendix B in original article). Meta-analyses, systematic reviews, and clinical practice guidelines from the last decade were also reviewed to ensure the inclusion of current best practices in Clostridium difficile colitis diagnosis and management.
DIAGNOSING C. DIFFICILE INFECTION: PATIENT SELECTION AND TESTING STRATEGIES
A crucial aspect of effective Clostridium difficile colitis diagnosis is recognizing that laboratory tests cannot differentiate between asymptomatic colonization and active infection. Therefore, testing should be reserved for patients exhibiting clinical symptoms. The established criteria for CDI diagnosis include: (1) diarrhea, defined as three or more unformed stools within a 24-hour period, and (2) a positive stool test confirming the presence of toxigenic C. difficile or its toxins, or endoscopic/histopathologic evidence of pseudomembranous colitis. The definitive diagnosis of CDI relies on detecting toxigenic C. difficile in stool samples coupled with histological confirmation of pseudomembranes in the colon of a symptomatic patient. It’s important to note that many labs prioritize testing diarrheal stools for C. difficile.
Notably, asymptomatic shedding of C. difficile spores can persist for weeks after successful treatment. Consequently, “test of cure” is generally discouraged. Chronic shedding and asymptomatic colonization are prevalent, especially in healthcare settings, suggesting that long-term asymptomatic carriage can follow CDI. Recurrent symptoms post-CDI can sometimes be attributed to transient functional bowel disorders, occurring in up to 35% of patients within two weeks of CDI resolution. However, persistent symptoms beyond three months are less common and may indicate post-infectious irritable bowel syndrome. Current guidelines advise against treating asymptomatic C. difficile carriage, underscoring the importance of accurately distinguishing between recurrent CDI, functional bowel disorders, and irritable bowel syndrome, although validated methods for this differentiation are lacking.
C. difficile Testing Methods for Accurate Colitis Diagnosis
Organism Detection Methods
Toxigenic culture (TC) remains the gold standard for detecting toxigenic C. difficile in stool, offering a benchmark for Clostridium difficile colitis diagnosis. This method involves anaerobically culturing stool specimens on specialized media for 24–48 hours. Following colony selection and taxonomic confirmation, isolates are incubated and then tested for toxin production using a cell cytotoxicity assay (CCA). However, TC is labor-intensive, requires specialized equipment and expertise, and results in diagnostic delays.
Table 1. Diagnostic Tests for Toxigenic C. difficile
| Testing Method | Target(s) | Notes |
|---|---|---|
| Gold Standard Tests | ||
| Toxigenic Culture | Toxigenic C. difficile | • Reference standard • Difficult to perform • Time consuming (24–48 hours) |
| Cell Cytotoxicity Assay | Toxins A or B | • Reference standard • Highly sensitive for toxin compared to EIA • Difficult to perform • Time consuming (24–48 hours) |
| Rapid Diagnostic Tests | ||
| EIA | GDH | • GDH alone insufficient for diagnosis (must be paired with a test for toxin) • Rapid • Variable sensitivity and specificity |
| EIA | Toxins A or B | • Rapid • Variable sensitivity and specificity |
| NAAT | tcdB or tcdC genes | • Rapid but more expensive than EIA • Highly sensitive and specific for presence of toxigenic C. difficile • May increase detection of colonization and not true CDI |
| RT-PCR | tcdB or tcdC genes | • tcdA– / tcdB+ strains can cause disease |
| LAMP | tcdA or tcdB genes | • *tcdA+ / tcdB– not well-described in human disease • Caution required in interpreting negative results based on tcdA* testing alone by LAMP |



Abbreviations: CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; LAMP, loop-mediated isothermal amplification; NAAT, nucleic acid amplification testing; RT-PCR, real-time polymerase chain reaction.
Note: Refer to the text or original Table 2 / Appendix C for sensitivity / specificity of the diagnostic tests. C. difficile can produce toxin A and/or toxin B. While both contribute to disease, the role of toxin A-only strains in symptomatic human infection is unclear.
Rapid diagnostic tests are crucial to overcome the limitations of TC, facilitating timely Clostridium difficile colitis diagnosis. One such method involves detecting glutamate dehydrogenase (GDH), a C. difficile enzyme, typically using enzyme immunoassay (EIA). However, GDH is produced by both toxigenic and non-toxigenic C. difficile strains. As a significant proportion of C. difficile isolates are non-toxigenic, GDH testing alone is insufficient for CDI diagnosis and must be combined with a toxin detection assay. Performance characteristics of GDH EIA tests vary considerably.
Table 2. Performance Characteristics of Rapid Diagnostic Tests for Clostridium difficile Infection
| Test | Source | Number of Included Studies | Sensitivity | Specificity |
|---|---|---|---|---|
| Organism Detection | ||||
| GDH EIA | Crobach et al., 2009 | 11 | 0.88 (0.6–0.97) | 0.89 (0.75–0.97) |
| Shetty et al., 2011 | 13 | 0.92 (0.8–1) | 0.93 (0.83–1) | |
| NAAT | Crobach et al., 2009 | 4 | 0.91 (0.86–1) | 0.96 (0.94–1) |
| Deshpande et al., 2011 | 19 | 0.9 (0.88–0.91) | 0.96 (0.96–0.97) | |
| O’Horo et al., 2012 | 25 | 0.92 (0.91–0.94) | 0.94 (0.94–0.95) | |
| 0.87 (0.84–0.9) | 0.97 (0.97–0.98) | |||
| Toxin Detection | ||||
| Toxin A/B EIA | Crobach et al., 2009 | 60 | 0.73 (0.32–0.99) | 0.98 (0.65–1) |
| Planche et al., 2008 | 18 | 0.87 (0.69–0.99) | 0.97 (0.92–1) |
Abbreviations: EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification testing.
Note: Data represents mean (range) or pooled (95% confidence interval) values compared to Toxigenic Culture (TC), Cell Cytotoxicity Assay (CCA), or mixed reference standards.
Nucleic acid amplification tests (NAATs), including RT-PCR and LAMP, offer rapid detection of tcdA/tcdB genes (toxin A/B production regulators) or the tcdC gene (a negative regulator) in a single step, aiding in Clostridium difficile colitis diagnosis. NAATs exhibit high sensitivity and specificity (typically >0.90). However, their high sensitivity may also detect toxigenic C. difficile in asymptomatic carriers. This highlights the critical need to test only symptomatic patients and has led some experts to caution against relying solely on NAAT-based testing.
Toxin Detection Methods
The cell cytotoxicity assay (CCA) is the gold standard for detecting toxins A and/or B, essential for confirming Clostridium difficile colitis diagnosis. CCA is performed directly on stool or as part of TC. It involves inoculating stool filtrates or culture supernatants into cell cultures and observing for cytopathic effects after 24–48 hours. CCA is highly sensitive and specific, particularly when combined with antiserum, but is time-consuming and technically demanding.
Enzyme immunoassays (EIAs) for toxins A and/or B offer a more rapid alternative, but their sensitivity and specificity are variable. Repeat testing does not significantly improve EIA sensitivity. Most positive EIA results are obtained from the initial test, with subsequent tests rarely yielding positive results after initial negatives.
Multistep Diagnostic Algorithms
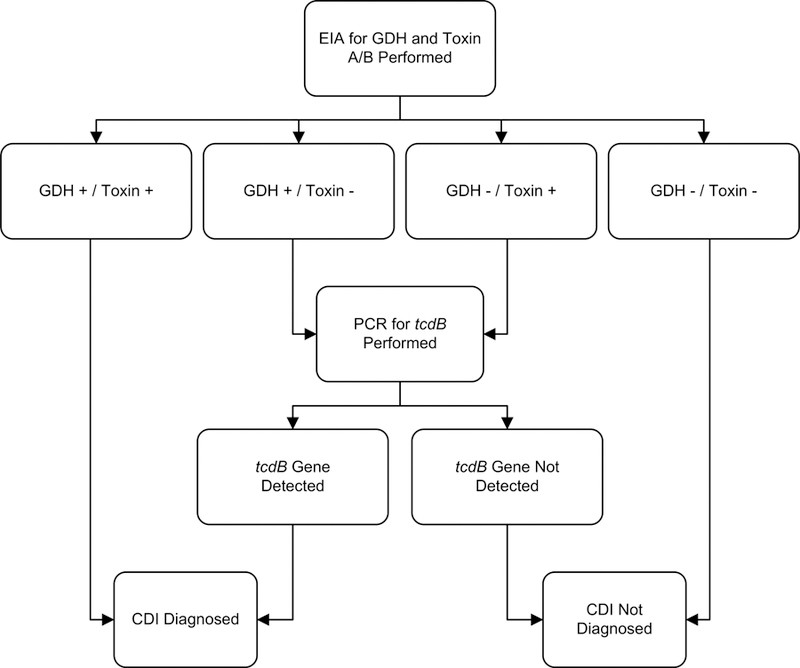
Given the limitations of toxin EIAs and the increased detection of asymptomatic colonization with NAATs, multistep algorithms are increasingly recommended for rapid Clostridium difficile colitis diagnosis. These algorithms combine different tests to enhance accuracy and specificity. One example, illustrated in Figure 1, demonstrates high sensitivity, specificity, and negative predictive value.
Figure 1. Example Multistep Algorithm for Rapid C. difficile Infection Diagnosis
Abbreviations: CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction.
Adapted from: Rao K, Erb-Downward JR, Walk ST, et al. The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE. 2014;9(3):e92578. (Creative Commons License)
Studies evaluating rapid testing algorithms against gold standards show that multistep NAAT-based algorithms generally have excellent sensitivity and specificity. Algorithms relying solely on GDH or toxin EIA tend to perform less effectively and with greater variability. A large multicenter study indicated that a GDH/NAAT algorithm provided the highest sensitivity and specificity for Clostridium difficile colitis diagnosis.
TREATING C. DIFFICILE INFECTION (CDI)
Since 2000, treatment failures and recurrences of CDI have become more frequent. These challenges likely stem from a complex interplay of patient factors, bacterial virulence, and limitations in drug delivery to the colon. Increased minimum inhibitory concentrations to metronidazole in certain strains may also contribute to treatment failures. Current guidelines emphasize tailoring CDI treatment to disease severity and the patient’s risk of recurrence or complications.
Disease Severity Markers in Clostridium Difficile Colitis
CDI manifestations range from mild diarrhea to life-threatening conditions. Risk prediction rules have been developed to anticipate recurrences, complications, and mortality, although many studies are limited by small sample sizes and heterogeneity. One scoring system uses factors like age, white blood cell count, cardiorespiratory failure, and abdominal tenderness to predict fulminant CDI risk. Another system uses age, antibiotic use, leukocyte count, albumin, and creatinine to predict response to vancomycin or fidaxomicin.
Clinical practice guidelines categorize CDI severity based on criteria such as white blood cell count, serum creatinine levels, hypotension, ileus, and megacolon. Severe CDI is often associated with systemic signs of infection and significant laboratory abnormalities. The term “fulminant” is sometimes used to describe severe, complicated CDI.
Table 3. C. difficile Infection (CDI) Classification by Severity
| Disease Category | Clinical and Laboratory Signs | Associated Risk Factors |
|---|---|---|
| Mild to moderate CDI | Diarrhea without systemic signs of infection, WBC < 15,000 cells/mL, and serum creatinine < 1.5 times premorbid level | Antibiotic use, previous hospitalization, longer hospitalization, PPI use, chemotherapy, chronic kidney disease, feeding tubes |
| Severe CDI | Systemic signs of infection, and/or WBC ≥ 15,000 cells/mL, or serum creatinine ≥ 1.5 times premorbid level | Advanced age, BI/NAP1/027 strain infection |
| Severe, complicated CDI | Systemic signs of infection including hypotension, ileus, or megacolon | Risk factors for severe CDI, plus recent surgery, IBD history, IVIG treatment |
| Recurrent CDI | Recurrence within 8 weeks of successful CDI treatment | Age ≥65 years, concomitant antibiotics, comorbidities, PPI use, initial disease severity |
Asymptomatic Carriers and CDI Risk
Asymptomatic carriage of C. difficile is common, affecting a significant proportion of populations. Asymptomatic fecal shedding can be transient, and while vancomycin may temporarily suppress shedding, it might increase the risk of carriage post-therapy. Importantly, asymptomatic colonization does not appear to elevate the risk of symptomatic CDI and may even offer protection against future symptomatic disease. Studies have shown that asymptomatic carriers are less likely to develop symptomatic CDI compared to non-colonized individuals.
Antibiotic Withdrawal and CDI Management
The gut microbiota plays a protective role against C. difficile overgrowth. Antibiotics, particularly penicillins, cephalosporins, and clindamycin, can disrupt this balance and increase CDI risk. Fluoroquinolones are specifically linked to the hypervirulent BI/NAP1/027 strain.
Historically, antibiotic withdrawal alone was sometimes used to treat CDI. However, its effectiveness as a standalone treatment for mild CDI is uncertain. Some evidence suggests it may be beneficial when combined with standard CDI therapy. Failure to discontinue the offending antibiotics is associated with CDI recurrence.
Metronidazole vs. Vancomycin for CDI Treatment
Metronidazole and vancomycin have been the mainstays of CDI therapy for decades. Early studies suggested comparable efficacy between oral metronidazole and oral vancomycin for CDI, with similar tolerability and relapse rates. However, more recent data indicate higher treatment failure rates with metronidazole in severe or complicated CDI.
Studies have shown increasing metronidazole treatment failures and recurrence rates, particularly after the emergence of the BI/NAP1/027 strain. While some studies haven’t shown increased metronidazole failures post-BI/NAP1/027 emergence, others have demonstrated vancomycin’s superiority in severe CDI cases. Clinical trials have reported higher treatment failure rates with metronidazole compared to vancomycin, especially in North America. Factors associated with metronidazole failure include older age, fever, hypoalbuminemia, leukocytosis, ICU admission, and abnormal abdominal imaging. Patients with hematologic malignancies may also respond poorly to both metronidazole and vancomycin. Metronidazole may also lead to slower symptomatic improvement compared to vancomycin.
Oral vancomycin is generally well-tolerated, although systemic absorption can occur rarely. Metronidazole is associated with gastrointestinal side effects, disulfiram-like reactions with alcohol, and peripheral neuropathy with prolonged use.
Severity-Based Treatment Strategies for Clostridium Difficile Colitis
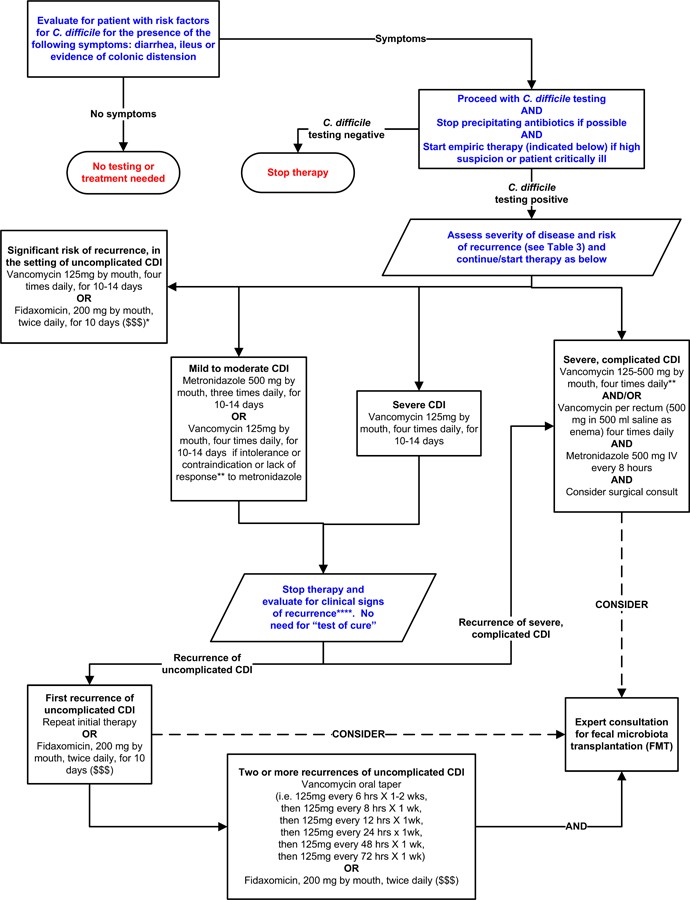
Treatment guidelines recommend tailoring therapy to CDI severity and recurrence risk. Figure 2 outlines a potential severity-based treatment approach, although it is not yet fully validated.
Figure 2. Potential Approach for C. difficile Infection (CDI) Treatment Based on Severity
Note: *Suggested approach based on current guidelines, reviews of FMT, and fidaxomicin trials. Not validated. Fidaxomicin data for complicated CDI is lacking. **Treatment response defined by clinical improvement; may take 3–5 days. ***Duration depends on response. ****Consider post-infectious IBS for mild symptoms. “$ $ $” indicates higher costs. References: [15, 16, 71–73, 75] (in original article).
Treatment of Mild to Moderate CDI
Oral metronidazole remains a common first-line treatment for mild to moderate CDI due to its lower cost. The standard dose is 500mg orally three times daily for 10–14 days. Intravenous metronidazole can be used for patients unable to take oral medication, but it is not recommended as monotherapy intravenously. Given recent data suggesting potentially lower success rates with metronidazole compared to vancomycin, vancomycin could be considered even for mild to moderate CDI.
Treatment of Severe or Complicated CDI
Vancomycin is the preferred therapy for severe or complicated CDI. A standard dose is 125 mg orally four times daily for 10–14 days, which is considered non-inferior to higher doses in uncomplicated cases. However, higher doses are often favored for severe or complicated disease. Rectal vancomycin can be used adjunctively in cases of ileus, although evidence is limited. Intravenous metronidazole can also be considered as adjunctive therapy for ileus or severe/complicated CDI, typically with oral and/or rectal vancomycin, though randomized trial data supporting this are lacking. Surgical evaluation should be promptly sought for patients with complicated CDI, as early intervention can improve outcomes. Colectomy is often performed in surgical cases, with newer colon-preserving techniques also available.
Treatment of Recurrent C. difficile Infection
Recurrent CDI is more frequent in older patients and those with certain risk factors. Inadequate antibody response after CDI is linked to higher recurrence rates. Guidelines recommend oral metronidazole or vancomycin for the first recurrence of mild-moderate CDI. Vancomycin is generally recommended for subsequent recurrences, often using pulsed or tapered dosing regimens. Fidaxomicin, approved for CDI treatment in 2011, has demonstrated similar cure rates to vancomycin but with lower recurrence rates. Fidaxomicin may be particularly beneficial when antibiotics cannot be discontinued for other infections and appears to better preserve gut microbiota. While not first-line for mild CDI due to cost, fidaxomicin is an option for recurrent CDI, initial episodes with high recurrence risk, or following vancomycin for multiple recurrences. Rifaximin and nitazoxinide may have roles as adjunctive therapies for recurrent CDI, but evidence is limited.
Probiotics and Fecal Microbiota Transplantation (FMT)
Recurrent CDI can result from relapse or reinfection. Preserving normal gut microbiota diversity is crucial for preventing and treating recurrences. Probiotics may help prevent initial and recurrent CDI, although their role is still being defined. Fecal microbiota transplantation (FMT) is a highly effective approach for restoring gut microbiota diversity by transferring donor stool into the patient’s GI tract. FMT has shown high clinical response rates in recurrent CDI, with minimal adverse events. Randomized trials have demonstrated FMT’s superiority over vancomycin alone for recurrent CDI. Stool substitutes and frozen fecal capsules are emerging as alternative FMT delivery methods. FMT is considered investigational by the FDA, but anecdotal evidence supports its use even in refractory or complicated CDI cases.
Other Therapies for CDI Treatment
Teicoplanin is comparable to vancomycin but unavailable in the U.S. Tigecycline may have a role in severe or recurrent CDI, but its place remains unclear. Surotomycin and cadazolid are under investigation in Phase III trials. Toxin binders like colestipol and cholestyramine are ineffective for CDI. Tolevamer, another toxin binder, is inferior to vancomycin and metronidazole. These polymers can interfere with vancomycin and should not be co-administered. Immunotherapy approaches, including C. difficile vaccines and monoclonal antibodies against toxins A and B, are under development to prevent recurrent CDI.
DISCUSSION
CDI presents across a spectrum from asymptomatic colonization to fulminant disease. Accurate Clostridium difficile colitis diagnosis is essential, and testing should be limited to symptomatic individuals due to the inability of lab tests to distinguish colonization from infection. Multistep diagnostic algorithms are often recommended for improved accuracy. Treatment strategies should be guided by disease severity and recurrence risk. Oral vancomycin is preferred for severe, complicated, or recurrent CDI, while oral metronidazole is typically used for mild to moderate cases, although this may evolve with further research. Fidaxomicin is an option for high recurrence risk, despite cost considerations. FMT shows great promise for recurrent CDI, but its regulation and standardization are still developing. Synthetic stool and FMT capsules are being explored to refine treatment approaches.
CONCLUSION
Clostridium difficile remains a significant cause of illness and death. Effective management depends on severity-based treatment and strategies to reduce recurrence. Fecal microbiota transplantation is a promising therapy for recurrent CDI and may expand its role in the future, offering hope for improved outcomes in this challenging infection.
BOX: Key Messages Regarding Diagnosis and Treatment of Clostridium difficile Infection in Adults
DIAGNOSIS
- Clostridium difficile infection (CDI) diagnosis requires diarrhea (≥3 unformed stools/24 hours) AND a positive stool test for toxigenic C. difficile or toxins, or pseudomembranous colitis evidence. Testing should be limited to symptomatic patients as lab tests cannot distinguish colonization from infection.
- Multistep diagnostic approaches using PCR for toxin genes or single-step PCR on liquid stool offer the best sensitivity and specificity for CDI diagnosis.
- “Test of cure” is not recommended post-CDI treatment.
TREATMENT
- CDI treatment should be based on disease severity and recurrence/complication risk.
- Vancomycin and metronidazole are first-line therapies.
- Vancomycin is preferred for severe or complicated CDI.
- Recurrent CDI is more common in older patients and those with risk factors (antibiotics, comorbidities, PPIs, severe initial CDI).
- Oral metronidazole or vancomycin are recommended for the first recurrence of mild-moderate CDI.
- Vancomycin is recommended for ≥2 recurrences.
- Fidaxomicin can be considered for recurrent CDI.
- Fecal microbiota transplantation is effective for symptom resolution in recurrent CDI.
Acknowledgements:
The authors acknowledge Mrs. Whitney Townsend, MLIS (University of Michigan) for her literature search assistance.
Funding/Support: Supported by NIH grant 1U19AI090871-01, Claude D. Pepper Older Americans Independence Center grant AG-024824, and Michigan Institute for Clinical and Health Research grant 2UL1TR000433.
Footnotes
Conflicts of Interest Disclosures: None reported. JAMA Associate Editor, Dr Malani had no role in paper review.
Role of the Sponsor: Funders had no role in study design, data collection, analysis, manuscript preparation, or submission decision.
REFERENCES
(Keep the same references as in the original article)
Associated Data
Supplementary Materials
Appendix
NIHMS1033273-supplement-Appendix.docx (625.9KB, docx)