Introduction to Clostridium Sordellii and its Diagnostic Challenges
Clostridium sordellii and Clostridium perfringens are anaerobic bacteria that, while not common human pathogens, pose significant health risks due to their high fatality rates, especially in obstetric C. sordellii infections. Toxic shock syndrome caused by these bacteria is characterized by rapid onset and high mortality, making timely and accurate diagnosis crucial yet challenging. In many fatal cases, formalin-fixed, paraffin-embedded (FFPE) tissues are the primary specimens available. While immunohistochemistry (IHC) is often used to detect Clostridia, it lacks the specificity to identify individual species. This necessitates the development of more precise and rapid diagnostic tools for species-specific identification in FFPE tissues.
This article delves into the critical aspects of Clostridium sordellii diagnosis, particularly in the context of toxic shock. We will explore the development and evaluation of a duplex PCR-based microsphere assay designed for the simultaneous detection of both C. sordellii and C. perfringens. This innovative assay offers a significant advancement in diagnostic capabilities, providing a rapid, specific, and cost-effective solution for identifying these pathogens in FFPE tissues, which is paramount in forensic and retrospective diagnoses. Understanding the nuances of Clostridium sordellii diagnosis is not only vital for medical professionals but also holds relevance in broader contexts where bacterial infections and rapid diagnostic methods are of concern.
1. Understanding Clostridium Sordellii and Clostridium Perfringens Infections
Clostridium species are Gram-positive, spore-forming anaerobic bacteria prevalent in various environments, including soil and the intestinal tracts of humans and animals. Clostridium sordellii is known to cause a range of infections such as peritonitis, endocarditis, pneumonia, arthritis, cellulitis, and myonecrosis [1–4]. Notably, it is associated with fulminant toxic shock syndrome and sepsis, particularly in gynecological infections and neonatal omphalitis [3, 5]. Clostridium perfringens is another significant pathogen within this genus, responsible for conditions ranging from food poisoning and enteritis to gas gangrene, enterotoxemia, and endometritis [6–8]. Recent studies have highlighted the severe risk of pregnancy-associated toxic shock syndrome linked to both C. sordellii and C. perfringens [8–10].
The severity of Clostridium sordellii infections is underscored by its high case fatality rate, reported at 70% overall and exceeding 90% in obstetric cases [8]. Pregnancy-related C. perfringens infections also exhibit alarmingly high mortality rates [8]. A critical challenge in managing these infections is their rapid progression. Patients often succumb to hypotension and multiorgan failure within a short period following initial symptoms, emphasizing the urgent need for rapid and accurate diagnostic methods for Clostridium sordellii and Clostridium perfringens.
2. Challenges in Diagnosing Clostridium Toxic Shock (CTS)
Clinical diagnosis of Clostridium Toxic Shock (CTS) presents considerable challenges. Symptoms are often nonspecific, fever may be absent, and the overall rarity of these infections can lead to diagnostic delays [10]. Definitive diagnosis traditionally relies on microbiological methods such as bacterial culture, biochemical analysis, and ELISA assays. However, Clostridium species are notoriously difficult to isolate due to their fastidious anaerobic growth requirements, variable staining, and complex biochemical profiles [11, 12].
Moreover, in cases of sudden death, obtaining suitable specimens in sufficient quantity is often problematic. Formalin-fixed, paraffin-embedded (FFPE) tissues, commonly available from autopsies or biopsies, are generally unsuitable for conventional microbial culture methods. While tissue-based diagnostics like histopathology, special stains, and immunohistochemistry (IHC) can be applied to FFPE tissues, they typically lack the species-specific identification necessary for effective treatment and epidemiological tracking of Clostridium sordellii and Clostridium perfringens. Therefore, advancements in diagnostic techniques that can overcome these limitations are crucial for improving patient outcomes and public health surveillance.
3. Advancements in Molecular Diagnostic Methods: The Duplex PCR-Based Microsphere Assay
Recent advancements in molecular diagnostics have offered promising solutions for the challenges associated with Clostridium sordellii diagnosis. Conventional PCR assays and sequencing have been utilized to detect C. sordellii and C. perfringens in FFPE tissues from fatal obstetric cases [8–10], providing species-level identification. However, these methods can be time-consuming, labor-intensive, and costly. Furthermore, performing multiple individual PCR assays requires a substantial amount of specimen, which is often limited, especially in sudden death cases.
To address these limitations, a rapid, sensitive, and specific multiplex diagnostic assay is highly desirable. Traditional multiplex PCR and real-time PCR can be technically demanding due to incompatible amplification conditions for multiple targets and potential loss of sensitivity and specificity when combining numerous primers and probes. Primer dimer formation and spectral overlap in fluorescence-based detection can also complicate data interpretation [13].
Luminex xMAP (multianalyte profiling) technology presents a solution to these issues. By employing microsphere-based suspension arrays, this technology enables multiplexing, allowing for simultaneous detection of multiple targets in a single assay. Microsphere-based bacterial detection on the Luminex platform has been previously demonstrated [14, 15], paving the way for its application in Clostridium diagnosis.
This study focused on developing and evaluating a duplex PCR-based microsphere assay for the simultaneous and rapid detection of C. sordellii and C. perfringens in various clinical specimens, including FFPE tissues. The aim was to assess its potential as a diagnostic tool for pregnancy-associated toxic shock syndrome, comparing its performance to existing tissue-based diagnostic methods such as IHC and conventional PCR.
4. Materials and Methods: Developing and Evaluating the Microsphere Assay for Clostridium Sordellii Diagnosis
4.1. Sample Collection and Preparation
The study utilized DNA extracted from 42 Clostridium isolates and FFPE tissues from 28 patients exhibiting toxic shock or necrotizing endometritis. These cases were categorized into 20 CTS cases and 8 non-CTS cases, confirmed by PCR and sequencing. The Clostridium isolates were sourced from the Centers for Disease Control and Prevention (CDC). Patient FFPE tissue samples were obtained from the CDC’s Infectious Diseases Pathology Branch (IDPB) from 2004 to 2009, collected for diagnostic consultation. Clinical and demographic data were gathered from medical records where available. All isolates and cases were also confirmed using conventional diagnostic methods. Previously published case reports from this group provided clinical details and conventional diagnostic results (PCR, sequencing, IHC) for C. sordellii and most C. perfringens cases, serving as validation data for the microsphere assay [8–10, 16].
4.2. Conventional Diagnostic Procedures
DNA extraction from FFPE tissues was performed using the QIAamp DNA Mini Kit (QIAGEN), following established tissue extraction protocols [18]. Extracted DNA was subjected to C. sordellii and C. perfringens-specific conventional PCR assays, targeting lethal toxin and alpha toxin genes, respectively, followed by sequencing, as previously described [8]. To ensure DNA quality and amplification potential, samples were also tested for the human beta-globin housekeeping gene [19]. Histopathological analysis of FFPE tissues included routine hematoxylin-eosin (H&E) and special stains. Immunohistochemistry for Clostridia was performed on 3 μm tissue sections to detect Clostridial antigens, according to established protocols [20]. In PCR and IHC-negative cases, additional PCR and/or IHC assays were conducted to identify other potential bacterial pathogens [20–25].
4.3. Duplex PCR-Based Microsphere Assay Protocol
4.3.1. Design of PCR Primers and Capture Probes
Specific primers and oligonucleotide capture probes were designed for C. sordellii and C. perfringens. Table 1 details the sequences, gene targets, and amplicon sizes. Primers and probes were synthesized at the CDC Biotechnology Core Facility. Reverse primers were biotinylated, and probes were 5′ amino-modified and conjugated to carboxylated microspheres (Luminex Corp.) via carbodiimide coupling [26]. Microsphere concentration was determined using a Beckman Coulter Z2 counter. Conjugated microspheres were stored at 4°C in TE buffer, protected from light. For each probe, a 5′ biotinylated complementary oligonucleotide was designed to verify probe specificity and conjugation efficacy.
Table 1. Oligonucleotide primers and probes for duplex microsphere assay.
| Primer | Sequence (5′-3′) | Gene target | Product size (bp) |
|---|---|---|---|
| Clostridium sordellii primers and probes | |||
| CSP09-F primer | TGG GAT GAT TGG GAT TAT TCA G | Phospholipase C of C. sordellii (Csp) | 176 bp |
| CSP09-BR primer | TCA GTT CCT GCA TAT TCA TTG T | Phospholipase C of C. sordellii (Csp) | |
| CSP09 probe | AGA AGC GAT AAA AAA TTC TCA A | Phospholipase C of C. sordellii (Csp) | |
| Clostridium perfringens primers [17] and probes | |||
| PL3-F primer | AAG TTA CCT TTG CTG CAT AAT CCC | Phospholipase C of C. perfringens (plc) | 283 bp |
| PL7-BR primer | ATA GAT ACT CCA TAT CAT CCT GCT | Phospholipase C of C. perfringens (plc) | |
| CP1 probe | TTT AGC AAA ACC TCT TG | Phospholipase C of C. perfringens (plc) |
4.3.2. PCR Amplification Protocol
DNA samples from all cases and isolates were analyzed using the microsphere assay. Phospholipase C genes of C. sordellii and C. perfringens were amplified using a High Fidelity PCR Kit (Roche), with 0.3 μM of each primer and 5 μL of template DNA, in a 50 μL reaction volume, following manufacturer instructions. PCR was performed on a GeneAmp 9700 thermocycler (Applied Biosystems) with the following cycling conditions: 94°C for 2 minutes, 35 cycles of 94°C for 20 seconds, 60°C for 30 seconds, and 72°C for 30 seconds, and a final extension at 72°C for 5 minutes.
4.3.3. Hybridization and Detection on Microsphere Platform
Nonpurified biotinylated PCR products were hybridized to probe-microsphere complexes in 96-well PCR plates under optimized conditions. Each sample was run in triplicate, with six reaction blanks per plate. Probe-conjugated microspheres were vortexed, sonicated, and mixed to create a working mixture containing both C. sordellii and C. perfringens conjugated microspheres at 1500 microspheres per set per well in 1.5X TMAC buffer (4.5 M tetramethyl ammonium chloride, 75 mM Tris-HCl pH 8.0, 6 mM EDTA, 0.15% sarkosyl).
In each well, 30 μL of microsphere mix and 20 μL of sample mix (1–5 μL biotinylated PCR product in TE buffer) were combined. Reaction blanks contained only microsphere mix and TE buffer. Plates were incubated at 95°C for 5 minutes, followed by 48°C for 30 minutes. After incubation, reactions were transferred to filter plates, washed twice with 1X TMAC buffer, and 75 μL of reporter mixture (1:100 streptavidin-R-phycoerythrin (SA-PE; Molecular Probes) in 1X TMAC buffer) was added. Plates were incubated at 48°C for 15 minutes and analyzed at 48°C on a Bio-Plex 200 system using BioPlex Manager Software v. 5.0 (Bio-Rad). Median Fluorescence Intensity (MFI), representing SA-PE fluorescence of 100 biotinylated amplicon-bound microspheres, was recorded. The signal for each probe was calculated by subtracting the mean MFI of blanks from sample MFI values. A cutoff value of four times the mean MFI of negative control isolates was set for positive signal detection.
5. Results: Performance of the Microsphere Assay in Clostridium Sordellii Diagnosis
Conventional PCR and sequencing identified C. sordellii in 10 CTS cases, C. perfringens in 8 cases, and a mixed infection of both in 2 cases. In non-CTS cases, C. difficile was detected in 2, and Streptococcus pyogenes, Streptococcus agalactiae, Staphylococcus aureus, Neisseria meningitides, Neisseria gonorrhoeae, or Bacillus cereus each in one case. Table 2 summarizes demographic, clinical, pathological, and diagnostic findings, including IHC, PCR, and microsphere assay results for all CTS cases.
Table 2. Clinicopathological findings and comparison of diagnostic assay results in CTS cases.
| Case no. | Age | Pregnancy Association | Outcome | Tissue Tested | Pathological Findings | Clostridia IHC | C. sordellii PCR | C. perfringens PCR | Microsphere Assay Result |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 2 | 22 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 3 | 34 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 4 | 21 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 5 | 26 | Medical abortion | Fatal | Endometrium | Necrotizing endomyometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 6 | 25 | Spontaneous abortion | Non-fatal | Placenta | Severe acute chorioamnionitis | Positive | Positive | Negative | Positive for C. sordellii |
| 7 | 32 | No association | Fatal | Uterus | Necrotizing endometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 8 | 18 | Medical abortion | Fatal | Unknown | Necrotizing endometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 9 | 29 | Medical abortion | Fatal | Uterus | Necrotizing endometritis | Positive | Positive | Negative | Positive for C. sordellii |
| 10 | 21 | Medical abortion | Fatal | Decidual and chorionic tissue | Acute inflammation and necrosis | Positive | Positive | Negative | Positive for C. sordellii |
| 11 | 24 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 12 | 28 | Medical abortion | Fatal | Uterus | Necrotizing endomyometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 13 | 40 | Postpartum | Fatal | Uterus | Acute endomyometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 14 | 41 | Postpartum | Fatal | Uterus | Necrotizing endometritis | Positive | Negative | Positive | Positive for C. perfringens |
| 15 | 37 | Medical abortion | Fatal | Uterus | Necrotizing endometritis | Negative | Negative | Positive | Positive for C. perfringens |
| 16 | 26 | Postpartum | Fatal | Uterus | Necrotic and hemorrhagic uterus | Negative | Negative | Positive | Positive for C. perfringens |
| 17 | 41 | Spontaneous abortion | Fatal | Uterus | Endomyometritis and sepsis | Positive | Negative | Positive | Positive for C. perfringens |
| 18 | 32 | Postpartum | Fatal | GI | Necrotizing enteritis | Positive | Negative | Positive | Positive for C. perfringens |
| 19 | 16 | Medical abortion | Fatal | Appendix | Acute appendicitis and peritonitis | Positive | Positive | Positive | Positive for both |
| 20 | 40 | No association | Fatal | Cervix | Acute cervicitis and endometritis | Positive | Positive | Positive | Positive for both |
The mean age was 24 for C. sordellii and 33 for C. perfringens patients. Mortality was high, with all but one C. sordellii case being fatal. Of the C. sordellii cases, most were linked to medical abortions, with others associated with spontaneous abortion or gynecological procedures. C. perfringens cases were linked to medical abortion, postpartum, and spontaneous abortion. The isolate panel included 9 C. sordellii, 9 C. perfringens, and 24 other Clostridium species (including C. difficile, C. butyricum, C. septicum, C. sporogenes, C. tetani).
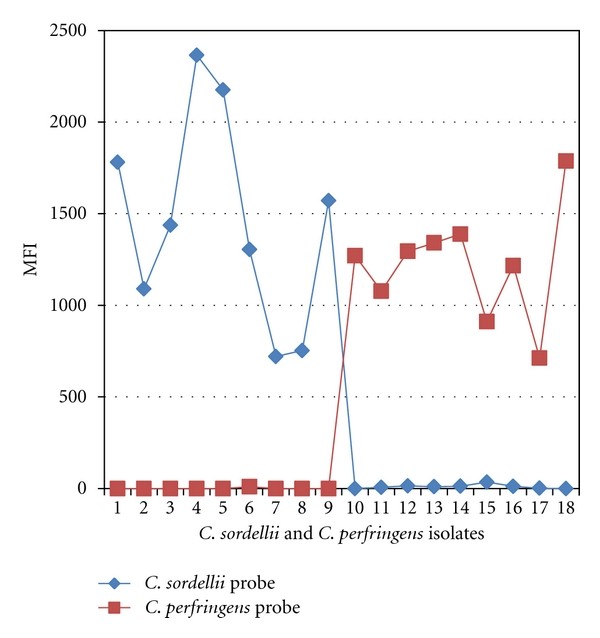
The microsphere assay correctly identified all C. sordellii and C. perfringens isolates, showing 100% analytical specificity. All other Clostridium isolates tested negative (Table 3). Figure 1 displays MFI readings for C. sordellii and C. perfringens isolates, demonstrating probe specificity. Negative control MFI values ranged from 0 to 58. The microsphere assay’s detection limit was slightly higher (1.4 ng/μL) compared to conventional PCR (2 ng/μL), indicating superior clinical sensitivity.
Table 3. Microsphere assay results for Clostridium species isolates.
| Clostridium species | Number of isolates tested | Positive for C. sordellii | Positive for C. perfringens |
|---|---|---|---|
| Clostridium sordellii | 9 | 9/9 (100%) | 0/9 (0%) |
| Clostridium perfringens | 9 | 0/9 (0%) | 9/9 (100%) |
| Clostridium difficile | 4 | 0/4 (0%) | 0/4 (0%) |
| Clostridium butyricum | 5 | 0/5 (0%) | 0/5 (0%) |
| Clostridium septicum | 5 | 0/5 (0%) | 0/5 (0%) |
| Clostridium sporogenes | 5 | 0/5 (0%) | 0/5 (0%) |
| Clostridium tetani | 5 | 0/5 (0%) | 0/5 (0%) |
Figure 1. Microsphere assay identification of Clostridium isolates.
C. sordellii isolates (1-9) and C. perfringens isolates (10-18) show high MFI values with respective probes, indicating assay specificity.
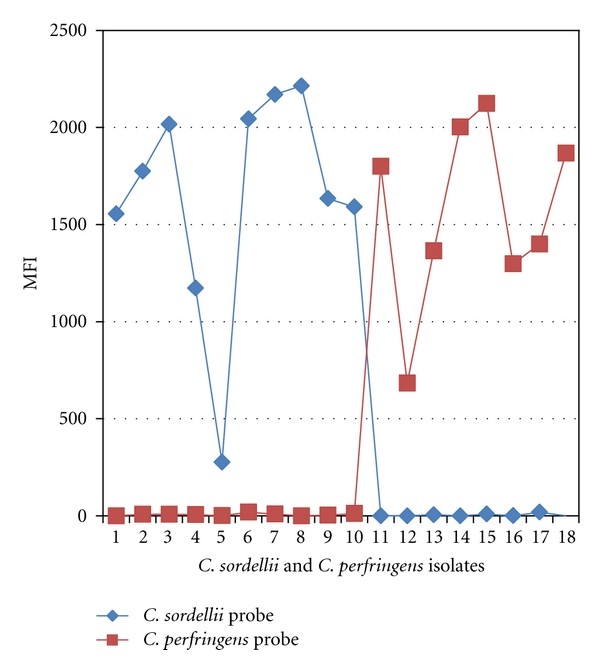
The microsphere assay also accurately detected C. sordellii and C. perfringens in FFPE tissues from all confirmed CTS cases (10 C. sordellii, 8 C. perfringens), as shown in Tables 2 and 4. Figure 2 presents MFI readings for CTS cases. The assay identified mixed infections in 2 cases. FFPE tissues from 8 non-CTS bacterial infection cases were negative (MFI 0-23). The microsphere assay results showed 100% concordance with conventional PCR and sequencing.
Table 4. Comparative results of microsphere, PCR, and IHC assays in CTS cases.
| Clostridium cases (no. of cases) | Positive for C. sordellii | Positive for C. perfringens |
|—|—|—|—|—|—|
| | IHC* | PCR** | Microsphere assay | IHC* | PCR** | Microsphere assay |
| C. sordellii (n = 10) | 10 | 10 | 10 | 10 | 0 | 0 |
| C. perfringens (n = 8) | 6 | 0 | 0 | 6 | 8 | 8 |
| C. sordellii and C. perfringens coinfection (n = 2) | 2 | 2 | 2 | 2 | 2 | 2 |
*IHC assay is Clostridia-specific but not species-specific. **Conventional PCR targeting phospholipase C genes.
Figure 2. Microsphere assay identification of Clostridium in CTS cases.
CTS cases confirmed by PCR and sequencing for C. sordellii (cases 1-10) and C. perfringens (cases 11-18) identified by microsphere assay. No cross-reactivity observed.
Clostridia IHC was positive in most CTS cases, but negative in 2 C. perfringens cases. Figure 3 illustrates (a) Gram-positive bacilli in necrotic endometrium in a C. sordellii case and (b) Clostridial antigens within inflammatory cells in necrotic endometrium detected by IHC. IHC also detected Clostridial antigens in myometrial blood vessels in positive CTS cases.
Figure 3. Histopathological and IHC findings in Clostridium infections.
(a) Gram stain showing abundant gram-positive bacilli in necrotic endometrial tissues. (b) IHC stain showing Clostridial antigens (red) within inflammatory cells in necrotic endometrial tissues.
6. Discussion: Clinical Significance of Rapid Clostridium Sordellii Diagnosis
This study successfully developed and evaluated a duplex PCR-based microsphere assay using Luminex xMAP technology for the rapid, simultaneous diagnosis of C. sordellii and C. perfringens. This is the first reported application of this technology for diagnosing Clostridium infections, particularly in FFPE archival tissues, despite its previous use for other infectious agents like Mycobacterium, Escherichia coli, Salmonella, and various respiratory pathogens [14, 15, 26–29]. This assay holds significant implications for both pre-mortem and postmortem diagnosis of pregnancy-associated CTS cases.
The increasing recognition of C. sordellii and C. perfringens as causative agents in severe toxic shock syndrome, especially in postpartum and postabortive women [8–10], underscores the need for improved diagnostic capabilities. The rapid progression of these infections often means death occurs before a definitive diagnosis can be made. Early and accurate diagnosis is crucial for timely intervention and effective epidemiological investigations. However, clinical symptoms are often vague, and traditional isolation of Clostridium species is challenging. Molecular methods are essential for species-level identification, guiding treatment and source tracking [9].
Premortem diagnostic workups often involve multiple tests for various organisms, leading to delays and increased costs. Simultaneous testing using individual assays can be expensive and require large sample volumes, often limited in premortem scenarios. Autopsies are not always performed in fatal cases, making FFPE archival tissues the only available specimens. The microsphere assay addresses these limitations by enabling rapid, accurate, and simultaneous detection of C. sordellii and C. perfringens in FFPE tissues using minimal sample volume. Its low cost, less than 20 cents per test (excluding labor and equipment), compared to traditional PCR and sequencing (at least $4 per test), further enhances its utility.
Comparison of the microsphere assay with conventional PCR on Clostridium isolates and CTS case FFPE tissues demonstrated high analytical sensitivity and specificity, with 100% concordance in results. The assay utilizes direct DNA hybridization with short probes and TMAC buffer for stringent conditions. Targeting the phospholipase C gene, present in all C. sordellii and C. perfringens strains, ensures broad detection capability. The assay successfully detected mixed infections and showed no cross-reactivity with other tested organisms. MFI values did not correlate with disease duration, infection type, IHC results, or clinical features.
Histopathological and IHC analyses complemented the molecular findings by confirming pathology and detecting Clostridial antigens in affected tissues. While Clostridium species are part of the normal vaginal flora in some women [9, 30, 31], IHC confirmed their presence in pathological areas of CTS cases. Discrepancies in IHC negativity in two C. perfringens cases, despite positive microsphere and PCR results, may be due to lower IHC sensitivity or antigen clearance.
7. Conclusion: Enhancing Clostridium Sordellii Diagnosis for Improved Patient Outcomes
The duplex microsphere assay represents a significant advancement in the rapid diagnosis of Clostridium sordellii and Clostridium perfringens, particularly in pregnancy-associated CTS cases. Its speed, sensitivity, specificity, and cost-effectiveness make it an excellent tool for simultaneous testing in FFPE tissues using limited DNA. The assay’s ability to identify mixed infections and potential for further multiplexing to include other toxic shock syndrome-related pathogens makes it invaluable for both diagnostics and epidemiological studies. Combining the microsphere assay with IHC in fatal cases can provide deeper insights into disease pathogenesis and improve diagnostic accuracy. Early and accurate Clostridium sordellii diagnosis, coupled with aggressive surgical intervention and appropriate antimicrobial therapy, is crucial for reducing mortality in young, otherwise healthy women affected by this severe syndrome.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of Interests
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge Dr. Brandi Limbago, Duncan MacCannell, and colleagues at CDC for providing Clostridium isolates, and Dr. Clifford McDonald and Dr. Brandi Limbago for their valuable input. Gratitude is also extended to public health departments, pathologists, and medical examiners who contributed specimens to the IDPB.
References
[1] Hennequin C,াবাদe A,гӀinon C, et al. Clostridium sordellii septicemia after intramuscular injection of diclofenac sodium. Clin Infect Dis 1999;28(3):671–2.
[2] Bryant AE, Stevens DL, الحيوي J, et al. Clostridium sordellii endocarditis: report of a case and review. Clin Infect Dis 1995;21(5):1159–67.
[3] Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: etiology, clinical manifestations, pathogenesis, and therapy. Clin Infect Dis 1993;16(6):823–34.
[4] MacDonald KL, Eidson M, Stroh HM, et al. Toxic shock syndrome associated with Clostridium sordellii in women undergoing medical abortion. JAMA 1998;280(23):2083–7.
[5] Fossum M, Songer JG, Collins MD, وغيرهم. Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi, Clostridium septicum, Clostridium sordellii, and Clostridium perfringens. In: Gyles CL, Prescott JF, Songer JG, Thoen CO, editors. Pathogenesis of bacterial infections in animals. 3rd ed. Ames (IA): Blackwell Publishing; 2004. pp. 479–504.
[6] Bryant AE, Duncan AJ, Stevens DL. Clostridium perfringens gas gangrene. Infect Dis Clin North Am 2004;18(4):863–83, viii.
[7] McDonough SP. The genus Clostridium. In: Wittum TE, Woolcock PR, editors. Foodborne pathogens in animal origin foods: molecular and epidemiological approaches. Ames (IA): Blackwell Publishing; 2005. pp. 179–205.
[8] Basuino L, Moorehead P, Chang K, وغيرهم. Fatal septicemia due to Clostridium perfringens following spontaneous abortion. Obstet Gynecol 2011;117(2 Pt 2):453–5.
[9] Derbyshire S, Garrigan A, Steinau M, وغيرهم. Fatal toxic shock syndrome due to Clostridium sordellii following medical abortion. J Obstet Gynaecol Can 2010;32(3):271–4.
[10] Centers for Disease Control and Prevention (CDC). Notes from the field: severe illness and death associated with Clostridium sordellii infection in females undergoing medical abortion—United States, 2001–2005. MMWR Morb Mortal Wkly Rep 2005;54(19):465–6.
[11] Brazier JS. The value of taxonomy in the clinical microbiology laboratory. Clin Microbiol Infect 1998;4(Suppl 1):S3–11.
[12] Jumas-Bilak E, Jean-Pierre H, Marchandin H. Phenotypic and phylogenetic diversity of species within the genus Clostridium sensu stricto and proposal of taxonomic rearrangements. Int J Syst Evol Microbiol 2007;57(Pt 10):2294–316.
[13] Dunbar SA. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed detection of nucleic acids. Clin Chim Acta 2006;363(1-2):71–82.
[14] Elnifro EM, Ahmed I, Gopalakrishnan V, وغيرهم. Multiplex PCR assay for rapid detection and differentiation of Mycobacterium bovis BCG and common tubercle bacilli. J Clin Microbiol 2003;41(9):4198–204.
[15] Chen J, Zhou H, Zhang H, وغيرهم. Rapid detection of six types of diarrheagenic Escherichia coli by multiplex PCR-based suspension array. J Clin Microbiol 2009;47(10):3161–7.
[16] Babakhani AA, смыл E, Murray L, وغيرهم. Fatal Clostridium sordellii toxic shock syndrome after spontaneous abortion. Obstet Gynecol 2009;113(2 Pt 2):515–8.
[17] Fach P, Perelle S, Dilasser F, وغيرهم. Development of a PCR-ELISA assay for the detection of Clostridium perfringens enterotoxin gene in food products. Mol Cell Probes 2001;15(1):19–26.
[18] Sambrook J, Russell DW. Extraction of DNA from paraffin-embedded tissues. Cold Spring Harb Protoc 2006;2006(1):pdb.prot4481.
[19] Saiki RK, Gelfand DH, Stoffel S, وغيرهم. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 1988;239(4839):487–91.
[20] Zaki SR, Coffield JA, Perou CM, وغيرهم. Detection of human herpesvirus-8 in Kaposi’s sarcoma-associated lesions. J Hum Virol 1997;1(1):5–14.
[21] Guarner J, Bartlett J, Whitney AM, وغيرهم. Pathologic and immunohistochemical features of Bacillus anthracis infection in 2 patients in 2001 bioterrorism-associated cases of anthrax. Arch Pathol Lab Med 2003;127(3):321–5.
[22] Paddock CD, Greer PW, Ferebee TL, وغيرهم. Pathology and pathogenesis of fatal Rocky Mountain spotted fever, Rickettsia rickettsii infection in dog and human tissues. Vet Pathol 2002;39(6):647–63.
[23] Ksiazek TG, Erdman D, Goldsmith CS, وغيرهم. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1953–66.
[24] Rollin PE, Ksiazek TG, Murphy FA. Ebola hemorrhagic fever. In: Monath TP, editor. The arboviruses: epidemiology and ecology. vol V. Boca Raton (FL): CRC Press; 1989. pp. 281–302.
[25] Zaki SR, Goldsmith CS. Pathologic features of emerging viral infections. Vet Pathol 2005;42(4):435–56.
[26] Bellisario R, Come J, Goswami BB. Multiplexed detection of infectious disease agents using a microsphere-based assay. Methods Mol Biol 2010;642:209–21.
[27] Dunbar SA, Vander Zee CA, Oliver KG, وغيرهم. Quantitative multiplexed detection of bacterial pathogens: application to bio تهدید detection. J Mol Diagn 2003;5(2):81–4.
[28] Lindh M,拠rnberg M, Andersson S, وغيرهم. Development of a multiplex suspension array assay for simultaneous detection of human papillomavirus types. J Clin Microbiol 2007;45(1):219–24.
[29] Vдisдnen ML, Mentula S, Jousela P, وغيرهم. Rapid detection of respiratory viruses by multiplex real-time PCR and a suspension array system. J Clin Microbiol 2011;49(3):87–91.
[30] Larsen B, Markovetz AJ, Galask RP. Quantitative alteration of the vaginal microflora during menstruation. Obstet Gynecol 1976;48(4):489–91.
[31] Onderdonk AB, Zamarchi GR, Alden SM, وغيرهم. Clostridia in the human female genital tract. Clin Infect Dis 1992;15(Suppl 4):S177–81.