Computer-aided diagnosis (CAD) has emerged as a pivotal research area within medical imaging and diagnostic radiology. This article explores the foundational motivations and principles behind the initial development of CAD systems, alongside their current standing and future possibilities within a Picture Archiving and Communication System (PACS) framework. In the CAD paradigm, radiologists leverage computer-generated outputs as a “second opinion,” retaining ultimate diagnostic authority. This approach distinguishes itself from automated computer diagnosis, which relies solely on computer algorithms. CAD’s efficacy is not predicated on outperforming clinicians but rather on complementing their expertise. Notably, numerous CAD systems are now integral in assisting clinicians in the early detection of breast cancer via mammography.
CAD systems utilizing lateral chest images show promise in enhancing the overall detection of lung nodules when integrated with CAD for posterior-anterior (PA) chest images. Furthermore, computers can reliably detect vertebral fractures on lateral chest radiographs, suggesting CAD’s potential to improve radiologists’ accuracy in this domain and facilitate earlier osteoporosis diagnosis. In Magnetic Resonance Angiography (MRA), CAD systems are being developed to aid in the detection of intracranial aneurysms. For monitoring temporal changes, CAD schemes using temporal subtraction images have been designed for sequential bone scans. Looking ahead, comprehensive CAD packages are envisioned as components of PACS. A chest CAD package, for example, might encompass computerized detection of lung nodules, interstitial opacities, cardiomegaly, vertebral fractures, and temporal changes in chest radiographs, as well as the classification of nodules and differential diagnosis of interstitial lung diseases. To further refine differential diagnosis, PACS could potentially incorporate image retrieval functionalities, allowing for the comparison of new cases with a database of pathologically confirmed images based on visual similarity metrics.
1. Introduction
Computer-aided diagnosis (CAD) is increasingly becoming a standard component of clinical practice for breast cancer detection in mammography across numerous screening centers and hospitals in the United States (1–25). This trend indicates a broader application of CAD in identifying and differentiating various abnormalities in medical images acquired through diverse imaging modalities and examinations. Indeed, CAD has solidified its position as a major focus of research in medical imaging and diagnostic radiology (12–25). While initial attempts at computerizing medical image analysis date back to the 1960s (26–32), the systematic exploration of CAD began in the 1980s. This era marked a crucial conceptual shift, moving away from automated computer diagnosis towards computer-aided diagnosis. This article will delve into the motivations and philosophical underpinnings of early CAD development, its current state, and its future prospects within the evolving landscape of picture archiving and communication systems (PACS).
2. Historical Evolution of CAD Development
Extensive research and development of diverse CAD systems commenced in the early 1980s at the Kurt Rossmann Laboratories for Radiologic Image Research, within the Radiology Department at the University of Chicago. This initiative built upon a foundation of basic research into the impact of digital images on radiologic diagnosis (33–48), coinciding with parallel advancements in PACS research in the US and Europe (49). Despite PACS’s evident utility in radiology department image management and potential economic benefits for hospitals, its direct clinical advantages for radiologists remained unclear at the time. This prompted the pivotal question: “How can the benefits of digital images directly enhance radiologists’ diagnostic capabilities in their daily practice?” This inquiry naturally led to the conceptualization of computer-aided diagnosis.
The 1980s inherited the concept of automated diagnosis, or automated computer diagnosis, from 1960s studies (26–31, though these earlier endeavors had not achieved widespread success. (The distinctions and commonalities between automated diagnosis and computer-aided diagnosis will be elaborated in the subsequent section). The complexity of computerized lesion analysis in medical images was apparent. Consequently, the success of CAD development was far from guaranteed. Therefore, research efforts were strategically directed towards areas with significant medical impact potential. Cardiovascular diseases, lung cancer, and breast cancer were identified as critical areas of focus. This led to the establishment of three primary research projects: vascular imaging lesion detection and quantification by Fujita et al. (50) and Hoffmann et al. (51); lung nodule detection in chest radiographs by Giger et al. (52,53; and clustered microcalcification detection in mammograms by Chan et al. (54).
Our CAD research and development has consistently been guided by three core principles. Firstly, our fundamental strategy for devising methods and techniques for lesion detection and quantification in medical images is rooted in understanding radiologists’ image reading processes (20,25). This approach is inherently logical because radiologists are experts in complex image interpretation and diagnosis. We posited that computer algorithms should be developed based on mimicking and augmenting human image reading, understanding how radiologists detect lesions, potential reasons for missed abnormalities, and strategies for differentiating benign from malignant lesions.
Secondly, we considered how to measure the success of CAD development. The ultimate validation, we believed, would be the routine clinical integration of CAD in hospitals globally. To achieve this, commercialization by medical industry partners would be essential. Therefore, we prioritized securing intellectual property rights for core CAD technologies through patents and actively engaged with medical industry stakeholders to foster CAD product commercialization. Our initial CAD patent (55), filed in 1987, has since become a widely referenced patent in CAD technology.
Thirdly, we aimed to promote broad acceptance of the CAD concept and encourage global CAD research collaboration. Given the vast scope of CAD research – encompassing diverse lesion types, modalities, observer performance studies, clinical trials, and commercialization – success would necessitate a collaborative, global effort. We viewed other CAD researchers as collaborators and promoters, not competitors. For over two decades, starting from the early stages of CAD research, we maintained extensive scientific exhibits at the Radiological Society of North America (RSNA) Annual Meetings in Chicago. These exhibits showcased comprehensive CAD research in chest, breast, and vascular imaging. In 1993, we demonstrated real-time CAD for clustered microcalcification detection on mammograms using a film digitizer and computer, inviting 118 breast radiologists to test the CAD system with their cases at the RSNA meeting. The informal validation results were promising (56). From 1996 to 2001, we conducted observer performance studies with numerous participants at RSNA meetings, evaluating lesion detection on chest images with and without CAD output, allowing radiologists to experience CAD firsthand. The utility of CAD was clearly demonstrated (57,58. These demonstrations effectively disseminated the CAD concept widely and rapidly.
In lung cancer CAD research, we focused on developing computerized lung nodule detection in chest radiographs. Lung nodule detection is known to be challenging for radiologists, with miss rates up to 30%. Missed nodules are often obscured by overlapping normal anatomical structures, effectively camouflaging them against the complex background of chest images (59–63). We anticipated that this background complexity would pose a significant obstacle for computer-based detection as well. Thus, the initial step in our computerized lung nodule detection scheme was background structure removal or suppression in chest radiographs. The difference-image technique (64–68), which subtracts a nodule-suppressed image from a nodule-enhanced image, proved effective in enhancing lesions and reducing background noise. This technique proved useful not only for lung nodules (64,65) but also for microcalcifications (54,66) and masses (67) in mammograms, and nodules in CT (68).
By the mid-1980s, the Rossmann Laboratories had developed foundational schemes for lung nodule detection in chest images (52,53 and clustered microcalcification detection in mammograms (54). While these schemes demonstrated relatively high sensitivity, they also produced a high number of false positives. The practical clinical utility of these computerized outputs was uncertain. For example, clustered microcalcification detection by computer yielded an average of four false positives per mammogram, despite achieving approximately 85% sensitivity. To assess the clinical potential of CAD, we conducted an observer performance study with and without computer output. Surprisingly, radiologists’ performance in detecting clustered microcalcifications significantly improved with CAD assistance, even with the high false-positive rate. These findings, published by H.P. Chan et al. in 1990 (66), provided the first scientific evidence for the benefits of CAD in lesion detection.
3. Computer-Aided Diagnosis versus Automated Computer Diagnosis
Early research into quantitative analysis of medical images by computers emerged in the 1960s (26–31). The prevailing expectation was that computers could eventually replace radiologists in abnormality detection, based on the premise that machines excel at specific tasks compared to humans. This led to the concept of computer diagnosis, or automated diagnosis, in radiology. While these initial efforts yielded interesting results, they ultimately fell short of widespread success due to limitations in computer processing power, the immaturity of advanced image processing techniques, and the limited accessibility of digital images. However, a more fundamental flaw was an overestimation of computer capabilities. In fact, numerous attempts at automated computer diagnosis have been explored across various medical fields since the 1950s. In hematology, for example, R.L. Engle (32), reflecting on 30 years of experience, concluded, “Thus, we do not see much promise in the development of computer programs to simulate the decision making of a physician. —- However, after many years we have concluded that we should stop trying to make computers act like diagnosticians.”
However, the 1980s saw the rise of an alternative approach: computer-aided diagnosis (CAD). This concept posited that computer outputs could assist, rather than replace, radiologists. CAD gained rapid and widespread adoption. Initially, some computer scientists criticized CAD, predicting its failure – a prediction that has proven demonstrably false. This early criticism may have stemmed from the failures of prior automated computer diagnosis attempts, suggesting that the failure of one research direction can create an unfounded bias against seemingly similar approaches.
Despite the rise of CAD, the two concepts—automated computer diagnosis and computer-aided diagnosis—persist today. Some researchers continue to pursue automated computer diagnosis. Furthermore, some believe that current CAD technologies could evolve into primary diagnostic tools, effectively becoming automated computer diagnosis systems. During panel discussions at the 2002 CARS meeting in Paris and the AAPM meeting in Montreal, approximately half of the participants predicted CAD transitioning to automated computer diagnosis within 50 years, while the other half disagreed. Therefore, understanding both the similarities and differences between CAD and automated computer diagnosis remains crucial. Both approaches rely on quantitative computer analysis of digital medical images, requiring the development of sophisticated computer algorithms.
The key distinction lies in how computer output is utilized in diagnosis. In CAD, radiologists interpret computer outputs as a “second opinion” while retaining final diagnostic authority. In cases where radiologists are confident, they may concur with or disregard computer findings. However, in ambiguous cases, CAD is intended to improve diagnostic accuracy. This improvement hinges on computer accuracy; higher computer performance leads to greater overall diagnostic benefit. Crucially, CAD does not require computers to match or exceed radiologist performance. CAD’s value derives from the synergistic effect of combining human and computer strengths, leading to its widespread clinical adoption.
Automated computer diagnosis, conversely, demands very high computer performance. If computer sensitivity for lesion detection falls below average physician sensitivity, its clinical utility becomes questionable. Patients in developed nations expect diagnostic standards at least comparable to those provided by physicians. Furthermore, insurance reimbursement for automated computer diagnosis might be denied if it offers inferior performance. A high false-positive rate, even one or more per case, also hinders automated computer diagnosis implementation. Physicians would need to verify every computer-flagged case, negating the automation aspect. Therefore, automated computer diagnosis necessitates both high sensitivity and specificity – extremely challenging benchmarks for algorithm developers. While CAD for breast cancer detection in mammography is widely used, current computer performance levels do not yet support automated computer diagnosis in most developed countries.
Another critical difference lies in performance evaluation. Automated computer diagnosis performance is solely defined by computer accuracy. CAD performance, however, is measured by the performance of the physician using CAD output as a second opinion. Effective physician utilization of computer results is paramount for CAD success. Quantitative evaluation, using Receiver Operating Characteristic (ROC) analysis (69–71), is essential to determine if CAD improves physician performance. Remarkably, even with computer ROC curves significantly below radiologist performance (e.g., in clustered microcalcification detection), radiologists using CAD can achieve improved ROC curves. H.P. Chan et al.’s 1990 study (66) provided statistical evidence of this CAD benefit in lesion detection. Subsequently, numerous studies have confirmed CAD’s effectiveness in detecting various lesions, including clustered microcalcifications (66) and masses (72) in mammograms, lung nodules (73,74 and interstitial opacities (75) in chest radiographs, lung nodules in CT (76, and intracranial aneurysms in MRA (77. These findings accelerated CAD research and development across diverse examinations and lesion types.
In summary, while CAD and automated computer diagnosis share common ground, fundamental differences exist. Automated computer diagnosis is algorithm-centric, whereas CAD is rooted in a collaborative physician-computer model. This distinction significantly impacts required computer performance levels. Automated computer diagnosis demands computer performance comparable to or exceeding physician performance. CAD, however, requires computers to complement, not necessarily surpass, physician capabilities. The widespread adoption of CAD systems for early breast cancer detection in mammography in the US and Europe highlights this point. These systems, despite not outperforming radiologists individually, prove clinically valuable by enhancing overall diagnostic accuracy.
4. Current Status of CAD Research
Table 1 illustrates the number of CAD-related papers presented at RSNA meetings from 2000 to 2005. Chest, breast, and colon were the most frequently studied organs, but research also extended to the brain, liver, skeletal, and vascular systems. Cancer detection in the breast (1–6), lung (78–84, and colon (85,86) are key targets for screening programs (87,88). Screening examinations often yield predominantly normal results, making the detection of rare suspicious lesions challenging and time-consuming for radiologists. Therefore, CAD’s initial clinical adoption has focused on these screening contexts. Commercial CAD systems for these cancers are now clinically available. Figure 1 compares the 1993 performance level (87% sensitivity at 1.0 false positive per image) for clustered microcalcification detection by computer (when CAD technology was first licensed) with the estimated current performance (98% sensitivity at 0.25 false positive per image) of advanced commercial CAD systems. This demonstrates substantial performance improvement driven by industry efforts, leading to successful clinical CAD systems. This exemplifies how academic research “seeds” can flourish through commercialization.
Table 1. CAD Papers by Organ System at RSNA Meetings (2000-2005)
| Year | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 |
|---|---|---|---|---|---|---|
| Chest | 22 | 37 | 53 | 94 | 70 | 48 |
| Breast | 23 | 28 | 32 | 37 | 48 | 49 |
| Colon | 4 | 10 | 21 | 17 | 15 | 30 |
| Brain | – | 4 | 2 | 10 | 9 | 15 |
| Liver | 3 | – | 5 | 9 | 9 | 9 |
| Skeletal | 2 | 7 | 7 | 9 | 8 | 5 |
| Vascular etc | 5 | – | 12 | 15 | 2 | 7 |
| Total | 59 | 86 | 134 | 191 | 161 | 163 |
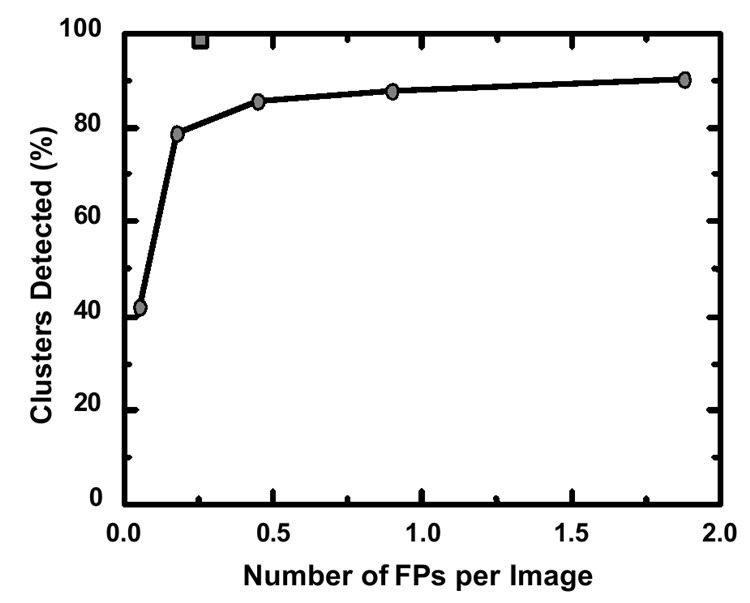
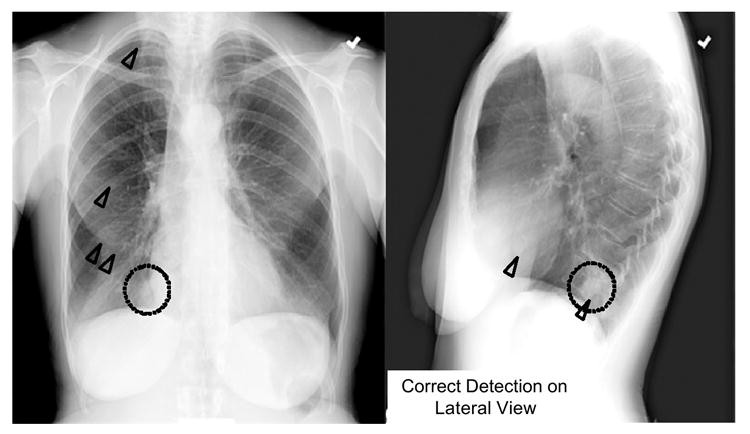
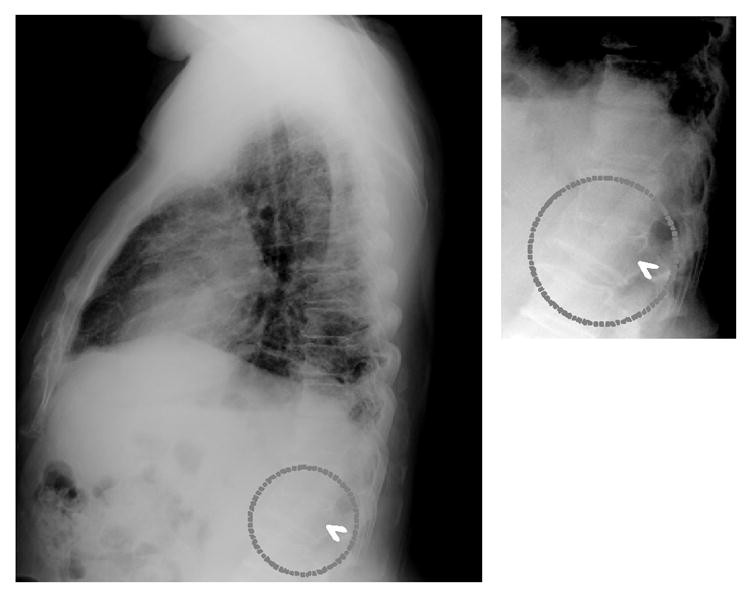
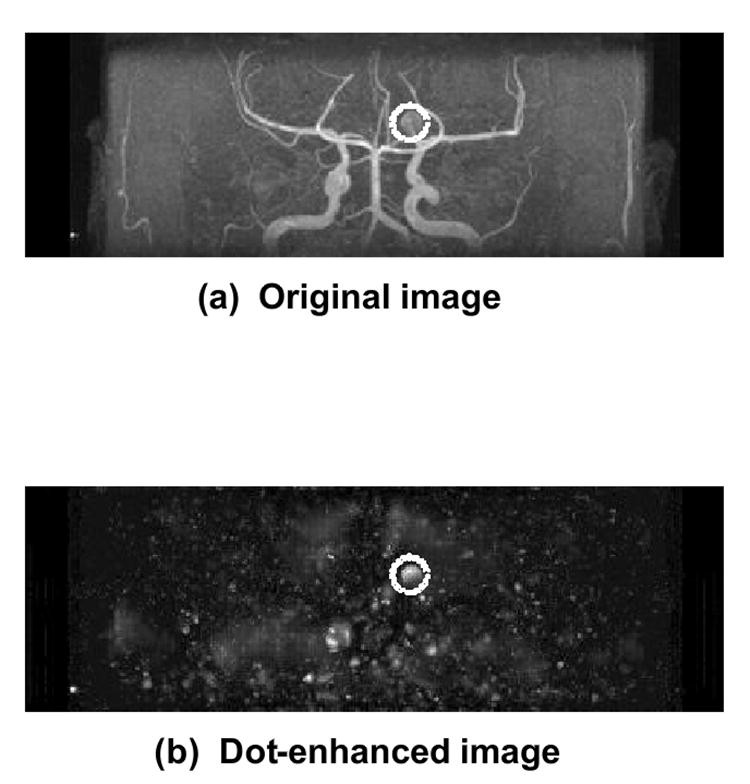
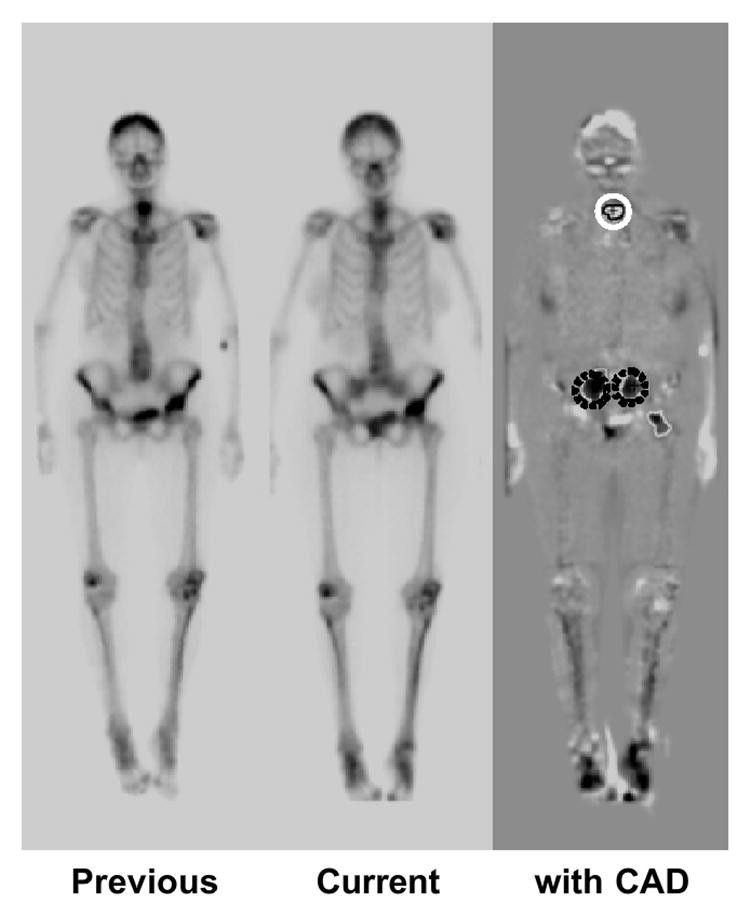
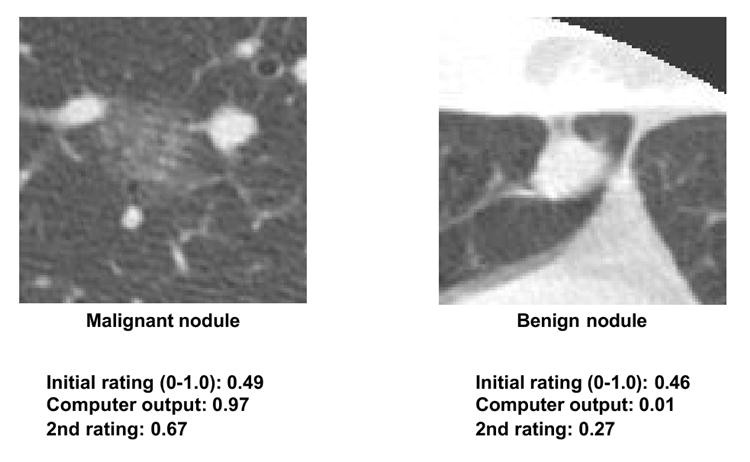
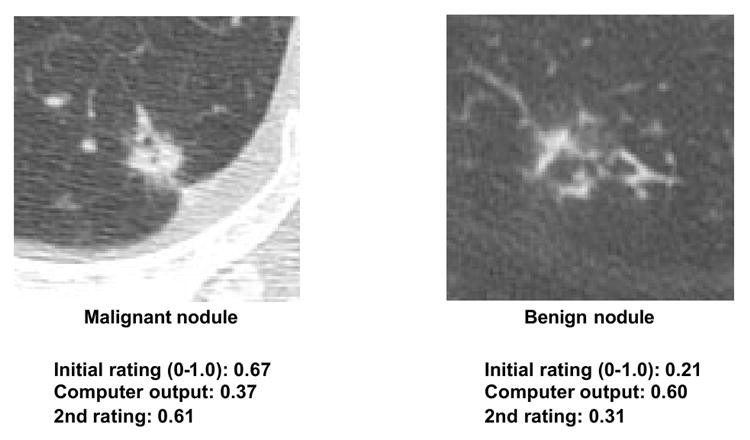
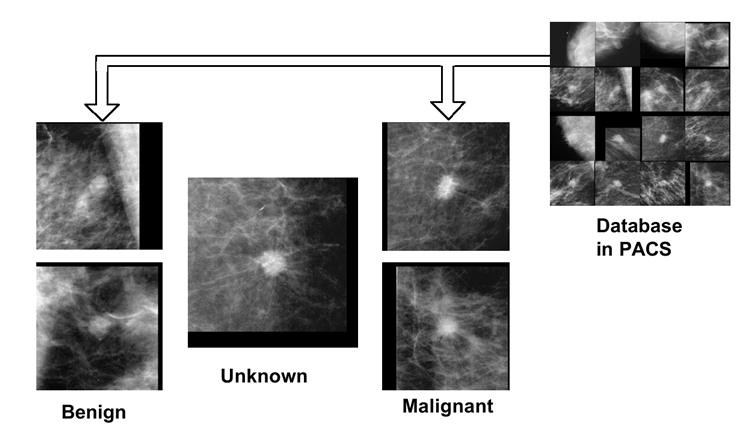
Fig. 1. CAD Performance Improvement in Microcalcification Detection
Comparison of CAD performance in clustered microcalcification detection between 1993 (circles) and current commercial systems (square), demonstrating significant improvement in sensitivity and false positive rate.
In mammography, prospective studies involving large cohorts (8,682 to 115,571 screenees) have assessed CAD’s impact on breast cancer detection rates (Table 2). While results vary, all studies reported increased cancer detection rates with CAD. Cupples et al. (4) reported a 164% increase in small (<1 cm) invasive cancer detection and a 5.3-year reduction in mean patient age at diagnosis when CAD was used in mammography.
Table 2. Prospective Studies of CAD in Screening Mammography
| Authors | Cases | Cancer Detection Rate Gain | Recall Rate Increase |
|---|---|---|---|
| Freer et al. (1) | 12,860 | 19.5% | 18.8% |
| Gur et al. (2) | 115,571 | 1.7% | 0.1% |
| Birdwell et al. (3) | 8,682 | 7.4% | 7.6% |
| Cupples et al. (4) | 27,274 | 16.1% | 8.1% |
| Morton et al. (5) | 18,096 | 7.6% | 10.8% |
| Dean et al. (6) | 9,520 | 10.8% | 26.0% |
This special issue of the Journal of Computerized Medical Imaging and Graphics includes review articles on CAD for mammography, chest radiography, thoracic CT, CT colonography, and multi-organ CAD. Therefore, the following sections highlight selected recent findings.
4.1 Lung Nodule Detection in PA and Lateral Chest Radiographs
CAD schemes are well-developed for chest radiography and thoracic CT, with some commercial systems available. Chest radiography CAD includes detection and differential diagnosis of lung nodules (89–91 and interstitial lung diseases (92–96), as well as detection of cardiomegaly (97,98), pneumothorax (99), and temporal changes (100–105. However, these methods often utilize only posterior-anterior (PA) chest images, even when lateral views are available. Lateral chest views are commonly acquired alongside PA views in clinical practice. Despite differing opinions on the necessity of both PA and lateral views (106–111), incorporating lateral views can enhance CAD performance for lung nodule detection and improve radiologist decision-making when lateral views are already acquired.
Recently, Shiraishi et al. (112) developed a CAD scheme for lung nodule detection in lateral chest radiographs to complement PA-view CAD and improve overall performance. PA-view CAD alone achieved 70.5% sensitivity with 4.9 false positives per image. While lateral-view CAD had lower performance (60.7% sensitivity, 1.7 false positives per image), combined PA and lateral CAD improved overall sensitivity to 86.9% (6.6 false positives per two views). Notably, 20 of 122 nodules (16.4%) in 106 PA/lateral pairs were detected only on lateral views. Figure 2 shows subtle lung nodules in the right mediastinum, missed by PA-view CAD but correctly identified by lateral-view CAD. Lateral-view CAD, therefore, enhances lung nodule detection in chest radiography when used in conjunction with standard PA-view CAD.
Fig. 2. Lung Nodule Detection on Lateral Chest Radiograph
Subtle lung nodule (dotted circles) in the right mediastinum, detected by CAD (triangles) on the lateral view but missed on the PA view.
4.2 Vertebral Fracture Detection on Lateral Chest Radiographs
Vertebral fracture (or deformity) is a frequent consequence of osteoporosis, a major public health concern. Early vertebral fracture detection is crucial as timely intervention can reduce the risk of subsequent fractures (113–117. Vertebral fractures are often visible on lateral chest radiographs. However, studies (118–121) indicate that approximately 50% of vertebral fractures visible on lateral chest radiographs are underdiagnosed or underreported, even when severe. Kasai et al. (122,123) developed a computerized vertebral fracture detection method for lateral chest radiographs to aid radiologists and facilitate early osteoporosis diagnosis. Their method automatically extracts a curved search area encompassing vertebral endplates, which is then straightened to horizontally align the endplates. Horizontal line-enhancement filters highlight edge candidates, and multiple thresholding followed by feature analysis identifies vertebral endplates. Vertebral height is determined from endplate locations, and fractured vertebrae are identified by comparing measured height to expected height.
The study used 20 patients with severe vertebral fractures and 118 fracture-free patients, confirmed by radiologist consensus. Preliminary results showed 95% sensitivity (19/20 fracture cases) with 1.03 false-positive fractures per image (139/135 images). Validation using 32 independent fracture cases yielded 75% sensitivity (24/32) at 1.03 false positives per image (33/32 images). Figure 3 shows correct computer detection of a fractured vertebra. Computerized vertebral fracture detection can serve as a “second opinion,” improving radiologist accuracy and enabling earlier osteoporosis diagnosis from lateral chest radiographs.
Fig. 3. Vertebral Fracture Detection on Lateral Chest Radiograph
Computer-aided detection (arrowhead) of a fractured vertebra (dotted circles) on a lateral chest radiograph, serving as a second opinion to improve diagnostic accuracy for osteoporosis.
4.3 Intracranial Aneurysm Detection in MRA
“Less invasive” modalities like CT angiography (CTA) and magnetic resonance angiography (MRA) have gained prominence in intracranial aneurysm detection (124–131). However, detecting small aneurysms remains challenging and time-consuming, and even medium-sized aneurysms can be missed on maximum intensity projection (MIP) images due to vessel overlap and unusual locations. CAD can assist radiologists, especially in detecting small aneurysms in MRA. Arimura et al. (132,133) developed a CAD scheme for automated unruptured intracranial aneurysm detection in MRA, utilizing a 3D selective enhancement filter for dot-like structures (aneurysms). Isotropic 3D MRA images are processed using a selective, multi-scale enhancement filter (134, as shown in Figure 4. Initial candidates are identified using multi-level thresholding on dot-enhanced images and region-growing with image feature monitoring. Candidates are classified into four types based on size and local structure (large, short-branch small, single-vessel small, bifurcation small). False positives are then removed within each group using rules based on gray levels and morphology. Linear discriminant analysis further reduces false positives.
Fig. 4. Intracranial Aneurysm Detection in MRA using 3D Enhancement Filter
(a) Original isotropic 3D MRA image; (b) Dot-enhanced image after applying a 3D selective, multi-scale enhancement filter, highlighting an intracranial aneurysm (dotted circles).
CAD performance was evaluated on 207 aneurysm cases from three institutions using different MR equipment, employing a leave-one-out-by-patient method within each institution. Average sensitivity for 1-23 mm aneurysms was 96% with 3.2 false positives per patient. An observer study with 15 radiologists using 22 aneurysm and 28 non-aneurysm cases showed ROC curve area (Az value) improvement from 0.931 to 0.983 (P = 0.001) with CAD assistance (77). This CAD system can effectively aid radiologists in intracranial aneurysm detection in MRA.
4.4 Temporal Change Detection in Serial Bone Scans
Bone scintigraphy is a common nuclear medicine procedure, vital for diagnosing osseous metastases and monitoring tumor response to therapy. While highly sensitive for bone abnormalities, identifying multiple lesions (e.g., in prostate and breast cancer metastases) is time-consuming (135,136). Detecting subtle temporal changes between serial bone scans is particularly challenging due to patient variations, radioisotope accumulation, and gamma camera image quality. Shiraishi et al. (137) developed a CAD scheme for temporal change detection in serial whole-body bone scans using temporal subtraction images obtained with nonlinear image warping (103). The scheme involves image density normalization, image matching, temporal subtraction, initial change detection, feature extraction, rule-based false positive reduction (using 16 features), and computer output display.
Based on radiologist consensus, 107 “gold-standard” interval changes were identified in 58 pairs of serial bone scans (anterior and posterior views). These included 71 hot lesions (increased uptake or new uptake) and 36 cold lesions (decreased or disappeared uptake). Overall sensitivity for interval change detection (hot and cold lesions), evaluated by resubstitution and leave-one-case-out methods, was 95.3% (5.97 false positives/view) and 83.2% (6.02 false positives), respectively. Figure 5 shows a temporal subtraction image and correct computer detection of one cold and two hot lesions. Temporal subtraction enhances interval changes in serial bone scans, and CAD can effectively assist radiologists in their interpretation.
Fig. 5. Temporal Subtraction Image for Interval Change Detection in Bone Scans
Temporal subtraction image highlighting interval changes between serial bone scans. Computer-aided detection correctly identifies one cold lesion (white solid circle) and two hot lesions (dark dotted circles).
5. Future Potential of CAD in PACS Environments
CAD schemes are likely to be integrated with imaging modality workstations (e.g., digital mammography, CT, MRI). Furthermore, comprehensive CAD packages will be incorporated into PACS. A chest CAD package, for example, could include computerized detection of lung nodules, interstitial opacities, cardiomegaly, vertebral fractures, and interval changes, alongside nodule classification and differential diagnosis of interstitial lung diseases. All chest images could be automatically analyzed for various abnormalities, with potential lesion sites and relevant information (malignancy likelihood, disease probability) displayed at workstations. To be clinically viable, false positive rates must be minimized to avoid overwhelming radiologists with clinically insignificant findings, ensuring CAD highlights primarily relevant abnormalities.
Radiologists could utilize such CAD packages in two ways: (1) initial image review without CAD, followed by CAD output review before final decision-making. This may preserve initial impressions and prevent dismissing subtle lesions missed by CAD, but could increase reading time. (2) Initial CAD output display followed by radiologist review. This could reduce reading time but carries the risk of missing lesions due to computer false negatives. High sensitivity CAD packages could mitigate this risk. Combining complementary CAD schemes within PACS offers synergistic advantages. For instance, while individual CAD schemes may miss certain lung nodules or opacities, temporal subtraction of serial chest images can effectively highlight subtle interval changes.
CAD research has developed lesion detection and differential diagnosis schemes. While lesion detection CAD (e.g., in mammography) is clinically established, differential diagnosis CAD has not yet seen widespread clinical adoption or commercialization. However, differential diagnosis CAD performance is promising (138–143). For example, CAD for distinguishing benign from malignant lung nodules in CT, based on malignancy likelihood scores (142,143), has shown benefit in observer studies. Feng Li et al. (143) demonstrated that radiologists using CAD (Az = 0.853) outperformed both radiologists alone (Az = 0.785) and CAD alone (Az = 0.831). CAD influenced radiologists’ confidence levels appropriately based on malignancy likelihood, improving decisions in subtle cases (Figure 6). Importantly, CAD did not negatively impact decisions in obvious cases (Figure 7), allowing radiologists to maintain correct judgments even with incorrect CAD outputs. This synergistic improvement demonstrates CAD’s value as a “second opinion,” enhancing decisions in challenging cases while preserving radiologist expertise in clear-cut scenarios.
Fig. 6. CAD Assistance in Subtle Nodule Diagnosis
CAD improves radiologists’ diagnostic accuracy for subtle lung nodules by providing malignancy likelihood scores, leading to beneficial shifts in confidence levels towards correct diagnoses.
Fig. 7. Radiologist Judgment Override in Obvious Nodule Cases
Radiologists maintain correct diagnoses in obvious nodule cases, overriding incorrect CAD outputs, demonstrating CAD’s non-detrimental effect in straightforward scenarios.
To further enhance differential diagnosis, providing similar benign and malignant images alongside malignancy likelihood scores would be beneficial. Visual similarity to known cases can boost diagnostic confidence. The value of similar image retrieval has been demonstrated in observer studies (144). Figure 8 compares an unknown mammogram mass with similar benign and malignant masses. Observers correctly identified the unknown mass as more similar to malignant examples.
Fig. 8. Similar Image Retrieval for Differential Diagnosis
Example of similar image retrieval for differential diagnosis, comparing an unknown mammogram mass (center) with similar benign (left) and malignant (right) masses from PACS.
Two key challenges for similar image retrieval are: (1) developing a comprehensive image database within PACS and (2) creating sensitive tools for similarity assessment. PACS image archives represent a vast, largely untapped resource. Developing reliable methods for quantifying image similarity is crucial to unlock the potential of PACS for comparative diagnosis. Recent studies suggest that psychophysical measures, derived from neural networks trained on image features and radiologist similarity ratings, can quantify image similarity for lung nodules (144) and mammographic lesions (145,146). Further research is needed to validate the clinical utility of such similarity search tools within PACS.
6. Conclusion
Computer-aided diagnosis, while established in mammographic breast cancer detection, is still in the early stages of realizing its full potential across diverse lesion types and imaging modalities. CAD, fundamentally different from automated computer diagnosis, emphasizes the synergistic roles of physicians and computers. Future PACS integration of CAD packages, encompassing lesion detection and differential diagnosis, promises to enhance daily diagnostic workflows. CAD is poised to become an increasingly valuable tool in routine clinical practice, augmenting radiologist accuracy and efficiency in medical image interpretation.
Acknowledgments
The author expresses gratitude to over 200 individuals, including faculty, researchers, fellows, residents, staff, and visitors at the Department of Radiology and Medical Physics Programs, University of Chicago, for their contributions to CAD research; to Charles E. Metz, PhD, and Shigehiko Katsuragawa, PhD, for manuscript review and suggestions; and to Mrs. E. Lanzl for manuscript editing. This work was supported by USPHS Grants CA62625 and CA 98119. K.D. is a shareholder of R2 Technology, Inc. CAD technologies from the Kurt Rossmann Laboratories, University of Chicago, have been licensed to companies including R2 Technology, Deus Technology, Riverain Medical Group, Mitsubishi Space Software Co., Median Technologies, General Electric Corporation, and Toshiba Corporation, in accordance with University of Chicago policy on financial disclosures related to research activities.
Footnotes
Publisher’s Disclaimer: This is an accepted manuscript PDF, undergoing further editing and typesetting before final publication. Content may be subject to change during production, and standard journal disclaimers apply.
References
[References]
Note: Please replace “[References]” with the actual references from the original article in markdown format. Ensure the reference links are correctly formatted as in the original article (e.g., [1](#R1)). Due to the length of the references, they are omitted here for brevity but should be included in the final output.