1. Introduction
Computer-aided diagnosis (CAD) has transitioned from a promising concept to a clinically relevant tool, particularly in breast cancer detection through mammography across numerous screening centers and hospitals in the United States [1-25]. This widespread adoption signals a significant shift towards leveraging CAD across diverse medical imaging modalities and examinations for the detection and differential diagnosis of various abnormalities [12-25]. While the initial forays into computerized medical image analysis date back to the 1960s [26-32], the 1980s marked the beginning of serious, systematic CAD research. This era introduced a fundamental change in how computer outputs were utilized, moving away from automated computer diagnosis towards computer-aided diagnosis. This article will explore the motivations and underlying philosophy behind the early development of CAD systems, alongside their current standing and future prospects within Picture Archiving and Communication Systems (PACS).
2. Historical Review of CAD Development
The early 1980s witnessed the initiation of large-scale research and development efforts in CAD at the Kurt Rossmann Laboratories for Radiologic Image Research, within the Radiology Department of the University of Chicago. This period was preceded and accompanied by foundational research into the impact of digital images on radiologic diagnosis [33-48], and parallel advancements in PACS research across the United States and Europe [49]. Despite PACS’s evident utility in managing radiologic images and potential economic benefits for hospitals, its immediate clinical advantages for radiologists seemed limited at the time. This realization spurred the critical question: “How can digital images tangibly improve the daily diagnostic work of radiologists?” This inquiry directly led to the inception of computer-aided diagnosis.
The 1980s context included the already explored concept of automated diagnosis, or automated computer diagnosis, from studies in the 1960s [26-31]. However, these early attempts had not achieved widespread success. (The distinctions and overlaps between automated diagnosis and computer-aided diagnosis will be further discussed in the subsequent section.) The complexity of computer analysis on medical image lesions was evident, making the success of CAD development uncertain. Consequently, research efforts were strategically directed towards areas with the potential for significant medical impact. Cardiovascular diseases, lung cancer, and breast cancer were identified as critical areas, leading to the establishment of three primary research projects focusing on: vascular imaging lesion detection and quantification (Fujita et al. [50] and Hoffmann et al. [51]); lung nodule detection in chest radiographs (Giger et al. [52, 53]); and clustered microcalcification detection in mammograms (Chan et al. [54]).
From its inception, CAD research and development has been guided by three core principles. Firstly, the methodology for lesion detection and quantification in medical images is deeply rooted in understanding radiologists’ image reading processes [20, 25]. This strategy acknowledges the complex and demanding nature of radiologists’ tasks in image interpretation and diagnosis. It posits that effective computer algorithms should be modeled on the cognitive processes of image reading – how radiologists identify lesions, why they might miss certain abnormalities, and their methods for distinguishing between benign and malignant conditions.
Secondly, the measure of success for CAD development was envisioned as its routine, daily clinical application in hospitals worldwide. Achieving this vision necessitated commercialization by medical industries. Therefore, the strategy included securing intellectual property for fundamental CAD technologies through patents and actively promoting these technologies within medical industries to foster commercial product development. This approach led to the first CAD patent [55], filed in 1987, which has since become a highly referenced patent in CAD technology.
Thirdly, promoting broad acceptance of the CAD concept and encouraging global CAD research across institutions was deemed essential. The multifaceted nature of CAD research, encompassing scheme development for various lesions across modalities, observer performance studies, clinical trials, and commercialization, required collaborative, widespread effort. Thus, a collaborative ethos was adopted, viewing other CAD researchers as colleagues rather than competitors. For over two decades, this collaborative spirit was manifested through extensive scientific exhibits at the Radiological Society of North America (RSNA) Annual Meetings in Chicago. These exhibits comprehensively showcased CAD research in chest, breast, and vascular imaging. In 1993, a real-time CAD demonstration for clustered microcalcification detection on mammograms was set up using a film digitizer and computer. Breast radiologists were invited to test the CAD scheme with their cases, yielding promising informal validation results [56]. From 1996 to 2001, observer performance studies were conducted at RSNA meetings, involving numerous radiologists in detecting lesions on chest images both with and without computer assistance. These studies effectively demonstrated CAD’s utility [57, 58], significantly contributing to the broad and rapid dissemination of the CAD concept.
In lung cancer CAD research, the initial focus was on developing a computerized scheme for lung nodule detection in chest radiographs. Lung nodule detection is known to be visually challenging for radiologists, with miss rates up to 30%. This is often due to the overlap of nodules with normal anatomical structures, effectively camouflaging them against the complex background of chest images [59-63]. It was anticipated that this background “noise” would pose a significant obstacle for computer detection as well. Consequently, the first step in computerized lung nodule detection was to address background structure interference. The difference-image technique [64-68] emerged as a useful method for background suppression, enhancing lesions by subtracting a nodule-suppressed image from a nodule-enhanced one. This technique proved effective not only for lung nodules [64, 65] but also for microcalcifications [54, 66] and masses [67] in mammograms, and nodules in CT scans [68].
By the mid-1980s, the Rossmann Laboratories had developed foundational schemes for lung nodule detection in chest images [52, 53] and clustered microcalcification detection in mammograms [54]. Despite achieving relatively high sensitivity, these early schemes produced a large number of false positives. The practical clinical utility of these outputs remained uncertain. For instance, clustered microcalcification detection yielded an average of four false positives per mammogram with approximately 85% sensitivity. To assess clinical applicability, an observer performance study was conducted, comparing radiologist performance with and without computer output. Surprisingly, even with the high false positive rate, radiologists’ performance in microcalcification detection significantly improved with CAD assistance. This finding, published in 1990 by H.P. Chan et al. [66], provided initial scientific validation for the clinical benefits of CAD.
3. Computer-Aided Diagnosis versus Automated Computer Diagnosis
The 1960s saw the first reports on quantitative medical image analysis by computers [26-31]. The prevailing expectation was that computers could eventually replace radiologists in abnormality detection, leveraging their superior capabilities in certain tasks. This era established the concept of computer diagnosis, or automated diagnosis, in radiology. While these early efforts produced intriguing results, they ultimately fell short of success due to limitations in computer processing power, the immaturity of image processing techniques, and limited access to digital images. However, a critical flaw was the overambitious expectation placed on computers. Indeed, diverse approaches to automated computer diagnosis have been explored across various medical fields since the 1950s. In hematology, for example, R.L. Engle [32], reflecting on 30 years of experience, concluded, “Thus, we do not see much promise in the development of computer programs to simulate the decision making of a physician. —- However, after many years we have concluded that we should stop trying to make computers act like diagnosticians.”
The 1980s witnessed a paradigm shift, recognizing the computer’s role as an aid to, rather than a replacement for, radiologists. This concept, known as computer-aided diagnosis, rapidly gained traction and widespread adoption. Initially, some computer scientists critiqued CAD, predicting its failure – a prediction that has proven unfounded. This criticism may have stemmed from the previous unsuccessful attempts at automated computer diagnosis, highlighting how the failure of one research direction can create undue bias against seemingly similar, yet fundamentally different, approaches.
Despite the rise of CAD, the concept of automated computer diagnosis persists. Some researchers actively pursue its development, while others believe that current CAD technologies could evolve into primary diagnostic tools, essentially achieving automated computer diagnosis. Panel discussions at the 2002 CARS meeting in Paris and the AAPM meeting in Montreal revealed a split opinion, with roughly half of participants predicting CAD’s shift to automated computer diagnosis within 50 years, and the other half disagreeing. Understanding the commonalities and distinctions between CAD and automated computer diagnosis is therefore crucial. Both approaches rely on quantitative computer analysis of digital medical images, necessitating the development of sophisticated computer algorithms.
The key difference lies in how computer output is utilized diagnostically. In CAD, radiologists interpret computer output as a “second opinion,” retaining final diagnostic authority. In cases where radiologists are confident, they may either concur with or disregard the computer’s findings. However, in ambiguous cases, CAD output is intended to enhance diagnostic accuracy, contingent on the computer’s correctness. Higher computer performance naturally leads to greater overall diagnostic improvement. Crucially, CAD computer performance does not need to equal or surpass that of radiologists; the synergistic effect of combining radiologist expertise with computer capabilities is the core advantage. This multiplicative benefit has driven CAD’s widespread clinical adoption.
Automated computer diagnosis, conversely, demands very high computer performance. If computer sensitivity for lesion detection were lower than that of physicians, its clinical utility would be questionable. Patients in developed countries would likely not accept diagnostic outcomes inferior to those achievable by physicians. Furthermore, insurance reimbursement for automated computer diagnosis might be denied if performance benchmarks are not met. High false-positive rates, such as one or more per case, would also hinder implementation, as physicians would need to verify every computer-flagged case, negating the automation aspect. Therefore, automated computer diagnosis requires both high sensitivity and high specificity – extremely challenging targets for algorithm developers in radiologic image analysis. While CAD has achieved clinical integration in mammographic breast cancer detection, current computer performance levels generally do not support automated computer diagnosis in most advanced healthcare systems.
Another critical distinction lies in performance evaluation. Automated computer diagnosis performance is solely measured by computer accuracy. CAD performance, however, is assessed by the overall diagnostic accuracy of the physician using computer output as a second opinion. Effective physician utilization of computer results is therefore paramount for CAD success. Quantitative evaluation, using Receiver Operating Characteristic (ROC) analysis [69-71], is essential to determine the degree to which computer output enhances physician performance. Even with suboptimal computer performance (e.g., low sensitivity and specificity), physicians can effectively integrate computer findings. For example, a computer might detect subtle lesions missed by physicians, while physicians can easily disregard obvious computer false positives, leading to overall diagnostic gain. However, poor computer performance inherently precludes successful automated computer diagnosis.
Thus, CAD evaluation must assess both computer performance and the resultant physician performance improvement. ROC analysis quantitatively demonstrates whether physician performance is enhanced by computer assistance. Chan et al.’s 1990 study [66] provided the first scientific evidence of CAD benefits, showing statistically significant improvement in radiologists’ microcalcification detection accuracy when using CAD, even when computer ROC performance alone was lower than radiologists’. Subsequent studies have confirmed CAD’s utility across various lesion types, including microcalcifications [66] and masses [72] in mammography, lung nodules [73, 74] and interstitial opacities [75] in chest radiographs, lung nodules in CT [76], and intracranial aneurysms in MRA [77]. These findings have accelerated CAD research and development across diverse medical imaging applications.
In summary, while CAD and automated computer diagnosis share quantitative computer analysis of medical images, fundamental differences exist. Automated computer diagnosis is algorithm-centric, whereas CAD is physician-computer collaborative. This distinction dictates vastly different performance requirements. Automated computer diagnosis demands computer performance comparable to or exceeding physician capabilities. CAD, however, only requires computers to provide complementary performance, not necessarily superior performance. The widespread adoption of CAD systems in breast cancer detection in the US and Europe, despite computer performance being below average physician performance, underscores its clinical utility as an assistive tool.
4. Current Status of CAD Research
Table 1 presents the number of CAD research papers presented at RSNA meetings from 2000 to 2005, categorized by organ system. Chest, breast, and colon dominated the research landscape, but CAD applications for brain, liver, skeletal, and vascular systems were also explored. Notably, breast [1-6], lung [78-84], and colon [85, 86] cancer detection, often involving screening examinations [87, 88], received significant attention. Screening examinations, characterized by a high proportion of normal results and the need to detect subtle suspicious lesions, are particularly well-suited for initial CAD implementation. Commercial CAD systems for these cancers are now clinically available, marking the successful translation of research into practice. Figure 1 illustrates the performance improvement in clustered microcalcification detection since CAD technology was first licensed in 1993 (87% sensitivity at 1.0 false positive per image) to current commercial systems (estimated 98% sensitivity at 0.25 false positive per image). This significant advancement, driven by industry efforts, demonstrates the successful evolution of CAD from academic concept to clinical tool. It exemplifies how academic innovation can be significantly amplified through commercialization.
Table 1. Number of CAD Papers at RSNA Meetings (2000-2005)
| Year | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 |
|---|---|---|---|---|---|---|
| Chest | 22 | 37 | 53 | 94 | 70 | 48 |
| Breast | 23 | 28 | 32 | 37 | 48 | 49 |
| Colon | 4 | 10 | 21 | 17 | 15 | 30 |
| Brain | – | 4 | 2 | 10 | 9 | 15 |
| Liver | 3 | – | 5 | 9 | 9 | 9 |
| Skeletal | 2 | 7 | 7 | 9 | 8 | 5 |
| Vascular etc | 5 | – | 12 | 15 | 2 | 7 |
| Total | 59 | 86 | 134 | 191 | 161 | 163 |
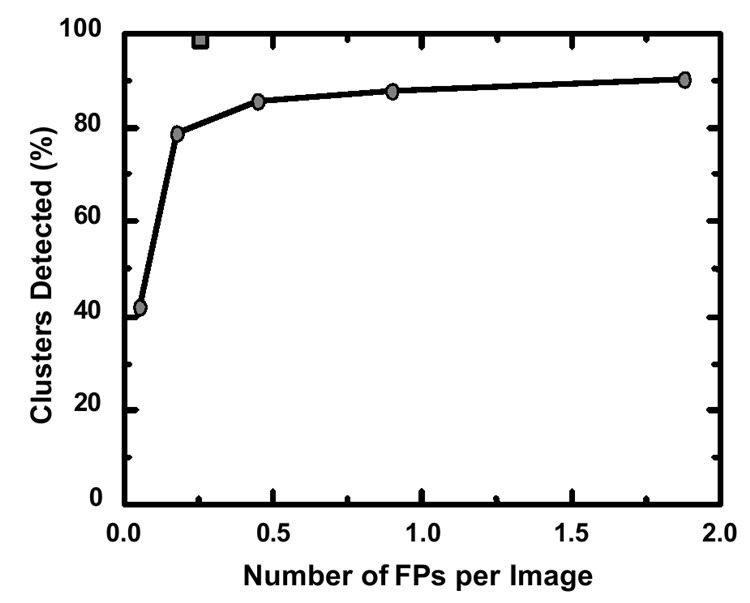
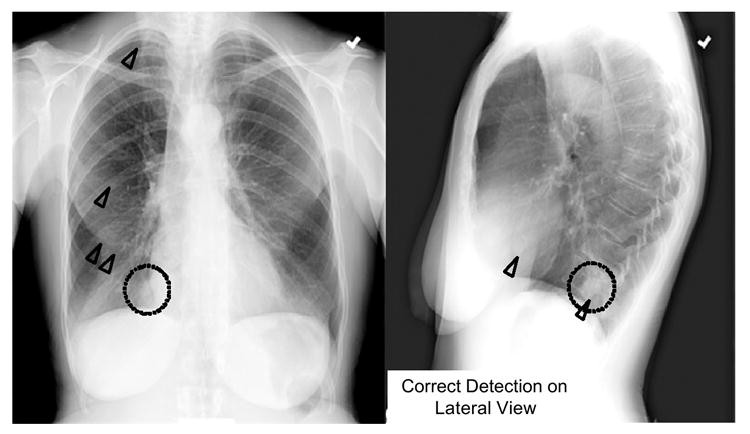
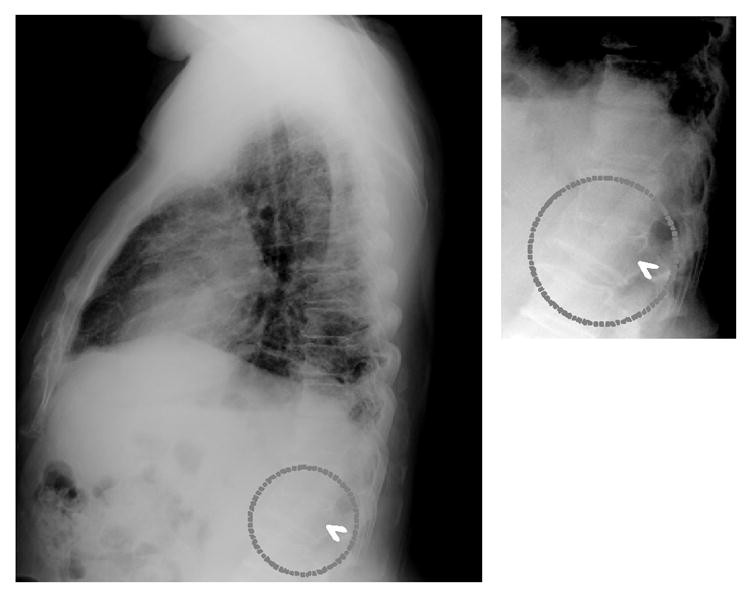
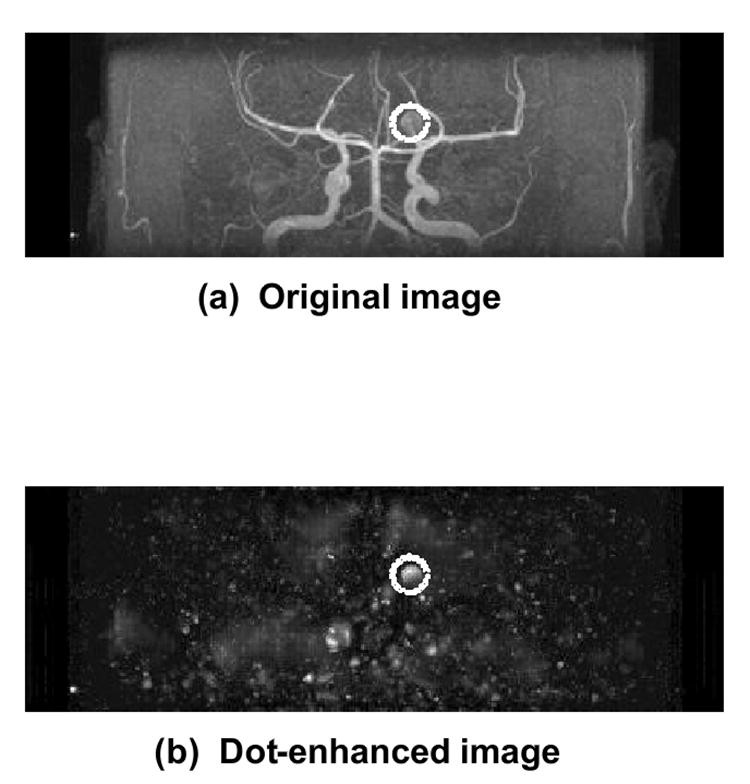
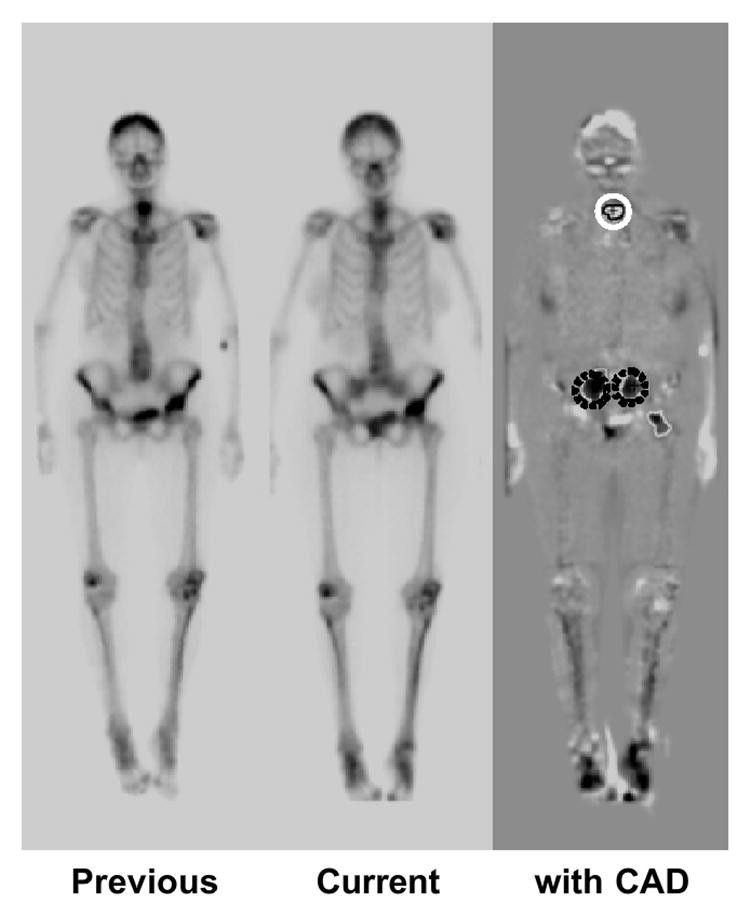
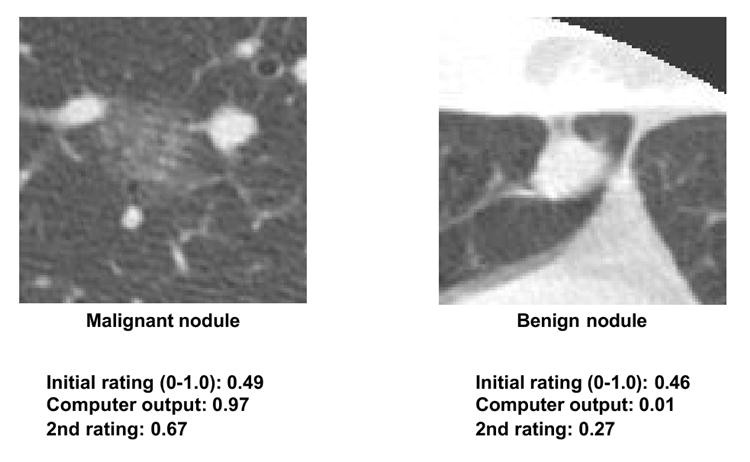
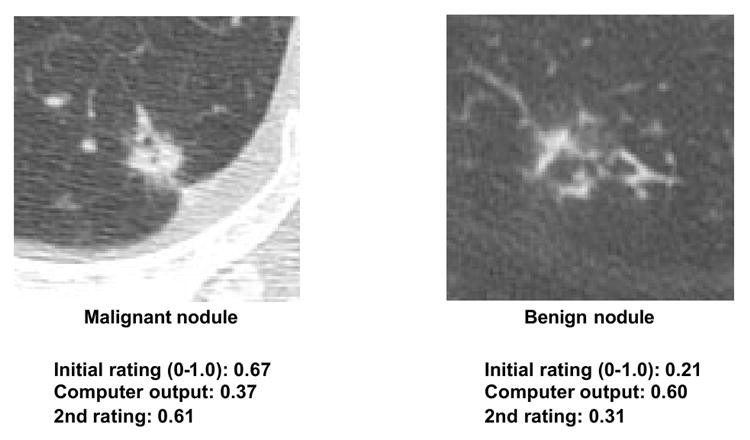
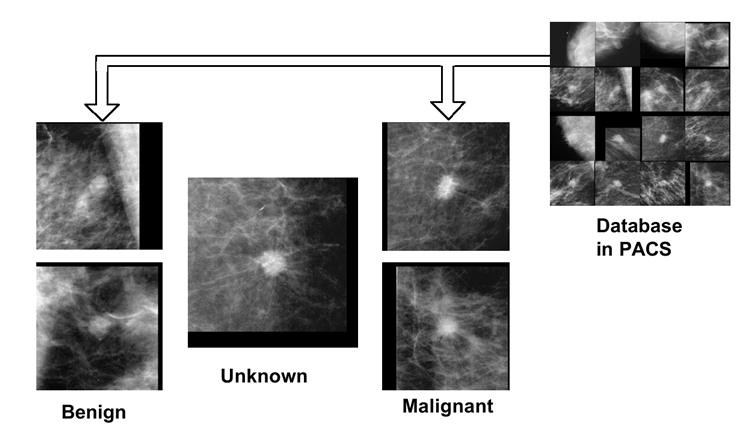
Fig. 1. CAD Performance Improvement in Microcalcification Detection
Comparison of clustered microcalcification detection performance: 1993 CAD technology (circles, 87% sensitivity at 1.0 false positive/image) vs. current commercial CAD (square, estimated 98% sensitivity at 0.25 false positive/image).
Prospective studies on large-scale mammography screening programs (8,682 to 115,571 screenees) have investigated CAD’s impact on breast cancer detection rates (Table 2). While results vary, all studies indicate an increase in cancer detection rates with CAD. Cupples et al. [4] reported a 164% increase in detecting small (<1 cm) invasive cancers and a 5.3-year reduction in mean patient age at detection when CAD was used.
Table 2. Prospective Studies on CAD for Breast Cancer Detection in Mammography
| Authors | No. of cases | Gain in cancer detection rate | Increase in recall rate |
|---|---|---|---|
| Freer et al. [1] | 12,860 | 19.5% | 18.8% |
| Gur et al. [2] | 115,571 | 1.7% | 0.1% |
| Birdwell et al. [3] | 8,682 | 7.4% | 7.6% |
| Cupples et al. [4] | 27,274 | 16.1% | 8.1% |
| Morton et al. [5] | 18,096 | 7.6% | 10.8% |
| Dean et al. [6] | 9,520 | 10.8% | 26.0% |
This special issue of the Journal of Computerized Medical Imaging and Graphics includes review articles on CAD for mammography, chest radiography, thoracic CT, CT colonography, and multi-organ, multi-dimensional CAD, thus the following sections will focus on selected topics with recent advancements.
4.1 Lung Nodule Detection on PA and Lateral Chest Radiographs
Chest radiography and thoracic CT have seen significant CAD development, with several commercial systems available. Chest radiography CAD applications include detection and differential diagnosis of lung nodules [89-91] and interstitial lung diseases [92-96], as well as detection of cardiomegaly [97, 98], pneumothorax [99], and interval changes [100-105]. However, many early CAD methods focused solely on posterior-anterior (PA) chest images, even when lateral views were available. Despite differing historical opinions on the necessity of both PA and lateral views [106-111], utilizing lateral views can reasonably enhance lung nodule detection CAD performance and aid radiologist decision-making, especially when lateral views are already acquired.
Shiraishi et al. [112] recently developed a CAD scheme for lung nodule detection specifically in lateral chest radiographs, aiming to improve overall performance when combined with PA view CAD. Using PA view CAD alone yielded 70.5% sensitivity with 4.9 false positives per image. While lateral view CAD performance was lower (60.7% sensitivity, 1.7 false positives/image), the combined sensitivity significantly improved to 86.9% (6.6 false positives per two views). Notably, 20 (16.4%) of 122 nodules (across 106 PA/lateral pairs) were detected only on lateral views. Figure 2 illustrates subtle lung nodules in the right mediastinum, missed by PA view CAD but correctly identified on the lateral view. Lateral view CAD thus offers potential for enhancing overall lung nodule detection in chest radiographs when integrated with standard PA view CAD.
Fig. 2. Lung Nodule Detection on Lateral View
Subtle lung nodule (dotted circles) in the right mediastinum, detected by CAD (triangles) on lateral view but missed on PA view.
4.2 Vertebral Fracture Detection on Lateral Chest Radiographs
Vertebral fracture, a common consequence of osteoporosis, is a major public health concern. Early detection is crucial for timely intervention to reduce subsequent fracture risk [113-117]. Vertebral fractures are often visible on lateral chest radiographs, yet studies [118-121] indicate that approximately 50% are underdiagnosed or underreported, even when severe. Kasai et al. [122, 123] developed a CAD method for vertebral fracture detection on lateral chest radiographs to assist radiologists and improve early osteoporosis diagnosis. The method automatically extracts a curved search area encompassing vertebral endplates, straightens it to horizontally orient endplates, enhances edge candidates using a horizontal line-enhancement filter, and employs multiple thresholding and feature analysis to identify endplates. Vertebral height is then determined from endplate locations, and fractures are detected by comparing measured height to expected height.
The study used 20 patients with severe fractures and 118 fracture-free patients (confirmed by radiologist consensus). Preliminary results showed 95% sensitivity (19/20) with 1.03 false-positive fractures per image. Validation testing on 32 independent fracture cases yielded 75% sensitivity (24/32) at 1.03 false positives per image. Figure 3 shows correct CAD detection of a vertebral fracture. Computerized vertebral fracture detection provides a “second opinion” for radiologists, potentially improving their detection accuracy on lateral chest radiographs and facilitating earlier osteoporosis diagnosis.
Fig. 3. Vertebral Fracture Detection by CAD
CAD detection (arrowhead) of a fractured vertebra (dotted circles) below the diaphragm on a lateral chest radiograph, serving as a second opinion to improve radiologist accuracy and early osteoporosis diagnosis.
4.3 Intracranial Aneurysm Detection in MRA
The past decade has seen increasing interest in less-invasive imaging modalities like CT angiography (CTA) and MR angiography (MRA) for intracranial aneurysm detection [124-131]. However, detecting small aneurysms, and even medium-sized ones obscured by vessel overlap or unusual locations on maximum-intensity-projection (MIP) images, remains challenging and time-consuming. CAD can assist radiologists, particularly in detecting small aneurysms using MRA. Arimura et al. [132, 133] developed a CAD scheme for automated unruptured intracranial aneurysm detection in MRA, using a 3D selective enhancement filter for dot-like structures (aneurysms). Isotropic 3D MRA images are processed with a selective, multi-scale enhancement filter [134], as shown in Figure 4. Initial candidates are identified via multi-level thresholding on dot-enhanced images and region-growing with feature monitoring. Candidates are classified into four types based on size and local structure (large, short-branch small, single-vessel small, bifurcation small). False positives are then reduced within each group using rules based on localized image features (gray levels, morphology) and linear discriminant analysis.
Fig. 4. Intracranial Aneurysm Detection in MRA with 3D Filter
(a) Isotropic 3D MRA image processed with a selective, multi-scale enhancement filter for intracranial aneurysm detection (dotted circles), resulting in (b) a dot-enhanced image.
CAD scheme performance was evaluated on 207 aneurysm cases from three institutions using different MR equipment, tested independently via leave-one-out-by-patient method. Average sensitivity for 1-23mm aneurysms was 96% with 3.2 false positives per patient. An observer study with 15 radiologists using 22 aneurysm and 28 aneurysm-free cases showed ROC curve area (Az value) improvement from 0.931 to 0.983 (P = 0.001) with CAD assistance [77]. This demonstrates CAD’s utility in assisting radiologists with intracranial aneurysm detection in MRA.
4.4 Interval Change Detection in Successive Bone Scans
Bone scintigraphy is a common nuclear medicine procedure, crucial for diagnosing osseous metastases and monitoring osseous tumor response to therapy. While highly sensitive for bone abnormalities, identifying multiple lesions (e.g., prostate and breast cancer metastases [135, 136]) and subtle interval changes between successive scans is time-consuming and challenging. Variations in patient condition, radioisotope accumulation, and gamma camera image quality complicate interval change detection. Shiraishi et al. [137] developed a CAD scheme for interval change detection in successive whole-body bone scans using temporal-subtraction images obtained with nonlinear image-warping [103]. The scheme includes image density normalization, image matching, temporal subtraction via nonlinear warping, initial interval change detection, feature extraction, rule-based false-positive reduction using 16 features, and computer output display.
Based on three-radiologist consensus, 107 “gold-standard” interval changes were identified in 58 pairs of successive bone scans (anterior and posterior views). These included 71 hot lesions (increased uptake or new uptake) and 36 cold lesions (decreased or disappeared uptake). Overall interval change detection sensitivity, evaluated by resubstitution and leave-one-case-out methods, was 95.3% (5.97 false positives/view) and 83.2% (6.02 false positives), respectively. Figure 5 shows a temporal subtraction image highlighting interval changes, with CAD correctly detecting one cold and two hot lesions. Temporal subtraction enhances interval changes in successive bone scans, and CAD can effectively assist radiologists in interpreting these changes.
Fig. 5. Interval Change Detection in Bone Scans via Temporal Subtraction
Temporal subtraction image from previous and current bone scans, highlighting interval changes. CAD correctly detects one cold lesion (white solid circle) and two hot lesions (dark dotted circles).
5. Future Potential of CAD in PACS
CAD schemes are likely to be integrated with modality-specific workstations (e.g., digital mammography, CT, MRI) and PACS. Comprehensive CAD packages for modalities like chest imaging may include computerized detection of lung nodules, interstitial opacities, cardiomegaly, vertebral fractures, and interval changes, along with nodule classification and differential diagnosis of interstitial lung diseases. All chest images could be automatically analyzed for various abnormalities, with potential lesion sites and associated information (malignancy likelihood, disease probability) displayed at workstations. For clinical practicality, minimizing false positives is critical to avoid overwhelming radiologists with clinically insignificant findings, ensuring CAD highlights primarily relevant abnormalities.
Radiologists could utilize such CAD packages in two ways: (1) initial image review without CAD, followed by CAD output review before final decision; (2) immediate display of CAD output upon image loading, influencing initial interpretation. The first approach might mitigate the risk of CAD-induced oversight of subtle lesions (due to false negatives), but could increase reading time. The second approach could reduce reading time but carries the risk of missing lesions due to computer false negatives. High sensitivity across a comprehensive CAD package could minimize this risk. For example, temporal subtraction techniques, highly sensitive to subtle changes, could compensate for missed nodules or opacities by other CAD components, highlighting interval changes. This synergistic potential is a key advantage of integrated CAD packages in PACS.
CAD research has focused on lesion detection and differential diagnosis (benign vs. malignant, or differentiation between diseases). While lesion detection CAD is clinically established (e.g., mammography), differential diagnosis CAD is not yet widely implemented commercially, despite reported high performance [138-143]. A CAD scheme for distinguishing benign from malignant lung nodules in CT [142, 143], based on malignancy likelihood prediction, demonstrated effectiveness in observer performance studies [143]. Radiologist performance with CAD (Az = 0.853) surpassed radiologists alone (Az = 0.785) or computer alone (Az = 0.831), with statistically significant Az value differences. CAD-generated malignancy likelihood influenced radiologists’ confidence levels, generally improving diagnostic accuracy, particularly in subtle cases (Figure 6). Importantly, CAD did not negatively impact radiologist accuracy in obvious cases, even with incorrect CAD output (Figure 7), as radiologists maintained their correct initial judgments. This synergistic improvement highlights CAD’s value as a “second opinion,” enhancing decisions in complex cases while preserving accuracy in straightforward ones.
Fig. 6. CAD Assistance in Subtle Nodule Diagnosis
Subtle, challenging HRCT nodules. Correct CAD malignancy likelihood output aids radiologists in improving decisions, indicated by beneficial confidence level changes towards correct diagnoses for both malignant and benign nodules.
Fig. 7. Radiologist Accuracy Maintained in Obvious Nodule Cases Despite CAD Errors
“Obvious” HRCT nodules. Radiologists maintain correct initial ratings for both malignant and benign nodules, even with incorrect CAD output, demonstrating no detrimental CAD effect.
Further enhancing differential diagnosis could involve providing similar benign and malignant images from PACS for comparison with new cases. Image similarity could supplement malignancy likelihood scores. A study [144] demonstrated improved Az values in distinguishing benign and malignant CT nodules using similar images. Figure 8 shows an unknown mammogram mass compared to similar benign and malignant masses, aiding correct classification.
Fig. 8. Similar Image Retrieval from PACS for Differential Diagnosis
Unknown mammogram mass (center) compared to similar benign (left) and malignant (right) masses retrieved from PACS, aiding diagnostic classification.
Two key challenges for similar image utilization are: (1) developing a comprehensive image database within PACS and (2) creating sensitive tools for similarity-based image retrieval. PACS image archives, largely untapped beyond same-patient comparisons, represent a vast resource. Developing reliable methods to quantify image (or lesion) similarity for visual comparison is crucial to unlock this potential. Recent studies suggest psychophysical measures, derived from neural networks trained on image features and radiologist similarity ratings, can quantify similarity in lung nodules [144] and mammographic lesions [145, 146]. Further research is needed to validate the clinical utility of such tools for PACS-based similar image retrieval.
6. Conclusion
Computer-aided diagnosis, while clinically established in mammographic breast cancer detection, is still in the early stages of its full potential across diverse modalities and lesion types. CAD, fundamentally a physician-computer partnership, differs distinctly from automated computer diagnosis. Future CAD integration within PACS, encompassing lesion detection and differential diagnosis packages, promises to be a valuable tool in daily diagnostic practice.
Acknowledgments
The author expresses gratitude to over 200 individuals from the Department of Radiology and Medical Physics programs at the University of Chicago for their contributions to CAD research and development, and to Charles E. Metz, PhD, and Shigehiko Katsuragawa, PhD, for manuscript review and suggestions. Thanks also to Mrs. E. Lanzl for manuscript editing. This work was supported by USPHS Grants CA62625 and CA 98119. K.D. is a shareholder of R2 Technology, Inc. CAD technologies from the Kurt Rossmann Laboratories have been licensed to various companies. The University of Chicago mandates public disclosure of potential financial interests related to research activities.
Footnotes
Publisher’s Disclaimer: This is an unedited manuscript accepted for publication, provided as an early service. The manuscript will undergo copyediting, typesetting, and proof review before final publication. Errors may occur during production, and standard journal disclaimers apply.
References
(References are identical to the original article and are therefore omitted for brevity as per the user’s request to focus on content generation, not reference formatting.)