Introduction
Cytomegalovirus (CMV) pneumonia poses a significant and increasingly prevalent threat to individuals with hematologic malignancies. In these immunocompromised populations, CMV infection can manifest in a spectrum of severity, ranging from asymptomatic viremia to severe end-organ diseases, with CMV pneumonia carrying a particularly high mortality rate, reported as high as 57%. While advances in immunosuppressive therapies and the heightened utilization of CMV polymerase chain reaction (PCR) testing in bronchoalveolar lavage (BAL) fluid and serum have likely contributed to the observed rise in CMV pneumonia cases among non-transplant hematologic malignancy patients, diagnostic methodologies have not kept pace. The definitive diagnosis of CMV pneumonia necessitates a synthesis of clinical pulmonary symptoms and signs with the detection of CMV within BAL fluid or lung tissue. Traditional detection methods encompass virus isolation, histopathology, immunohistochemistry, or in situ hybridization.
Since its introduction in the early 1990s, PCR has become the cornerstone of CMV detection due to its superior sensitivity. Quantitative real-time PCR (qRT-PCR) further refines this capability by quantifying viral loads in blood and BAL fluid. Blood viral load monitoring is a well-established tool for predicting CMV disease and guiding pre-emptive therapy, especially post-bone marrow transplantation (BMT). However, qRT-PCR analysis of BAL fluid, while highly sensitive and possessing a strong negative predictive value, exhibits limitations in specificity and positive predictive value.
Adding complexity, asymptomatic pulmonary CMV infections have been documented in both immunocompromised and immunocompetent individuals, blurring the lines between CMV infection and clinically significant CMV end-organ disease. A critical question remains: do CMV pneumonia patients exhibit higher viral titers compared to those with asymptomatic pulmonary shedding? The lack of standardized methods for measuring CMV DNA viral loads and the absence of established viral cut-off values to differentiate CMV infection from CMV pneumonia underscore a significant gap in diagnostic precision. Currently, outside of CMV DNAemia diagnosis via blood PCR, defined viral load thresholds for diagnosing CMV disease in hematologic malignancy patients are lacking, particularly for respiratory samples. While CMV culture of BAL fluid has shown sensitivity in lung transplant recipients, histological assessment of transbronchial biopsies remains more clinically relevant. A 2005 study using a quantitative hybrid capture assay suggested a BAL fluid CMV load of >500,000 copies/mL as indicative of CMV pneumonia with high sensitivity and specificity when compared to lung biopsies. However, robust data establishing definitive qRT-PCR cut-off values in BAL fluid for CMV pneumonia diagnosis remains scarce.
This study endeavors to address this critical gap by evaluating the diagnostic utility of CMV qRT-PCR in bronchial washing fluid for CMV pneumonia in hematologic malignancy patients. The primary aim is to define viral load cut-off values in bronchial washing fluid that can reliably differentiate CMV pneumonia from CMV infection in this vulnerable patient population.
Materials and Methods
Patient Cohort and Data Collection
This retrospective study examined data from 565 adult patients (aged >15 years) with hematologic malignancies who underwent bronchoscopy at Seoul St. Mary’s Hospital (a high-volume BMT center) between March 2008 and June 2014. Bronchoscopies were performed to identify pneumonia-causing pathogens. Pneumonia diagnosis was based on new chest radiograph infiltrates coupled with clinical signs of lower respiratory tract infection and/or fever. Microbiological findings from bronchial washing fluids were retrospectively analyzed alongside blood CMV qRT-PCR results and lung biopsy data. Clinical data collected included age, sex, hematologic diagnosis, prior hematologic treatments, and transplantation details.
The study received ethical approval from the Institutional Review Board of Seoul St. Mary’s Hospital (No. KC15RISI0153), with a waiver for informed consent granted due to the retrospective nature of the analysis.
Bronchoscopy Procedure and Bronchial Washing Fluid Collection
Fiber-optic bronchoscopy was performed following chest computed tomography (CT) identification of pulmonary infiltrates. Experienced bronchoscopists utilized a flexible bronchoscope (BF-1T60t, Olympus, Tokyo, Japan). Bronchial washing was performed by wedging the bronchoscope into a segmental bronchus corresponding to the new infiltrate on chest CT. Ten mL of normal saline was repeatedly instilled and aspirated until at least 20 mL of aspirate was collected.
Microbiological and CMV Assays
Bronchoscopic washing fluid specimens underwent direct examination and culture for bacteria, fungi, and viruses. Bacterial, mycobacterial, and fungal detection involved Gram, Ziehl–Neelsen, Periodic-acid Schiff, and Gomori methenamine silver staining, followed by culture. Respiratory virus detection employed a multiplex qPCR assay (AdvanSure RV Real-time PCR kit, LG Life Sciences, Seoul, Korea) for a panel of common respiratory viruses. PCR assays were also used for Pneumocystis jirovecii and mycobacteria detection.
CMV detection methods on bronchial washing fluid included qRT-PCR for CMV DNA, shell vial culture, and immunohistochemical (IHC) staining. Hematoxylin-eosin and IHC staining for CMV were performed on transbronchial lung biopsies and reviewed by a lung pathologist. For CMV qRT-PCR, DNA was extracted from 200 μL of whole blood or bronchial washing fluid using the QIAamp DNA Blood Kit (Qiagen, Valencia, CA, USA). RT-PCR was performed using the ExiCycler™ 96 instrument (Bioneer Corporation, Daejeon, Korea) and AccuPower® CMV Quantitative PCR Kit (Bioneer). The limit of detection (LoD) was determined to be 380 copies/mL through probit analysis. Standardization was achieved using the WHO International Standard for human CMV (NIBSC code: 09/162), establishing a conversion factor of 7.3 International Units (IU) per copy.
CMV Pneumonia Definition
CMV pneumonia suspicion arose in patients presenting with pneumonia signs and symptoms and chest CT findings consistent with viral pneumonia. CMV pneumonia diagnosis followed established criteria and was categorized as proven, probable, possible, or indeterminate by an infectious disease specialist (Lee DG) based on retrospective chart review and chest CT findings (Table 1). Patients with co-detection of pathogens like Aspergillus spp. and typical radiological signs of invasive pulmonary aspergillosis (IPA) were excluded.
Table 1: Diagnostic Classification of CMV Pneumonia
| Classification | Criteria |
|---|---|
| Proven | Positive CMV virus culture from bronchial washing fluid OR intranuclear inclusion bodies or CMV detection by IHC in lung biopsy. |
| Probable | Intranuclear inclusion bodies or CMV detection by IHC in bronchial washing fluid cytology. |
| Possible | Does not meet criteria for proven or probable CMV pneumonia. |
| Indeterminate | Does not meet criteria for proven or probable and another common respiratory virus (non-CMV) is isolated. |
Statistical Analysis
Statistical analyses were performed using SPSS software (ver. 18.0.0 for Windows; SPSS, Inc., Chicago, IL, USA). Data are presented as means ± SEM or proportions. CMV qRT-PCR results are given as means, medians, and ranges. The Mann–Whitney U-test was used to compare CMV PCR titers between CMV pneumonia and non-pneumonia groups. All tests were two-sided, with P < 0.05 considered statistically significant. Receiver operating characteristic (ROC) curves were used to determine optimal CMV load cut-off values in bronchial washing fluid for CMV pneumonia diagnosis.
Results
Patient Demographics and Clinical Characteristics
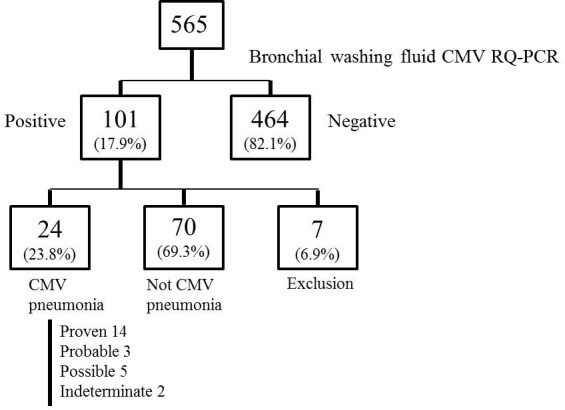
Bronchoscopy was performed on average 2.54 ± 2.9 days after new pulmonary infiltrates were identified. Of the 565 patients, 464 (82.1%) were CMV negative or had CMV loads below 380 copies/mL. Based on consensus criteria, 24 patients (23.8%) were diagnosed with CMV pneumonia, 70 (69.3%) were classified as not having CMV pneumonia, and 7 (6.9%) were excluded due to suspected IPA. Among the CMV pneumonia cohort, classifications were: 14 proven (58.3%), 5 possible (20.8%), 3 probable (12.5%), and 2 indeterminate (8.3%) (Figure 1, Supplementary Figure 1). Proven cases were based on positive CMV culture (bronchial washing fluid, n=9; lung biopsy, n=5). Indeterminate cases involved co-infections with coronavirus and rhinovirus.
Table 2 outlines baseline clinical characteristics. The mean age was 48 ± 3.0 years, and 75% were male. The most common underlying hematologic malignancies were acute myeloid leukemia (25.0%), hemophagocytic lymphohistiocytosis (16.7%), non-Hodgkin’s lymphoma (12.5%), and aplastic anemia (12.5%). Sixty-six point seven percent were on immunosuppressants at CMV pneumonia diagnosis, and 46.2% had received systemic chemotherapy within 30 days, including alemtuzumab (n=2) and steroid pulse therapy (n=3). All CMV pneumonia patients received antiviral treatment, with 58.3% experiencing pneumonia aggravation and 41.7% improvement. During a 122-day mean follow-up, 62.5% died, with a 28-day mortality rate of 45.8%. CMV pneumonia was the cause of death in 58.3%. Co-infections were present in 62.5%, involving bacteria (46.7%), viruses (20.0%), fungi (20.0%), and Mycobacterium species (13.3%).
Table 2: Baseline Clinical Characteristics of CMV Pneumonia Patients (n=24)
| Characteristic | Value |
|---|---|
| Age (yr) | 48 ± 3.0 |
| Male, n (%) | 18 (75.0) |
| Underlying hematologic disease, n (%) | |
| Acute myeloid leukemia | 6 (25.0) |
| Hemophagocytic lymphohistiocytosis | 4 (16.7) |
| Non-Hodgkin’s lymphoma | 3 (12.5) |
| Aplastic anemia | 3 (12.5) |
| Acute lymphoblastic leukemia | 2 (8.3) |
| Myelodysplastic syndrome | 2 (8.3) |
| Multiple myeloma | 1 (4.2) |
| Chronic myelogenous leukemia | 1 (4.2) |
| Chronic lymphocytic leukemia | 1 (4.2) |
| Primary myelofibrosis | 1 (4.2) |
| Prior treatment, n (%) | |
| Bone Marrow transplantation | 16 (66.7) |
| Use of immunosuppressant agent | 16 (66.7) |
| Systemic chemotherapy (last 30 days) | 12 (46.2) |
| Alemtuzumab chemotherapy | 2 (8.3) |
| Steroid pulse therapy (last 30 days) | 3 (12.5) |
| History of preemptive CMV therapy, n (%) | 4 (16.7) |
| Prognosis of pneumonia, n (%) | |
| Improved | 10 (41.7) |
| Aggravated | 14 (58.3) |
| Follow up periods (days) | 122.0 ± 65.0 |
| Death, n (%) | 15 (62.5) |
| Survived | 9 (37.5) |
| Before 28 days of diagnosis | 11 (45.8) |
| After 28 days of diagnosis | 4 (16.7) |
| Death due to the pneumonia, n (%) | 14 (58.3) |
Table 3 details transplantation characteristics. Sixteen of the 24 CMV pneumonia patients had undergone BMT. The mean time to CMV pneumonia diagnosis post-BMT was 167.7 ± 60.9 days, with most diagnoses (68.8%) occurring within 100 days post-BMT.
Table 3: Transplantation Characteristics of CMV Pneumonia Patients (n=16)
| Characteristic | Value |
|---|---|
| Donor type, n (%) | |
| Sibling | 5 (31.3) |
| Unrelated | 4 (25.0) |
| Familial mismatched transplantation | 4 (25.0) |
| Cord | 1 (6.3) |
| Autologous | 2 (12.5) |
| Source of graft, n (%) | |
| Bone marrow | 2 (12.5) |
| Peripheral blood stem cell | 13 (81.3) |
| Cord blood stem cell | 1 (6.3) |
| Risk of CMV disease, n (%) | |
| High risk† | 10 (62.5) |
| Low risk‡ | 6 (37.5) |
| Time since BMT (days) | 167.7 ± 60.9 |
| Before 100 days after BMT, n (%) | 11 (68.8) |
Bronchial Washing Fluid qRT-PCR and Viral Load Comparison
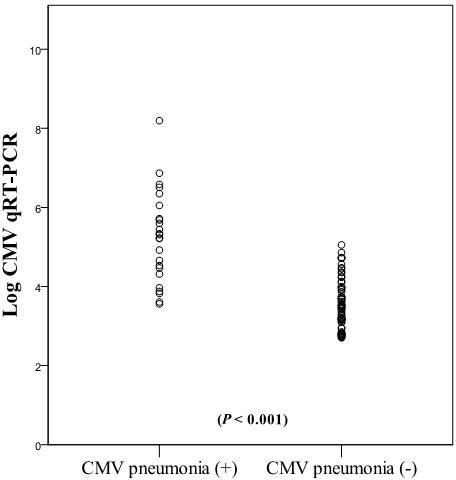
Figure 2 illustrates the distribution of CMV qRT-PCR viral load in bronchial washing fluid. Median log10 (CMV qRT-PCR copies/mL) values were significantly higher in CMV pneumonia patients (5.3, range 3.56–8.19, n=24) compared to those without CMV pneumonia (3.4, range 2.7-5.05, n=70) (P < 0.001).
Table 4 compares qRT-PCR results between CMV pneumonia (n=24) and non-CMV pneumonia (n=70) groups. Median CMV loads in bronchial washing fluid were significantly elevated in the CMV pneumonia group (1.8 × 105 copies/mL vs. 3.0 × 103 copies/mL; P < 0.001). Similarly, blood CMV loads were also significantly higher in the CMV pneumonia group (3.4 × 104 copies/mL vs. 5.4 × 103 copies/mL; P = 0.006). CMV pneumonia patients exhibited a 61-fold higher median bronchial washing fluid CMV load and a 6-fold higher median blood CMV load than those without CMV pneumonia.
Table 4: Quantitative PCR Results Comparison: CMV Pneumonia vs. Non-CMV Pneumonia
| CMV Pneumonia (n=24) | Not CMV Pneumonia (n=70) | P Value | |
|---|---|---|---|
| Bronchial washing fluid (copies/ml) | |||
| Mean | 7,378,508.6 | 10,899.2 | < 0.001 |
| Median | 187,224.5 | 3,055 | |
| Min – Max | 3,642 – 156,666,945 | 506 – 113,000 | |
| Blood (copies/ml) | |||
| Mean | 683,659.1 | 20,915.4 | 0.006 |
| Median | 33,839.5 | 5,486.5 | |
| Min – Max | 882 – 5,570,000 | 689 – 280,870 |
CMV DNA Cut-Off Value Determination
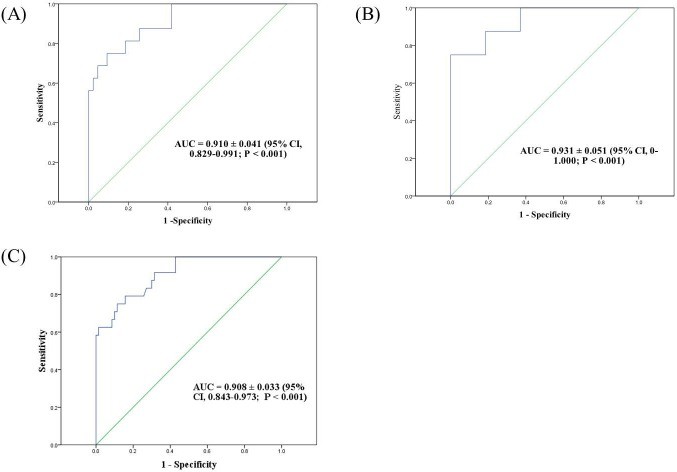
ROC curve analysis was performed to determine optimal bronchial washing fluid CMV load cut-off values for CMV pneumonia diagnosis. The analysis included 94 patients (35 no-BMT, 59 post-BMT, with 16 post-BMT patients diagnosed with CMV pneumonia). For post-BMT patients, the optimal cut-off was 18,900 copies/mL (137,970 IU/mL), yielding an AUC of 0.91 (Figure 3A). In no-BMT patients, the cut-off was significantly higher at 316,415 copies/mL (2,309,825 IU/mL), with an AUC of 0.93 (Figure 3B). Considering all patients, a cut-off of 28,774 copies/mL (210,054 IU/mL) demonstrated an AUC of 0.908 (Figure 3C).
Figure 3: Receiver-operator characteristics (ROC) curves for CMV pneumonia diagnosis using bronchial washing fluid CMV load. (A) Post-BMT patients (cut-off 18,900 copies/mL). (B) No-BMT patients (cut-off 316,415 copies/mL). (C) All patients (cut-off 28,774 copies/mL).
Discussion
This study provides compelling evidence that CMV loads in bronchial washing fluid, as quantified by qRT-PCR, serve as a robust indicator of lung involvement in CMV disease. Patients diagnosed with CMV pneumonia exhibited significantly elevated qRT-PCR CMV titers in both bronchial washing fluid (61-fold higher) and whole blood (6-fold higher) compared to those without CMV pneumonia. ROC curve analysis identified a clinically useful cut-off value of 28,774 copies/mL (210,054 IU/mL) CMV DNA in bronchial washing fluid, demonstrating 75% sensitivity and 88.6% specificity for CMV pneumonia diagnosis. Given the 0.85%-0.86% prevalence of CMV pneumonia at our BMT center, this cut-off yields a positive predictive value of 5.3% and a negative predictive value of 99.8%. These findings offer valuable clinical utility by providing an optimal CMV DNA cut-off point in hematologic malignancy patients, potentially facilitating earlier and less invasive CMV pneumonia diagnosis. While pathological confirmation remains the gold standard, lung biopsy carries inherent bleeding risks in this patient population. Our data suggests that bronchial washing, a less invasive procedure, can be a valuable alternative for CMV pneumonia diagnosis. These qRT-PCR cut-off values offer supplementary information for early CMV detection in pulmonary infiltrates of hematologic malignancy patients.
Early studies in the 1990s evaluated PCR for CMV detection in BAL fluid of BMT recipients. Cathomas et al. reported 100% PCR sensitivity for CMV pneumonia diagnosis, but noted lower specificity and positive predictive value, which were improved with adjunct CMV immunostaining. Hohenthal et al. also observed CMV PCR positivity in BAL fluid from hematologic malignancy patients with pneumonia, but the clinical significance remained unclear in most cases. Limited subsequent research has focused on PCR-based CMV detection in BAL fluid for this patient group. In lung transplant recipients, Chemaly et al. proposed a much higher viral load cut-off of 500,000 copies/mL in BAL fluid. The discrepancy between their cut-off and ours may be attributed to differences in host immune status and smaller sample size in Chemaly’s study. Notably, our study is unique in its quantitative qRT-PCR approach for differentiating CMV pneumonia from CMV infection and its inclusion of a larger patient cohort compared to prior studies.
BAL is considered the standard method for viral pneumonia pathogen detection in hematologic malignancy patients. Our center utilizes a modified bronchial washing procedure, wedging the bronchoscope into a selective segmental bronchus, aiming to identify pathogens in pulmonary infiltrates while minimizing alveolar damage. The CMV pneumonia incidence in our study aligns well with previous reports, despite the simplified bronchial washing technique compared to traditional BAL.
This study acknowledges several limitations. First, the ROC curve analysis included 10 patients with clinically diagnosed CMV pneumonia not confirmed by lung biopsy or culture. To mitigate this, a specialist rigorously excluded other infectious and non-infectious etiologies. All included patients received anti-CMV therapy and did not spontaneously improve. The diagnosed specimen percentage (4.2%) is consistent with prior studies. Second, 8 of the 24 diagnosed patients were not BMT recipients. Our center’s risk-adapted pre-emptive therapy post-BMT, initiated in 2000, meant that 4 CMV pneumonia patients had prior pre-emptive therapy. Non-BMT patients, lacking established prevention strategies, had no pre-emptive therapy history. Separate ROC curve analyses based on BMT status revealed significantly different viral cut-offs: 18,900 copies/mL for post-BMT and 316,415 copies/mL for no-BMT patients, highlighting the clinical relevance of BMT status. Excluding no-BMT patients slightly lowered the overall cut-off to 18,900 copies/mL. However, the limited number of post-BMT CMV pneumonia cases (n=16) may affect ROC curve accuracy. Larger, stratified analyses accounting for BMT status and CMV disease risk are warranted. Prospective validation of these cut-offs is also crucial. Third, pulmonary hemorrhage, potentially influencing BAL viral load, was considered. Excluding patients with possible pulmonary hemorrhage resulted in a slightly adjusted cut-off of 19,420 copies/mL, maintaining a high AUC.
Conclusions
This study demonstrates that CMV loads in bronchial washing fluid and whole blood, quantified by qPCR, are valuable indicators of pulmonary CMV disease involvement. A bronchial washing fluid CMV DNA cut-off value of 28,774 copies/mL (210,054 IU/mL) is significantly correlated with CMV pneumonia diagnosis in patients with hematologic malignancies. This finding offers a clinically relevant, less invasive diagnostic adjunct for this high-risk patient population.
Supplementary Figure
Supplementary Figure 1: Patient classification and diagnostic categories.
Abbreviations
BMT: Bone marrow transplantation
CMV: Cytomegalovirus
CT: Computed tomography
GVHD: Graft-versus-host disease
IHC: Immunohistochemical
PCR: Polymerase chain reaction
qRT-PCR: Quantitative real-time PCR
Footnotes
Author contributions
HY Lee and CK Rhee drafted the manuscript. CK Rhee designed the study, HY Lee and JY Choi collected the patients’ data. HY Lee helped performing statistical analysis. DG Lee reviewed and coordinated the data and drafted the manuscript. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.
REFERENCES
[List of references from original article – keep as is]
