INTRODUCTION
Cushing’s disease (CD), a specific form of Cushing’s syndrome (CS) resulting from an ACTH-secreting pituitary tumor, necessitates precise diagnosis, thoughtful treatment strategies, and sustained management for optimal patient outcomes. Effective Cushing Diagnosis Guidelines are critical for clinicians to navigate this complex condition. This article provides an updated overview of cushing diagnosis guidelines, incorporating recent advancements and expert consensus to enhance diagnostic accuracy and patient care.
Compared to Cushing’s syndrome arising from adrenal issues, patients with CD often experience a diminished long-term quality of life (QoL). Given the evolution of diagnostic tools and therapeutic approaches since the last comprehensive clinical guidelines in 2015, a contemporary update on cushing diagnosis guidelines is essential for clinicians managing this challenging endocrine disorder.
In October 2020, The Pituitary Society convened a virtual workshop, bringing together over 50 academic researchers and clinical specialists. The aim was to critically assess current literature, discuss management strategies, and formulate evidence-based cushing diagnosis guidelines. The workshop focused on pituitary-derived CS, excluding adrenal or ectopic CS, and avoided topics recently addressed in other consensus documents.
This article summarizes recent evidence and recommendations for clinical practice in line with established cushing diagnosis guidelines. We evaluate the quality of evidence and strength of consensus recommendations, highlighting key considerations for laboratory testing and medical therapies. Table 1 and Table 2 summarize laboratory tests and medical therapies, respectively. Panel 1 and Panel 2 outline consensus recommendations for managing CD complications and the use of medical therapy. Figure 1 and Figure 2 present diagnostic and management algorithms for CS and CD. Finally, Panel 3 lists crucial areas for future research to refine cushing diagnosis guidelines and improve patient care.
Table 1.
Laboratory Tests for CS Diagnosis and Monitoring for CD Recurrence
| Diagnosis |
|---|
| Test |
| 1-mg DST |
| 24-hr UFC |
| LNSC |
| Clinical Considerations and RecommendationsIf CD is suspected: • Start with either UFC and/or LNSC; DST could also be an option if LNSC not feasible • Multiple LNSC may be easier for patient collectionIf confirming CD: • Use any test • UFC average 2–3 collections • LNSC ≥2 on consecutive days • DST useful in shift workers, not in women on estrogen-containing OC • Measuring dexamethasone level along with cortisol the morning after 1 mg dexamethasone ingestion improves test interpretabilityIf CS due to adrenal tumor is suspected: • Start with DST • LNSC has lower specificity in these patients |
| Monitoring for Recurrence |
| Test |
| LNSC |
| 24-hr UFC |
| Desmopressin |
| 1-mg DST |
| Clinical Considerations and Recommendations • LNSC most sensitive, should be done annually • DST and UFC usually become abnormal after LNSC • Consider which tests were abnormal at initial diagnosis |
This table summarizes key laboratory tests used in cushing diagnosis guidelines.
Based on Galm et al J Clin Endocrinol Metab 2020 and Hinojosa-Amaya et al Front Endocrinol (Lausanne) 2019. Sensitivity and specificity will vary based on assay used (Petersenn Best Pract Res Clin Endocrinol Metab 2021).
*Cut-offs specified are for adults. Some experts recommend using the same cutoffs for initial diagnosis and recurrence.
**Some studies use ACTH absolute cutoffs/increments.
Abbreviations: CD, Cushing’s disease; CS, Cushing’s syndrome; DST, dexamethasone suppression test; LNSC, late-night salivary cortisol; UFC, urinary free cortisol; ULN, upper level of normal.
Table 2.
Summary of Medical Therapies for CD
| Target | Drug | Commonly used doses | Efficacy | Adverse effects | Key considerations |
|---|---|---|---|---|---|
| Adrenal steroidogenesis | Ketoconazole | 400–1200 mg/d PO, dosing BID | Retrospective studies: ∼65% UFC normalization initially, but 15–25% escape | GI disturbances, ↑ liver enzymes, gynecomastia, skin rash, AI | • EMA approved for treatment of endogenous CS, off-label use in US• Increasing doses needed to counter escape• Needs gastric acid for absorption (avoid PPIs)• Decrease in testosterone would be preferred in women; men need follow-up for hypogonadism• Risk for serious hepatotoxicity; mostly transient but regular LFT monitoring required• Risk of QTc prolongation• Careful review of other medications for potential drug-drug interactions is essential |
| Osilodrostat | 2–7 mg/d BID PO as maintenance; 30 mg/d BID maximum | Phase 3 randomized withdrawal study: 86% UFC normalization | ↑ Androgenic and mineralocorticoid precursors (hirsutism, hypertension, hypokalemia), GI disturbances, asthenia, AI | FDA approved for patients with CD in whom pituitary surgery is not an option or has not been curative• EMA and Japan approved for treatment of endogenous CS• Not yet widely available• Rapid decrease in UFCHas• Risk for hypocortisolism, hypokalemia, and QTc prolongation• Cross-reaction in routine assays with 11-deoxycortisol• Careful monitoring for hyperandrogenism in women | |
| Metyrapone | 500 mg/d to 6 g/d; dosing q 6–8 h | UFC normalizationRetrospective studies: ∼70%Prospective study: 47% at week 12 | ↑ Androgenic and mineralocorticoid precursors (hirsutism, hypertension, hypokalemia), AI | • EMA approved for treatment of endogenous CS, off-label use in US• Rapid decrease in UFC, typically in first month• Possible cross reactivity with 11-deoxyxortisol in cortisol immunoassays• Hyperandrogenism needs to be monitored with long-term use in women | |
| Mitotane | 250–500 mg/d PO up to 8 g/d | Retrospective studies: ∼80% UFC normalization | GI disturbances, dizziness, cognitive alterations, AI ↑ Liver enzymes; treatment should be stopped if elevations are >5 × ULN | • FDA and EMA approved for treatment of adrenal cancer with endogenous CS• Slow onset of action, highly variable bioavailability• Narrow therapeutic window (dose titration based on mitotane plasma levels)• Neurological toxicity could be a limiting factor• Mitotane is teratogenic and an abortifacient. Because of its long terminal half-life, this may limit its use in women who desire future pregnancy.• Cross-reaction in routine assays with 11-deoxycortisol | |
| Etomidate | 0.04–1 mg/kg/h IV for patients in the ICU; 0.025 mg/kg/h for non-ICU patients | Retrospective studies: ∼100% serum cortisol control (10–20 μg/dL) | Sedation/anesthesia, AI Myoclonus, nausea, vomiting, and dystonic reactions at higher anesthetic doses | • Off-label use only Very rapid onset of action, appropriate for acute treatment of severe hypercortisolism• IV hydrocortisone required at high doses to avoid adrenal insufficiency | |
| Somatostatin receptor | Pasireotide | 0.3–0.9 mg/mL BID SC | Phase 3 study: 15–26% UFC normalization | Hyperglycemia, T2DM, diarrhea, nausea, abdominal pain, cholelithiasis, fatigue | • Widely approved for patients with CD in whom pituitary surgery is not an option or has not been curative• Decreases tumor volume• High risk for hyperglycemia requires careful patient selection• Risk of QTc prolongation |
| Pasireotide LAR | 10–30 mg monthly IM | Phase 3 study: 40% UFC normalizationClinical signs and symptoms of hypercortisolism improved | |||
| Dopamine receptor | Cabergoline | 0.5–7 mg weekly PO | Retrospective studies:∼40% UFC normalization initially, but ∼25–40% escapeClinical signs and symptoms of hypercortisolism improved | Headache, nasal congestion, hypotension, depression, dizziness | • Off-label use only for CD• Decreases tumor volume in up to 50% of the patients evaluated• Clinical signs and symptoms of hypercortisolism improved• Poor response may be due to under-titration• Risk for treatment-induced impulse-control disorder; unclear risk for cardiac valvulopathy |
| Glucocorticoid receptor | Mifepristone | 300–1200 mg/d PO | Open-label phase 3 study: significant improvement in glycemia (∼60%) and blood pressureClinical signs and symptoms of hypercortisolism improved | GI disturbances, headache, hypokalemia, arthralgia, peripheral edema, hypertension, vaginal bleeding, AI | • FDA approved for hyperglycemia associated with CS• No laboratory markers of efficacy• Challenging to use outside specialized clinical practice• Risk of hypokalemia and adrenal insufficiency; needs close monitoring• Careful review of other medications for potential drug-drug interactions is essential |
| Investigational drugs with completed phase 3 clinical trials | |||||
| Adrenal steroidogenesis | Levoketoconazole274,275 | 300–1200 mg/d, BID, PO | Phase 3 open label study: 31% UFC normalization primary end-point; 42% when using imputed data (comparable with other studies)Phase 3 randomized withdrawal study: 41% lost response with drug vs 96% with placebo Clinical signs and symptoms of hypercortisolism improved | GI disturbances, headache, edema, ↑ liver enzymes, AI | • Investigational; FDA and EMA orphan drug status for treatment of endogenous CS• Possible lower risk for hepatotoxicity than with ketoconazole based on animal models, although no head to head studies in humans available• Needs gastric acid, avoid PPIs• Risk of QTc prolongation• Careful review of other medications for potential drug-drug interactions is essential |
This table provides a summary of medical therapies relevant to cushing diagnosis guidelines and management.
Abbreviations: AI, adrenal insufficiency; BID, twice daily; CD, Cushing’s disease; CS, Cushing’s syndrome; EMA, European Medicines Agency; FDA, US Food and Drug Administration; GI, gastrointestinal; ICU, intensive care unit; IM, intramuscular; IV, intravenous; LAR, long-acting release; LFT, liver function test; PO, by mouth; PPI, proton pump inhibitor; q, every; SC, subcutaneous; UFC, urinary free cortisol.
Panel 1.
Complications of CD: Summary of Recommendations
| Hypercoagulation |
|---|
| • There is currently no standard practice for preoperative or postoperative thromboprophylaxis in patients with CD. Some experts hold estrogen therapy in women who are awaiting surgery, but care should be taken if it was being used as contraception, because pregnancy also is associated with increased risk of thrombosis (LQ, DR) |
| • Prophylactic anticoagulation should be considered for patients at risk for VTE, including history of embolism or abnormal coagulation testing; severe preoperative hypercortisolism; current use of estrogen or oral contraceptives; poor mobility; extended preoperative or postoperative hospital stay; and high postoperative cortisol levels or cortisol over-replacement in patients with AI (MQ, SR) |
| • Early postoperative ambulation and use of compression stockings should be encouraged for all patients (HQ, SR) |
| • If thromboprophylaxis is administered, there was strong consensus for preference of low molecular weight heparin over oral anticoagulants given the long half-life of the latter and the lack of therapy to reverse their effect, which may be especially concerning in the preoperative setting (LQ, DR) |
| • Anticoagulants may be discontinued before surgery to minimize intraoperative bleeding risk, but the timing of when to stop and when to reinitiate after surgery is unclear (LQ, DR) |
| • Among meeting participants, recommended anticoagulation duration ranged in the preoperative setting from 2–4 days to 1–2 weeks, and in the postoperative setting from 1–2 days of the hospital stay up to 2–4 weeks or even longer to 2–3 months (LQ, DR) |
| • Thromboprophylaxis should not be routinely used in pediatric patients due to bleeding risk but reserved for selected patients |
| Cardiovascular Disease |
| • Evaluate, monitor, and treat according to current guidelines for patients at high risk for cardiovascular disease (HQ, SR) |
| • Management approach should be individualized (HQ, SR) based on the complications present (e.g., hypertension or hyperlipidemia) and care should be coordinated with primary care and cardiology physicians as needed (VLQ, DR) |
| Bone Disease |
| • Risk assessment for bone loss and fracture recommended in all patients (HQ, SR) |
| • Given the risk for fracture even in patients without osteoporosis, standard DXA alone may not be sufficiently informative; bone quality (microscanner or trabecular bone score) or morphometric vertebral assessment is recommended where available (HQ, SR) and can be useful in detecting subclinical fractures (HQ, SR), but might not be practical for all patients. The FRAX tool to assess fracture risk is not validated for CD |
| • Monitor and follow-up as for all adult high-risk populations (HQ, SR) |
| • Consider conventional osteoporosis treatments, e.g., bisphosphonates, for patients with persistent CD even if BMD is normal because of increased fracture risk due to cortisol excess (HQ, SR) |
| GH Deficiency |
| • There is currently no standard practice for whether, when, and how to test for GHD in adults with CD. As postoperative HPA axis recovery is often delayed, we recommend waiting at least 6–12 months after surgery before considering GHD assessment (MQ, SR) |
| • Patients with macroadenomas and more aggressive surgical resection are at higher risk for hypopituitarism; patients with 3 or more pituitary hormone deficiencies are more likely to have GHD and do not need dynamic testing (HQ, SR) |
| • Serum IGF-I level alone is not likely to be a reliable indicator of GHD, as levels can be in the lower half of the normal range on dynamic tests |
| • Accessibility of GH replacement may be an important factor in determining testing and treatment considerations. If GH replacement is implemented earlier than 2 years after pituitary surgery, we recommend retesting periodically to determine whether GH secretion has normalized upon HPA axis recovery (MQ, SR) |
| • As CS-associated myopathy does not spontaneously resolve during remission, physical rehabilitation is recommended for all patients (HQ, SR). |
| • In children, evaluate for GHD 3–6 months after surgery and immediately initiate GH replacement if needed to ensure proper growth |
This panel summarizes recommendations for managing complications, relevant to holistic cushing diagnosis guidelines and patient care.
Abbreviations: AI, adrenal insufficiency; BMD, bone mineral density; CD, Cushing’s disease; DXA, dual x-ray absorptiometry; GHD, growth hormone deficiency; HPA, hypothalamus-pituitary-adrenal; VTE, venous thromboembolism.
Panel 2.
Medical Therapy for CD: Summary of Recommendations
| Which factors are helpful in selection of a medical therapy? |
|---|
| • If there is a need for rapid normalization of cortisol, we recommend an adrenal steroidogenesis inhibitor; osilodrostat and metyrapone have the fastest action and are orally available, while etomidate can be used intravenously in very severe cases (HQ, SR) |
| • In mild disease, if residual tumor is present and there is a potential for tumor shrinkage, consider pasireotide or cabergoline (MQ, SR) |
| • If there is a history of bipolar or impulse control disorder, consider avoiding cabergoline (MQ, SR) |
| • If an expert pituitary endocrinologist is not available to monitor treatment response, use mifepristone cautiously (LQ, DR); we recommend counseling patients that cortisol cannot be used to monitor treatment response or AI (SQ, SR). Drug-drug interactions must be considered when this medication is used. |
| • In pregnant women or those desiring pregnancy, consider cabergoline or metyrapone, although no CD medications are approved for use in pregnancy (LQ, DR) |
| • Drug intolerance or side effects as well as concomitant comorbidities such as T2DM and hypertension should further guide type of medication used (MQ, SR) |
| • Consider cost and estimated therapy duration, especially if definitive treatment (i.e., pituitary and adrenal surgery) is planned or while awaiting effects of radiotherapy (LQ, DR) |
| Which factors are used in selecting an adrenal steroidogenesis inhibitor? |
| • Rapidity of action, tolerability, ease-of-use, degree of likely biochemical normalization, and specific clinical improvement as well as local availability and cost of each drug should be considered at therapy start (MQ, SR) |
| • Ketoconazole may be favored for ease of dose titration; concern about inducing hepatotoxicity and the need to monitor liver enzymes may lead to under-dosing (MQ, SR). Drug-drug interactions must be considered and hypogonadism may occur in men |
| • Osilodrostat achieves high rates of cortisol normalization. Dosing schedule may be more convenient for patients compared with metyrapone, but neither metyrapone nor osilodrostat is limited by hypogonadism in men (HQ, SR) |
| • Mitotane is rarely used as monotherapy in CD in most centers (LQ, DR) |
| How is tumor growth monitored when using an adrenal steroidogenesis inhibitor or glucocorticoid receptor blocker? |
| • MRI is typically obtained 6–12 months after initiating treatment and repeated every few years depending on the clinical scenario (MQ, SR) |
| • It can be difficult to determine whether tumor progression is due to loss of cortisol feedback or reflects the underlying behavior of aggressive, recurrent disease (LQ, DR) |
| • We suggest monitoring ACTH levels, as progressive elevations in ACTH may be a sign of tumor growth and a need for MRI, although the half-life of ACTH is short, levels fluctuate and do not necessarily reflect tumor growth (LQ, DR) |
| • If progressive tumor growth is seen, medical treatment should be suspended and the management plan reassessed (MQ, SR) |
| When is preoperative medical therapy used? |
| • There are no rigorous data supporting use of preoperative medical therapy (MQ, SR) |
| • Most experts would consider use of adrenal steroidogenesis inhibitors if surgery is delayed, either because of scheduling or due to external factors (LQ, DR) |
| • Patients with severe CD who have potentially life-threatening metabolic, psychiatric, infectious, or cardiovascular/thromboembolic complications may benefit in select cases (LQ, DR) |
| How is treatment response monitored? Which factors are considered in deciding whether to use combination therapy or to switch to another therapy? |
| • Response should be defined based on a combination of clinical (improved phenotype, weight, hypertension, glucose metabolism, QoL) and biochemical endpoints or only clinical endpoints when glucocorticoid receptor blockers are used (MQ, SR) |
| • Cortisol levels are often measured by UFC (except when using mifepristone); UFC is not useful if AI is a concern (HQ, SR) |
| • Because of the loss of biologic circadian rhythm, it is unclear whether targeting diurnal secretion alone with morning cortisol and/or LNSC is meaningful (LQ, DR) |
| • Change in treatment should be considered if cortisol levels are persistently elevated after 2–3 months on maximum tolerated doses (MQ, SR) |
| • If cortisol does not normalize but is reduced and/or there is some clinical improvement, combination therapy can be considered (LQ, DR) |
| • If there is clear resistance to treatment despite dose escalation, we suggest switching to a different therapy (LQ, DR) |
| Which agents are used for optimal combination therapy? |
| • There are few rigorous data supporting specific regimens for combination therapy (HQ, SR) |
| • Many experts consider combining ketoconazole with metyrapone or potentially ketoconazole with osilodrostat to maximize adrenal blockade when monotherapy is not effective or to allow lower doses of both drugs (LQ, DR) |
| • Ketoconazole plus cabergoline or pasireotide, and pasireotide plus cabergoline may be rational combinations if there is visible tumor present (LQ, DR) |
| • Other combinations that may be used include triplets of cabergoline, pasireotide, plus ketoconazole, and ketoconazole, metyrapone, plus mitotane (LQ, DR) |
This panel summarizes medical therapy recommendations, crucial for comprehensive cushing diagnosis guidelines and treatment planning.
Abbreviations: ACTH, adrenocorticotropin; AI, adrenal insufficiency; CD, Cushing’s disease; LNSC, late-night salivary cortisol; MRI, magnetic resonance imaging; QoL, quality of life; UFC, urinary free cortisol.
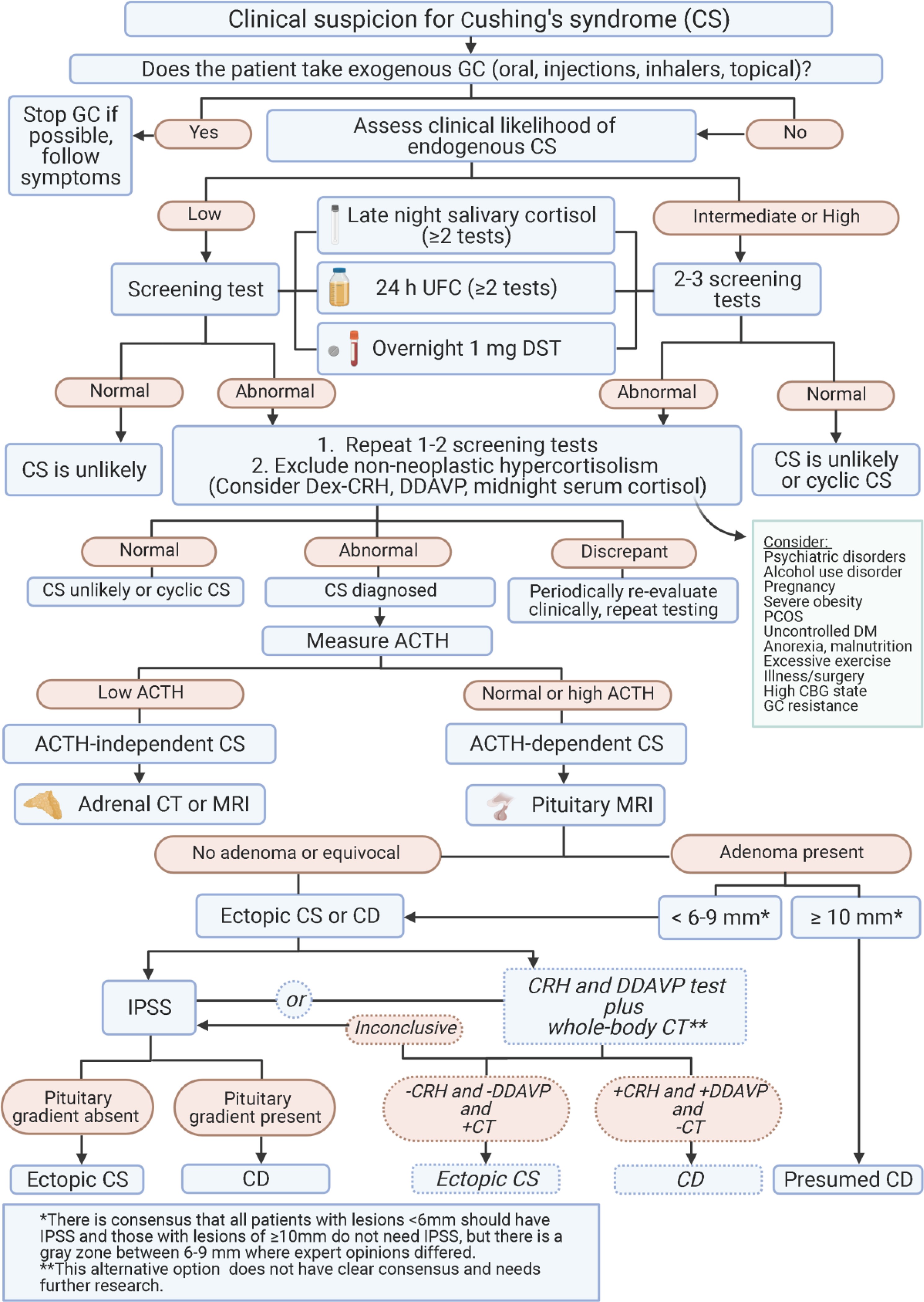
Figure 1. Algorithm for diagnosis of Cushing’s syndrome.
This algorithm visually represents the cushing diagnosis guidelines for Cushing’s syndrome.
Abbreviations: ACTH, adrenocorticotropin; CBG, corticosteroid binding globulin; CD, Cushing’s disease; CRH, corticotropin stimulating hormone; CS, Cushing’s syndrome; CT, computed tomography; Dex, dexamethasone; DM, diabetes mellitus; DST, dexamethasone suppression test; GC, glucocorticoid; IPSS, inferior petrosal sinus sampling; MRI, magnetic resonance imaging; PCOS, polycystic ovary syndrome; UFC, urinary free cortisol.
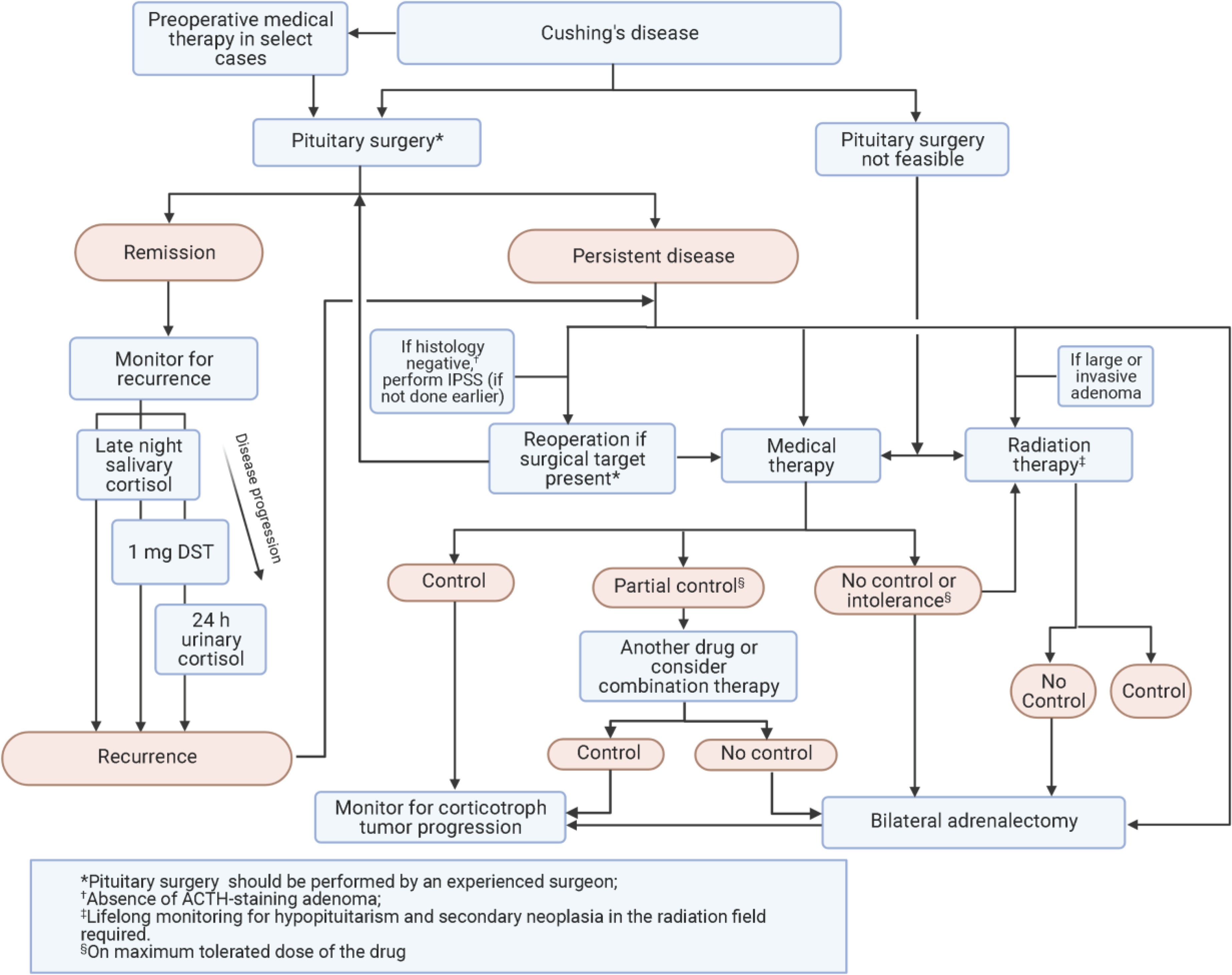
Figure 2. Algorithm for management of Cushing’s disease.
This algorithm details the management strategies following cushing diagnosis guidelines for Cushing’s disease.
Abbreviations: ACTH, adrenocorticotropin; DST, dexamethasone suppression test; IPSS, inferior petrosal sinus sampling.
Panel 3.
Future Research Topics Ranked of Highest Importance
| Screening and diagnosis of CS |
|---|
| • Optimize pituitary MR and PET imaging using improved data acquisition and processing to improve microadenoma detection |
| • Compare diagnostic algorithms for the differential diagnosis using invasive versus non-invasive strategies |
| • Identify additional corticotroph adenoma mutations and development of a comprehensive panel of genomic/proteomic tests for CD diagnosis |
| Complications of CD |
| • Define use of anticoagulant prophylaxis and therapy in different populations and settings |
| • Optimize the approach in managing long-term complications |
| Treatment of CD |
| • Determine clinical benefit of restoring the circadian rhythm, potentially with a higher nighttime medication dose |
| • Identify better markers of disease activity and control |
| • Develop new, better tolerated, more effective medical therapies |
| • Define populations that might benefit from preoperative medical treatment |
This panel outlines future research directions to refine cushing diagnosis guidelines and treatment approaches.
Abbreviations: CD, Cushing’s disease; CS, Cushing’s syndrome; MR, magnetic resonance; PET, positron emission tomography.
Recommendations presented here are intended for adult CD patients in clinical practice, always considering individual patient and disease characteristics for personalized care. Pediatric-specific considerations are also briefly addressed.
METHODS
Workshop co-chairs and steering committee members pinpointed 28 specific topics pertinent to CD diagnosis, complications, and treatment. Methodologies for literature review, pre-workshop lectures, and workshop discussions are detailed in the Appendix. A concise summary of the search strategy and selection criteria is provided below.
DIAGNOSIS OF CS: SCREENING, CONFIRMATORY, AND LOCALIZATION MODALITIES
Laboratory Tests (Table 1)
Background
Delays in CS diagnosis are common, often spanning years, partly due to limited awareness of the disease’s gradual progression and the complexities of diagnostic testing. Cushing diagnosis guidelines emphasize the importance of accurate and timely testing. Screening and diagnostic tests for CS evaluate cortisol secretion patterns, including disrupted circadian rhythm (late night salivary cortisol – LNSC), impaired glucocorticoid feedback (overnight 1-mg dexamethasone suppression test – DST or low dose 2-day dexamethasone test – LDDT), and elevated bioavailable cortisol (24-hour urinary free cortisol – UFC). In diagnostic settings, test sensitivities generally exceed 90%, with DST and LNSC showing the highest sensitivity, and UFC the lowest. Specificity varies, with LNSC being the most specific and DST and UFC the least.
LNSC
LNSC’s diagnostic value hinges on the principle that CS patients lose the typical circadian nadir of cortisol secretion. Cushing diagnosis guidelines often recommend at least two to three LNSC tests for accurate assessment. In mild CS cases, LNSC levels may be only marginally above the upper limit of normal (ULN). Collecting saliva at usual bedtime, rather than strictly at midnight, might reduce false positive results, as the cortisol nadir is closely linked to sleep onset. While mass spectrometry can detect both cortisol and cortisone, potentially mitigating contamination from topical hydrocortisone, it offers improved sensitivity but may compromise specificity compared to immunoassays. Serial, periodic LNSC measurements are particularly useful for monitoring cyclic CS, characterized by periods of normal cortisol secretion alternating with episodes of cortisol excess. Conversely, LNSC is unsuitable for individuals with disrupted day/night cycles, such as night-shift workers, as per cushing diagnosis guidelines.
Overnight 1-mg DST
In healthy individuals, a supraphysiologic dexamethasone dose effectively suppresses vasopressin and ACTH secretion, leading to reduced cortisol levels. Therefore, a serum cortisol level ≤1.8 μg/dL (50 nmol/L) following a 1-mg overnight DST is considered normal, as per cushing diagnosis guidelines. A negative DST result strongly suggests the absence of CS. Higher cortisol cutoffs, such as 5 μg/dL (138 nmol/L), may reduce DST sensitivity. Cortisol levels between 1.8–5 μg/dL may be indeterminate; in this range, values exceeding 5 μg/dL typically indicate dysregulated cortisol secretion from an incidentaloma alongside overt CS. False positives are possible due to rapid dexamethasone absorption/malabsorption (e.g., increased gut transit time, chronic diarrhea, celiac disease), concurrent use of CYP3A4 inducers (e.g., phenobarbital, carbamazepine, St. John’s wort), and elevated corticosteroid binding globulin (CBG) levels (e.g., oral estrogens, pregnancy, chronic active hepatitis), which can elevate total cortisol levels. Measuring dexamethasone levels alongside cortisol the morning after dexamethasone ingestion can improve test interpretation and reduce false-positive risks, aligning with cushing diagnosis guidelines. False negatives are less frequent, often stemming from inhibited dexamethasone metabolism by concomitant medications like fluoxetine, cimetidine, or diltiazem, resulting in higher biologically available dexamethasone doses. Reduced CBG and albumin levels, as seen in nephrotic syndrome, might also yield falsely low cortisol values.
UFC
Cushing diagnosis guidelines recommend at least two to three 24-hour urine collections to measure UFC, accounting for intra-patient variability. UFC’s advantage over DST lies in its independence from CBG alterations and dexamethasone compliance, reflecting overall cortisol production. However, despite averaging multiple collections, random variability can be as high as 50%. Similar to LNSC, UFC accuracy depends on proper patient collection technique.
Factors like sex, body mass index (BMI), age, very high or low urinary volume, and sodium intake can influence UFC levels and should be considered during interpretation, according to cushing diagnosis guidelines. Given the strong correlation between urine volume, glomerular filtration rate, and UFC, alternative screening tests like LNSC might be preferable for patients with renal impairment (CrCl < 30 mL/min or urine volume < 0.5 L/24 h).
Testing for non-neoplastic hypercortisolism (pseudo-CS)
Psychiatric disorders, alcohol use disorder, polycystic ovary syndrome, and obesity can activate the hypothalamic-pituitary-adrenal (HPA) axis, potentially mimicking CS. These conditions, termed pseudo-CS, can present with CS-like features common in the general population (e.g., weight gain), leading to biochemical screening. DST, LNSC, and UFC may yield positive (abnormal) results in pseudo-CS. Furthermore, co-administered medications could interfere with laboratory test results or exhibit steroid cross-reactivity. However, abnormal results in pseudo-CS tend to be mildly elevated, with UFC almost invariably remaining within 3-fold of normal. The combined LDDT-CRH (Dex-CRH) test, LDDT, or the desmopressin test may aid in differentiating ACTH-dependent CS from pseudo-CS, as per cushing diagnosis guidelines. The Dex-CRH test’s utility rests on the premise that only ACTH-dependent CS patients will show a cortisol response to CRH after dexamethasone suppression. However, test reliability can vary due to differing protocols, ovine or human CRH doses, cortisol and ACTH assay characteristics, and patient populations (e.g., hypercortisolism severity, adrenal vs. pituitary CS, underlying conditions). Desmopressin test utility is based on the finding that ACTH-secreting adenomas express vasopressin V1b (V3) receptors, triggering ACTH release upon desmopressin injection. The desmopressin test exhibits high specificity for CD, is less complex and costly than Dex-CRH, and both have demonstrated good diagnostic performance in distinguishing CS from pseudo-CS in some studies. Excellent agreement has been observed when both tests are performed.
Clinical Considerations and Recommendations
Screening and confirmatory testing for CS
Cushing diagnosis guidelines acknowledge the absence of a single, universally preferred diagnostic test for CS, nor is there complete consensus on when and how to test, despite efforts to develop diagnostic scoring systems. Clinical judgment and a high index of suspicion for CS are paramount, underscoring the need to personalize decisions regarding diagnostic testing timing and selection based on the clinical context.
When CS is suspected, any of the diagnostic tests (DST, UFC, and/or LNSC) can be valuable. Cushing diagnosis guidelines recommend initiating testing with DST, UFC, and/or LNSC, depending on local availability, with multiple LNSC tests potentially being more convenient for patients. If an adrenal tumor is suspected, DST is recommended as the initial test, and LNSC should only be used if cortisone levels can also be reported.
DST may be favored for shift workers and individuals with disrupted circadian rhythms due to irregular sleep patterns, but it may be unreliable in women using oral estrogen. Measuring dexamethasone levels alongside cortisol can be helpful if a false-positive DST is suspected based on the clinical scenario, as per cushing diagnosis guidelines. If UFC is used, two to three collections should be obtained to assess variability. For LNSC, at least two to three tests are recommended. Despite initial concerns about SARS-CoV-2 infection risk with LNSC, it remains safe for lab personnel when proper precautions are followed. Bilateral inferior petrosal sinus sampling (IPSS) should not be used for primary hypercortisolism diagnosis, as central-to-peripheral ACTH gradients in healthy controls and pseudo-CS overlap with those in CD patients, according to cushing diagnosis guidelines. In classic cyclic CD or patients with unpredictable cortisol fluctuations, dynamic and localization testing, including IPSS, should be preceded by confirmatory LNSC, DST, or UFC to document the active phase of hypercortisolism.
Current cushing diagnosis guidelines express no preference for mass spectrometry over immunoassay for cortisol measurement in initial diagnosis, ensuring mild hypercortisolism cases are not missed. However, normative data using contemporary assays are needed.
Ruling out pseudo-CS
Due to the diverse etiologies of pseudo-CS, a singular approach to rule it out is lacking. Cushing diagnosis guidelines recommend considering the patient’s clinical history, particularly symptom duration, and repeating tests to avoid inappropriate treatment if CS is not present. In most pseudo-CS cases, hypercortisolism is mild, and monitoring for 3–6 months to assess symptom resolution is reasonable; treating the underlying condition (e.g., depression) can restore normal HPA axis function and cortisol levels. Standard diagnostic testing is unreliable in this population. LDDT or serial LNSC over time may correlate better with the clinical picture. Desmopressin testing is simple and outpatient-friendly. Dex-CRH can be valuable in this context, but published diagnostic accuracy varies; use at expert centers with dexamethasone level measurement is advised, as are cortisol cutoff adjustments in severely obese patients. Ovine CRH is currently unavailable in the United States, Canada, Brazil, Argentina, Mexico, and some other countries.
Imaging and Tumor Localization
Background
MRI is the preferred imaging modality for detecting ACTH-secreting pituitary adenomas. However, given the small size of most lesions, standard 1.5T MRI detects only about 50% of microadenomas. Cushing diagnosis guidelines acknowledge the limitations of standard imaging.
Technical advancements like spoiled gradient–recalled (SPGR) acquisition echo with 1 mm slice intervals, fluid attenuation inversion recovery (FLAIR), and constructive interference in the steady state (CISS) may enhance detection. Variants of T1-weighted turbo spin echo (TSE) sequences and ultra-high field 3T and 7T magnets can improve microadenoma localization. Nevertheless, roughly one-third of scans in CD patients remain negative, and higher resolution with 3T or 7T magnets might increase the detection of incidentalomas unrelated to CD.
Importantly, tumor size does not consistently correlate with hypercortisolism severity in CD. Larger adenomas can sometimes present with milder hypercortisolism. Cushing diagnosis guidelines emphasize clinical-biochemical correlation alongside imaging.
Positron emission tomography (PET) has been explored as an adjunct to or alternative to MRI for corticotroph adenoma localization. 18F-fluoro-deoxy-glucose (18F-FDG) PET/CT shows comparable detection rates to standard fast spin echo MRI in some studies, while others find both inferior to SPGR MRI. Ovine CRH stimulation prior to 18F-FDG PET can enhance uptake and improve detection. PET coregistration with volumetric MRI (PET/MRCR) integrates functional and anatomical imaging, and 11C-methionine may offer more precise radiotracer uptake localization. In one series, this technique accurately localized corticotroph adenomas in de novo disease and persistent/recurrent hypercortisolism post-surgery, often in patients with negative or equivocal standard spin echo MRI. However, this approach is not universally available or approved. Alternative strategies, such as targeting CRH-R1 expression on corticotroph tumors, are under investigation.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines continue to recommend MRI as the primary imaging modality for ACTH-secreting pituitary adenomas. 3T MRI is suggested over 1.5T when available. 7T MRI is not widely accessible, and routine re-imaging on 7T if 1.5T/3T MRI is negative is not currently justified.
Functional imaging is likely to become a superior approach to MRI alone. However, more data are needed to define the optimal use of different ligands in various clinical scenarios. While advanced imaging technologies are available in some centers of excellence, the benefit of referring all patients for imaging beyond 3T MRI remains uncertain.
Distinguishing Between CD and Ectopic ACTH-dependent CS
Background
In CD, glucocorticoid (GC) receptors typically retain the capacity to inhibit ACTH secretion at high dexamethasone doses. V2 and V1b (V3R), along with CRH receptors, are overexpressed. Conversely, most (but not all) ectopic ACTH-secreting tumors lack these receptors. Desmopressin and CRH stimulation tests are therefore useful in differentiating pituitary from ectopic tumors, a key step in cushing diagnosis guidelines. Increased plasma ACTH and cortisol following CRH or desmopressin administration usually indicate CD. Combining multiple dynamic tests might improve diagnostic accuracy. However, well-differentiated neuroendocrine tumors (NETs) can also express these receptors, leading to false-positive results. High-dose DST, despite limited overall accuracy, remains in use in some countries. No single diagnostic test achieves 100% specificity, and discordant results can occur in up to one-third of patients. Ectopic tumor type, patient age, sex, and hypercortisolism severity can all influence test outcomes.
IPSS, measuring ACTH in pituitary versus peripheral venous drainage, remains the gold standard for excluding ectopic ACTH production, according to cushing diagnosis guidelines. It should ideally be performed at specialized centers due to potential patient risks. A central-to-peripheral ACTH gradient ≥2:1 (or ≥3:1 after CRH stimulation) strongly suggests CD, while a gradient <2:1 (or <3:1 after CRH) indicates ectopic ACTH source.
A non-invasive approach combining three or four tests—CRH and desmopressin stimulation plus MRI, followed by whole-body CT if diagnosis is unclear—correctly diagnosed CD in approximately half of patients in one series, potentially obviating IPSS. Interestingly, a positive CT scan despite negative CRH/desmopressin stimulation and MRI had a 100% negative predictive value for CD. Currently, this combination of laboratory and imaging testing as a noninvasive strategy to distinguish pituitary from ectopic ACTH-secreting tumors is likely limited to specialized centers. Cushing diagnosis guidelines acknowledge the potential for non-invasive strategies in specialized settings.
68Ga-DOTATATE, a modified octreotide molecule linked to a radioactive 68Ga isotope, binds to somatostatin receptors with high affinity. It serves as a tracer in PET imaging of ectopic ACTH-secreting NETs. 68Ga-DOTATATE localizes about 65% of these tumors, including those missed or not definitively identified on cross-sectional imaging, offering sharper images and greater sensitivity for small tumors compared to single photon 111In-DTPA-pentetreotide. False positives can occur due to chronic inflammation, and a positive scan does not definitively confirm the NET as the ACTH source. However, 68Ga-DOTATATE imaging can guide clinical management.
The 68Ga isotope, typically derived from decaying 68Ge, faces supply exhaustion. Cyclotron-generated 68Ga locally, or 64Cu (with a longer 12.7-hour half-life and central production potential), may be used as alternative DOTATATE, DOTATOC, or DOTANOC conjugates. Future cushing diagnosis guidelines may incorporate these evolving imaging techniques.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines emphasize that no single laboratory test or test combination can definitively distinguish between pituitary and ectopic ACTH-secreting tumors. Clinical context and test results should guide management. When prompt brain MRI access is limited, neck-to-pelvis thin-slice CT scan is valuable if ectopic ACTH syndrome suspicion is high, such as in males with very high UFC and/or profound hypokalemia.
If MRI detects a pituitary tumor ≥10 mm and dynamic testing aligns with CD, IPSS is not essential for diagnosis. Clinical presentation should always be considered, as a pituitary lesion on MRI could be an incidental nonfunctioning adenoma or another sellar mass alongside an ectopic ACTH source. Some studies suggest this is true for lesions >6 mm, but not all expert centers use this lower cutoff. Consensus was reached that all patients with lesions <6 mm should undergo IPSS, and that IPSS should be considered for lesions between 6 and 10 mm, especially if dynamic tests are inconclusive or suggest ectopic CS.
A noninvasive alternative using high-dose DST and CRH stimulation test predicts CD if both are positive. However, if tests are discordant, IPSS is necessary. Emerging data suggest CRH/desmopressin testing with pituitary MRI, followed by whole-body CT scan, might be a reliable alternative if interpreted by an experienced multidisciplinary team. Cushing diagnosis guidelines may evolve to incorporate these non-invasive approaches, particularly in specialized centers.
COMPLICATIONS OF CD
CD management strategies must address comorbidities and complications that can compromise patient health and QoL. Comorbidities often require management concurrently with, or even preceding, CD-specific treatments to restore normal cortisol levels, according to cushing diagnosis guidelines. Clinical considerations and recommendations are summarized in Panel 1.
Hypercoagulability
Hypercoagulability in CS, paradoxically coupled with increased bleeding tendency due to skin atrophy and capillary fragility, elevates thromboembolic event (TE) risk. Cushing diagnosis guidelines highlight thromboembolic risk assessment and management. Most patients exhibit an activated coagulation cascade, including shortened activated partial thromboplastin time and increased fibrinogen, von Willebrand factor, and factor VIII, alongside impaired fibrinolysis mediated by elevated plasminogen activator inhibitor-1 and antiplasmin. Increased thrombin, thromboxane 2, and platelets, with compensatory increases in anticoagulation factors like protein C and S, have also been implicated.
Venous thromboembolic event (VTE) incidence in endogenous CS patients is over 10-fold higher than in nonfunctioning adenoma patients undergoing surgery, and 18-fold higher compared to the healthy population. VTE risk persists in the initial months post-CD surgery, indicating that hypercoagulability does not immediately reverse with cortisol normalization. At 30 days post-adrenalectomy, VTE risk was 3.4 to 4.75%, and the odds ratio for TE after bilateral adrenalectomy (BLA) in a longer-term study was 3.74 (95% CI: 1.69–8.27). Biochemical remission via short-term medical therapy (pasireotide ± cabergoline ± ketoconazole) also did not seem to reverse VTE risk or alter pro-anticoagulation factors in a series of 17 patients; pulmonary embolism occurred in two patients despite marked UFC reduction.
Retrospective data suggest thromboprophylaxis can reduce postoperative VTE incidence, especially when extended to 30 days. Surveys indicate growing awareness of thromboprophylaxis and increased anticoagulation use in clinical practice, but strategies to identify patients most likely to benefit are still under development. Cushing diagnosis guidelines recommend considering thromboprophylaxis in high-risk CD patients.
Cardiovascular Disease
CD patients exhibit an adverse cardiovascular disease risk profile that may persist even after successful treatment. Cushing diagnosis guidelines emphasize cardiovascular risk management as an integral part of CD care. Visceral, subcutaneous, and total fat may decrease post-remission, but most patients remain overweight or obese. Type 2 diabetes mellitus (T2DM) is present in up to 30% of patients, and dyslipidemia (low HDL, high LDL, high triglycerides) in 16–64% at diagnosis. T2DM often resolves after remission, but not always. Structural cardiovascular changes improve, including left ventricular hypertrophy, concentric remodeling, dilated cardiomyopathy, increased intima media thickness, and atherosclerotic plaque formation, alongside clinical manifestations like hypertension and heart failure, but may not fully resolve despite hypercortisolism remission.
Myocardial infarction, stroke, and other vascular events are major contributors to increased standardized mortality ratio (SMR; 4.1 to 16) in active/persistent CD. Most studies show these rates do not fully normalize but are lowered upon remission. Patients in remission after single pituitary surgery had normal SMR at 10 years in one study. Cardiovascular risk factor screening and assessment before and after surgery is therefore essential, according to cushing diagnosis guidelines.
Bone Disease
Skeletal fragility is a frequent and early hypercortisolism complication, with fractures potentially being the initial clinical manifestation. Vertebral fractures occur in 30–50% of patients, largely correlating with hypercortisolism severity. Cushing diagnosis guidelines include bone health assessment and management. Suppression of the growth hormone (GH)/insulin-like growth factor (IGF)-I and hypothalamic-pituitary-gonadal axes, along with altered parathyroid hormone pulsatility, lead to decreased osteoblast number and function, evidenced by reduced serum levels of bone formation markers like osteocalcin and alkaline phosphatase. Dual X-ray absorptiometry (DXA) of the lumbar spine may show low bone mineral density (BMD), but fractures can occur even with BMD in the normal or osteopenic range. Although BMD increases are reported after hypercortisolism resolution, some patients retain high fracture risk, with men at higher risk than women. Conventional osteoporosis treatments, e.g., bisphosphonates, and supportive vitamin D and calcium, may accelerate BMD improvement compared to cortisol normalization alone, and could benefit patients with persistent postsurgical hypercortisolism to prevent further bone loss. Data on specific bone treatments for osteopenic patients in remission post-CD treatment are lacking.
Growth Hormone Deficiency
GCs, both endogenous and exogenous, inhibit GH secretion, reducing hepatic IGF-I production in CS patients. Cushing diagnosis guidelines recommend considering GH deficiency assessment in CD patients. Although GH production can fully recover in most patients post-successful therapy and HPA axis recovery, even years after remission, persistent GH deficiency (GHD) can worsen hypercortisolism complications like bone loss, myopathy, and memory deficits. GHD prevalence in adults varies with diagnosis timing, ranging from 50–60% when tested within 2 years post-surgery to 8–13% when tested >2 years post-surgery, using insulin tolerance or glucagon stimulation tests. A 65% GHD rate was observed with the GHRH-arginine test after a median 3-year post-surgery remission time, while 36% of patients were diagnosed with GHD at 99 months post-radiotherapy remission. Prevalence using the macimorelin stimulation test is unknown. Notably, IGF-I is an insensitive screening test for adult GHD diagnosis.
Compared to other GHD etiologies, GHD in CS patients is more common in women and younger individuals; these patients generally exhibit higher rates of T2DM, hypertension, low bone mass, fractures, and poorer QoL. Myopathy may be partially GHD-related in remission patients. While preoperative IGF-I levels during active CS did not predict long-term myopathy risk, lower 6-month postoperative IGF-I levels strongly predicted more severe long-term muscle atrophy and weakness after CS remission.
GH replacement ameliorates metabolic syndrome complications and cardiovascular/cerebrovascular disease risk. Studies show reduced body weight, waist circumference, total and LDL-cholesterol, and improved QoL and BMD. Conversely, it may worsen glucose metabolism in pre-existing glucose intolerance. GH treatment has not yet been shown in randomized, prospective trials to reverse metabolic syndrome and cardiovascular or cerebrovascular complications. Cushing diagnosis guidelines suggest considering GH replacement in CD patients with confirmed GHD, weighing benefits and risks.
Other Complications
Increased infection risk, dysfunction of pituitary axes (e.g., central hypothyroidism), gonadal function impairment, infertility, and other complications can occur in CD patients. Physical and psychological morbidity commonly impact QoL, even post-successful treatment in some. Persistent features associated with prior hypercortisolism, including affective disorders, cognitive dysfunction, and negative illness perception, can have lasting effects on well-being. Proximal myopathy, with impaired stair climbing and standing up, is characteristic of CS myopathy, with multifactorial pathology including protein degradation via the forkhead box O3 (FOXO3) pathway, intramuscular fat accumulation, and inactivity-related muscle atrophy. Furthermore, hypercortisolism remission can exacerbate pre-existing autoimmune disorders. Cushing diagnosis guidelines recommend considering these broader complications in comprehensive patient management.
Recent guidelines and reviews have addressed these complications extensively, and were not specifically addressed at the Workshop.
INITIAL TREATMENT OF CD AND MONITORING FOR RECURRENCE
Pituitary Surgery
Background
Transsphenoidal surgery (TSS) is the recommended first-line therapy for CD patients, according to cushing diagnosis guidelines. Remission, typically defined as postoperative serum cortisol <1.8–2 μg/dL (50–55 nmol/L), is achieved in 60–80% of patients in experienced centers. Remission rates are higher (70–90%) in microadenomas and lower (50–60%) in macroadenomas. Patients in remission require GC replacement until HPA axis recovery. Delayed remission is possible, and monitoring until postoperative cortisol nadir usually identifies such cases. Occasional patients with mild hypercortisolism, cyclic CD, or pre-surgical medical treatment may achieve remission without marked postoperative hypocortisolism. Treatment at high-volume centers by experienced surgeons and tumor characteristics (MRI detection, noninvasiveness, size) are associated with improved outcomes. Whether endoscopic approaches offer incremental benefit over microscopic for macroadenomas remains unclear. Overall complication rates are low, with even lower rates in experienced hands. New-onset hypopituitarism occurs in about 10% of patients, alongside permanent diabetes insipidus (DI), cerebrospinal fluid (CSF) leak, and VTE in <5%.
Measuring surgical expertise in CD remains challenging. Hospitals limiting neurosurgeons performing TSS show better outcomes, fewer complications, shorter stays, and lower costs. Surgeons performing >200 TSS have the lowest complication rates. Regionalized neurosurgery teams of 4–5 experts per 2.5–5 million inhabitants could potentially optimize outcomes, reduce costs, and enhance overall care quality. Cushing diagnosis guidelines recommend considering surgical expertise and center volume in treatment planning.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines strongly recommend patients with CD undergo surgery at specialized Pituitary Tumor Centers of Excellence (PTCOE) whenever feasible. Surgery should be performed by an experienced pituitary neurosurgeon, with multidisciplinary follow-up including a pituitary endocrinologist. Pituitary surgery outcomes and cost-effectiveness should be reported and publicly accessible.
Monitoring for Recurrence (Table 1)
Background
Recurrence post-successful pituitary surgery is defined as the reappearance of clinical and biochemical hypercortisolism features after initial remission. Low or undetectable cortisol immediately postoperatively is a remission criterion, but does not guarantee absence of recurrence. Some patients with early remission and very low postoperative cortisol may later experience recurrence. Published recurrence rates range from 5% to 35%, with half occurring within 5 years of surgery and half after up to 10 years or more. Cushing diagnosis guidelines stress the need for lifelong recurrence monitoring.
Lifelong recurrence monitoring is essential. In patients who preoperatively responded to desmopressin, early postoperative loss of desmopressin response (with/without dexamethasone or CRH) may predict recurrence risk, but is not consistently used or recommended by most experts.
LNSC, 1-mg DST, UFC, and desmopressin tests have lower sensitivity for recurrence than for initial CS diagnosis, but specificity remains high (up to 95%+). LNSC can detect postoperative elevated cortisol earlier than 1-mg DST, while UFC is typically the last test to become abnormal in recurrence. LNSC may enable earlier intervention, but serial tests are advised due to result variability.
Recurrence evaluation should commence after HPA axis recovery, then annually or sooner if clinically suspected. However, clinical manifestations and biomarkers may be discordant. Early recurrence diagnosis poses the additional challenge of determining when and how to intervene. Cushing diagnosis guidelines recommend careful monitoring and individualized intervention strategies.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines recommend lifelong CD recurrence monitoring. Postoperative dynamic testing may predict recurrence, but its clinical utility remains to be established, as some patients with low predicted recurrence likelihood may recur many years later.
Among available tests, LNSC is most sensitive for recurrence detection and should be performed annually after postoperative HPA axis recovery. LNSC usually becomes abnormal before DST and UFC, but recurrence monitoring should also consider which tests were abnormal at initial diagnosis. If only minor biochemical abnormalities are present without clinical hypercortisolism features, close monitoring with repeat testing and comorbidity management, rather than immediate treatment of the underlying disorder, can be considered.
Repeat Pituitary Surgery
Background
Repeat TSS can be considered for patients with biochemical recurrent CD and visible tumor on MRI. In select expert centers reporting successful reoperation despite absent adenoma on MRI, ACTH-staining adenoma on pathology or central ACTH gradient on IPSS at initial operation was often present. Cushing diagnosis guidelines consider repeat surgery in select recurrence cases.
Tumor factors (size, extrasellar extension) should be considered for reoperation eligibility, and neurosurgeon experience likely impacts outcomes. Remission rates after reoperation vary widely (37–88%), partly due to differing remission criteria and follow-up duration. While some report higher surgical (CSF leak, meningitis) and endocrinological (DI, hypopituitarism) complication rates with repeat versus initial surgery, significant pituitary function deterioration or serious morbidity is less likely in experienced hands.
Clinical Considerations and Recommendations
If no surgical contraindications exist, cushing diagnosis guidelines suggest repeat TSS for patients with biochemical recurrent CD if tumor is evident on MRI, particularly if the initial surgery was not performed at a PTCOE. If MRI is negative for tumor, reoperation may be appropriate if an experienced surgeon at a high-volume center deems it feasible and positive pathology or central gradient on IPSS was seen during the initial operation.
MEDICAL THERAPY FOR CD
Drugs for CD treatment target adrenal steroidogenesis, pituitary somatostatin and dopamine receptors, and GC receptors. Cushing diagnosis guidelines incorporate medical therapies for persistent/recurrent CD, patients unsuitable for or refusing surgery, and cortisol control during radiotherapy (RT). Available medications and investigational drugs with phase 3 trial results are summarized in Table 2.
Medical Therapy: Targeting Adrenal Steroidogenesis
Background
Adrenal steroidogenesis inhibitors (ketoconazole, metyrapone, mitotane, etomidate, and the recently approved osilodrostat) block adrenal enzymes, reducing GC and/or adrenal androgen synthesis/secretion. They effectively control cortisol excess but do not directly target the pituitary ACTH-secreting adenoma or restore HPA axis circadian rhythm. Cushing diagnosis guidelines include adrenal steroidogenesis inhibitors as a medical therapy option.
Dose titration to achieve cortisol normalization carries adrenal insufficiency (AI) risk from overtreatment. Block-and-replace regimens pose inappropriate GC over-replacement risk if blockade is incomplete. Adverse events (AEs) can relate to ACTH increase in CD patients and adrenal hormone buildup proximal to the blockade, with mineralocorticoid or androgenic activity. Drug-drug interaction potential is crucial in treatment selection and use.
Ketoconazole
Ketoconazole blocks multiple adrenal enzymes early in the steroid biosynthetic pathway, avoiding excess androgen and mineralocorticoid precursors. However, it can also reduce gonadal steroid synthesis; men may experience hypogonadism and gynecomastia, limiting prolonged use. A review of 310 CS patients treated with ketoconazole showed UFC normalization in 64.3%, but up to 23% of initial responders lost biochemical control (“escape”). Similar data from a large retrospective study of 200 CD patients showed 64.7% UFC normalization in 51 patients treated >24 months, but 15.4% escape. Clinical CS feature improvements (weight, blood pressure, glucose metabolism, muscle weakness) have been observed. Cushing diagnosis guidelines acknowledge ketoconazole’s efficacy but also limitations.
Hepatotoxicity (10–20%) is mostly asymptomatic, with mild/moderate liver enzyme increases (≤5 × ULN), typically appearing within 6 months of treatment and resolving 2–12 weeks after dose reduction/discontinuation. Serious hepatotoxicity has been reported, leading the FDA to issue a black-box warning and recommend weekly liver function test (LFT) monitoring for fungal infection treatment with ketoconazole. Ketoconazole use for CS is off-label in the US. Gastrointestinal disturbances and AI (5–20%), and skin rash (≈5%) are also common. Numerous drug-drug interactions exist; careful medication review for problematic interactions is vital.
Metyrapone
Metyrapone, an 11β-hydroxylase inhibitor, normalized UFC in 71% of 120 CS patients (5 studies), with up to 18% escape after initial response. A subsequent multicenter retrospective study of 164 CS patients reported 43% biochemical control with ≈8 months monotherapy. An observational study of 31 CS patients (20 CD) showed rapid UFC and LNSC reduction after 1 month (−67% and −57% from baseline), with sustained normalization in 70% and 37% at last visit, respectively. Three patients lost control at 9 months despite normal UFC at 6 months, and 2 also showed normal LNSC. 11-deoxycortisol may clinically cross-react with cortisol in blood and urine immunoassays. A recent prospective study of 50 CS patients showed 47% UFC normalization at 12 weeks; AI was reported in 12%. Cushing diagnosis guidelines consider metyrapone a valuable option, but with specific considerations.
Metyrapone typically improves clinical CS features (66% in the prospective study), such as blood pressure, glucose metabolism, psychiatric disturbances, and muscle weakness.
Hirsutism, dizziness, arthralgia, fatigue, hypokalemia, and nausea are common metyrapone AEs; AI, abdominal pain, and atopic dermatitis are less frequent. Hyperandrogenism-related AEs can limit prolonged use, especially in females.
Osilodrostat
Proof-of-concept and phase 2 studies showed osilodrostat, an 11β-hydroxylase and aldosterone synthase inhibitor, effectively reduces cortisol and is well-tolerated. A phase 3 randomized withdrawal study enrolled 137 CD patients. After 12 weeks of open-label dose titration and 12 weeks of dose optimization, 72 patients (53%) maintained normal UFC and were randomized. At week 34 (randomized treatment end), 86% randomized to osilodrostat maintained normal UFC versus 29% on placebo (OR 13.7 [95% CI: 3.7, 53.4]; p<0.001). Cushing diagnosis guidelines now include osilodrostat as an effective medical therapy.
Osilodrostat treatment also yielded clinical improvements. By week 48, patients showed significant reductions in body weight, blood pressure, total and LDL cholesterol, and decreased fasting serum glucose and HbA1c. QoL and depression scores also improved.
Nausea, anemia, and headache were reported in 8–11% of patients. Hypocortisolism-related AEs were reported in about half of patients, mostly during open-label dose titration, generally manageable with dose reductions/interruptions, although GC replacement was needed in 25 of 70 (36%) patients with hypocortisolism-related AEs. Additionally, 42% of treated patients in the phase 3 study showed effects from increased adrenal steroid precursors, including hypokalemia and hypertension; 11% of women reported hirsutism. Another large phase 3 study showed a significantly greater proportion of osilodrostat recipients (77.1%) achieved mean UFC ≤ ULN after 12 weeks versus placebo (8.0%), with clinical feature, cardiovascular marker, and QoL improvements. Hypocortisolism-related AEs occurred in 27.4% of patients, fewer than in the prior study.
Mitotane
Mitotane inhibits steroidogenic enzymes and has long-lasting adrenolytic action in steroid-secreting adrenocortical cells. It suppresses hypercortisolism in 80% of cases, but with slow onset and variable bioavailability. CYP3A4-mediated rapid cortisol inactivation requires 2- to 3-fold increased GC replacement dose for AI treatment or block-and-replace strategies. It is rarely used for CD. Most participants considered mitotane use should be limited to adrenal carcinoma patients. Cushing diagnosis guidelines generally do not favor mitotane for CD.
Etomidate
Etomidate, originally an anesthetic, rapidly normalizes cortisol, used for acute severe hypercortisolism control in hospitalized patients. Low-dose etomidate (0.04–0.05 mg/kg/h) provides partial blockade; high-dose (0.5–1 mg/kg/h) complete blockade, with IV hydrocortisone to avoid etomidate-induced AI. Very low doses (0.025 mg/kg/h) may be used in hospitalized non-ICU patients, depending on local practice. Cushing diagnosis guidelines recognize etomidate for acute, severe hypercortisolism.
Compared to lipid formulation, propylene glycol preparation is more associated with thrombophlebitis and pain on injection, and also with additional AEs like hemolysis, renal tubular injury, and lactic acidosis at high doses.
Medical Therapy: Targeting Pituitary Somatostatin and Dopamine Receptors
Background
Dopamine agonist cabergoline and somatostatin receptor ligand pasireotide are used in CD patients with persistent/recurrent hypercortisolism, although only pasireotide is approved for this indication. Tumor effect is clinically important for large residual tumors, corticotroph tumor progression, or Nelson’s syndrome. Cushing diagnosis guidelines include these agents for specific CD scenarios.
Pasireotide
In a phase 3 study of 162 CD patients treated with SC pasireotide, UFC normalized at month 6 in 15–26% without dose increases. Higher normalization rates were seen in those with baseline UFC ≥2 × ULN. Significant clinical improvement was noted in most patients. Cushing diagnosis guidelines acknowledge pasireotide’s role, particularly in certain CD subtypes.
A second phase 3 study treated 150 CD patients with 10 or 30 mg monthly IM pasireotide LAR. At month 7, 40% in both groups showed normalized UFC, regardless of dose titration, with higher response in those with baseline UFC ≥1.5 × ULN. At month 12, blood pressure improvements were greater in UFC-normalized patients; BMI, weight, waist circumference, and QoL improved regardless of UFC control. Long-term extension studies showed sustained biochemical and clinical improvements for up to five years in select patients. Limited real-world data exist on long-term treatment compliance, and discontinuation rates are high in several studies. Pasireotide LAR also decreased median tumor volume by 17.8% (10 mg) and 16.3% (30 mg), with 43% and 47% of patients showing ≥20% reduction, respectively.
A separate longitudinal study in CD patients with Nelson’s syndrome post-BLA showed pasireotide LAR rapidly suppressed ACTH levels, with sustained reductions over 24 weeks.
Between one- and two-thirds of CD tumors harbor a USP8 mutation, potentially predicting treatment response as these tumors may express higher SST5 levels, for which pasireotide has high affinity. USP8 mutational status may become a useful predictor of pasireotide response in future cushing diagnosis guidelines.
Hyperglycemia risk is high with pasireotide. Phase 3 studies reported hyperglycemia-related AEs in ≈70% of patients, with new antidiabetic medication or dose adjustments in ≈50%. High hyperglycemia rates are thought to result from insulin and incretin secretion inhibition combined with lesser glucagon inhibition. Management with GLP-1 receptor agonists or DDP-4 inhibitors is considered helpful.
Cabergoline
CD data on cabergoline are mainly from small retrospective studies, showing biochemical normalization in 25–40% of patients, with 20–40% loss of control in initially normalized patients. Cushing diagnosis guidelines consider cabergoline as a potential option, but with lower efficacy rates.
A retrospective multicenter cohort study of 53 patients treated with cabergoline (median 2.3 mg/wk) yielded normal UFC in 40% during the first year, but only 23% sustained normalization after median 32.5 months follow-up. Lower control rates may be due to under-titration; a smaller study of 20 patients on cabergoline titrated to max 7 mg/wk (median 3.5 mg/wk) showed 40% UFC normalization at 24 months. Weight, glycemic control, and hypertension improved in 25–40% of complete responders, and tumor shrinkage was reported in 50%. Nelson’s syndrome patients may also respond to cabergoline, with ACTH normalization and tumor shrinkage reported. Although off-label for CD, cabergoline has been used in pregnant patients with prolactinomas and other pituitary adenomas, including CD.
Cabergoline-induced impulse-control disorder is likely under-reported, manifesting as hypersexuality, pathological gambling, excessive alcohol consumption, overeating, and uncontrolled shopping. This behavior can occur within months of cabergoline initiation or later, improving/resolving upon discontinuation. Cushing diagnosis guidelines highlight the risk of impulse control disorders with cabergoline.
High cumulative doses of ergotamine-derived dopamine agonists in Parkinson’s disease patients were linked to cardiac valve regurgitation risk. While one prolactinoma study found moderate tricuspid regurgitation more frequent with higher doses, a large multicenter study found no association between cumulative cabergoline dose and age-corrected prevalence of valvular abnormalities. A meta-analysis suggests the clinical significance of such echocardiographic findings remains uncertain.
Medical Therapy: Targeting the Peripheral Tissue Glucocorticoid Receptor
Mifepristone
Glucocorticoid receptor blocker mifepristone effectively controls some hypercortisolism effects regardless of etiology. Cushing diagnosis guidelines include mifepristone for specific hypercortisolism management aspects.
An open-label study of 50 endogenous CS patients (43 CD) showed that after 24 weeks of mifepristone, 60% with T2DM or impaired glucose tolerance had ≥25% glucose AUC reduction during OGTT, and 38% with hypertension had ≥5 mm Hg diastolic blood pressure reduction. Insulin resistance, weight, waist circumference, and QoL also improved.
Twelve patients showed increased blood pressure, including 9 with hypokalemia requiring spironolactone, consistent with mineralocorticoid receptor activation. Endometrial hypertrophy and irregular menstrual bleeding were also reported, consistent with mifepristone’s anti-progesterone activity. Dexamethasone was given to 7 patients with AI signs/symptoms, highlighting the need for careful monitoring. Importantly, cortisol levels remain high, and typical low cortisol measures for AI confirmation with other medical therapies are not applicable with mifepristone. Clinical features are the sole guide for AI assessment. Cushing diagnosis guidelines emphasize the unique monitoring challenges with mifepristone.
Continued mifepristone treatment in 27 CD patients in a long-term extension study showed sustained ≥2-fold ACTH elevations, but tumor volume progression (in 3 macroadenoma patients up to 25 months from baseline) did not correlate with ACTH increases. Thyroid function should be closely monitored, and thyroid hormone replacement adjusted as needed. All concomitant medications should be carefully reviewed due to mifepristone’s drug-drug interaction potential.
Medical Therapy: Clinical Considerations and Recommendations
Cushing diagnosis guidelines recommend individualizing medical therapy for all CD patients based on clinical scenario, including hypercortisolism severity. Regulatory approvals, treatment availability, and drug costs vary by country, influencing treatment selection. Balancing treatment cost with ineffective/insufficient treatment’s adverse consequences is important. In severe disease, aggressive treatment to normalize cortisol (or cortisol action with mifepristone) is paramount. Serial UFC and LNSC tests monitor treatment outcomes.
Workshop discussions on integrating treatment options are summarized below and in Panel 2.
Initial treatment selection for medical therapy
Adrenal steroidogenesis inhibitors are usually the first-line choice due to reliable effectiveness. For mild disease without visible MRI tumor, ketoconazole, osilodrostat, or metyrapone are typically preferred. Cabergoline may also be used for mild CD; less effective and slower onset, but less frequent dosing. For mild-to-moderate disease with residual tumor, cabergoline or pasireotide may be preferred due to tumor shrinkage potential. However, pasireotide’s high hyperglycemia risk necessitates careful patient selection. Cushing diagnosis guidelines offer initial treatment selection guidance based on disease severity and patient factors.
For severe disease, rapid cortisol normalization is crucial. Osilodrostat and metyrapone typically elicit response within hours, ketoconazole within days. Etomidate is also rapid-acting and usable if hospitalized and unable to take oral medications. Severe hypercortisolism may require steroidogenesis inhibitor combinations. If very severe and unresponsive to optimized medical therapy (including combinations), BLA should be considered to avoid worsening outcomes.
Other patient factors influence initial treatment selection. Cabergoline should be avoided in patients with bipolar or impulse control disorders, but may be preferred for young women desiring pregnancy. Although no CD drugs are specifically approved for pregnancy, metyrapone may be considered with precautions in select pregnant women. In such cases, higher normal pregnancy cortisol levels require a higher cortisol target cutoff, such as 1.5 × ULN. Cushing diagnosis guidelines consider patient-specific factors in therapy choice.
Mifepristone improves key hypercortisolism clinical features, specifically hyperglycemia and weight gain. However, it can be challenging in standard clinical practice, often worsening hypokalemia. No reliable biochemical markers exist for cortisol level monitoring, increasing AI risk from overtreatment. Its long half-life necessitates several days of stress-dose GC replacement (preferably dexamethasone) if AI ensues. Because cortisol measurements are unhelpful for dosing or safety monitoring, mifepristone should only be used by clinicians with extensive CD experience; patient counseling about unreliable cortisol monitoring, especially for AI, is also important.
Limited rigorous data support specific combination therapy regimens, but several have been described. Many experts combine ketoconazole with metyrapone to maximize adrenal blockade when monotherapy is ineffective or to allow lower doses of both drugs. A steroidogenesis inhibitor plus tumor-targeting agent (e.g., ketoconazole plus cabergoline) is also rational, especially with visible tumor. Other combinations include triplets of cabergoline, pasireotide, plus ketoconazole, and metyrapone, ketoconazole, plus mitotane. Combination therapy AE potentiation risk, such as QTc prolongation, should also be considered, as per cushing diagnosis guidelines.
Selecting an adrenal steroidogenesis inhibitor
Ketoconazole and metyrapone have the longest clinical experience as adrenal steroidogenesis inhibitors. They are approved for CD in Europe, but not the US (where only osilodrostat is approved), and may be unavailable in some countries. Ketoconazole is favored for dose titration ease, but often under-dosed due to hepatotoxicity concerns. LFTs should be regularly monitored, but treatment need not be stopped if LFTs are mildly elevated yet stable. Osilodrostat and metyrapone can rapidly control cortisol in most patients. They are not limited by LFT monitoring, and hypogonadism does not occur in men. Osilodrostat is expected to see increased use as availability expands, given its high efficacy and twice-daily dosing. AI and osilodrostat effects on androgens require monitoring, but whether patient sex should influence long-term treatment selection is unknown. Mitotane, rarely used for CD, has slower onset. Cushing diagnosis guidelines offer considerations for selecting specific adrenal steroidogenesis inhibitors.
Block-and-replace regimens may be considered for severe disease, cyclic CS, and surgery-ineligible patients. This may be useful if monitoring visits are infrequent due to external factors (e.g., pandemic, transportation issues). Caution is needed to avoid GC over-replacement and iatrogenic CS.
Monitoring response to medical therapy
Regular monitoring of treatment efficacy is required for all patients, including cortisol measures (except with mifepristone) and patient symptoms/comorbidities, especially weight, glycemia, and blood pressure. QoL, preferably via patient-reported outcomes, is also important. Cortisol levels are often measured by UFC; notably, UFC is unhelpful for AI diagnosis. Morning cortisol and/or LNSC can be alternatives, but circadian rhythm loss makes targeting diurnal secretion alone questionable. Nevertheless, morning cortisol values may be relevant in patients taking higher medication doses in the evening versus morning. Patients normalizing both UFC and LNSC with pasireotide LAR showed better clinical outcomes than those normalizing UFC alone. Higher bedtime treatment doses for twice-daily medications may help restore circadian rhythm, but rigorous evidence is lacking. Cushing diagnosis guidelines emphasize comprehensive monitoring strategies.
Direct comparison of treatment outcomes (efficacy and AEs) is difficult due to varying study designs, medication up-titration schemes, comparator arms, inclusion/exclusion criteria, and primary endpoints. Furthermore, some drugs lack prospective CS studies. Using UFC normalization as a target, osilodrostat shows highest efficacy in prospective trials, followed by metyrapone (retrospective and prospective data), ketoconazole (retrospective), pasireotide (prospective), and cabergoline (retrospective and prospective). Mifepristone efficacy markers are clinical CS feature and diabetes improvements, hindering direct biochemical efficacy comparison with other treatments.
Treatment change should be considered if cortisol remains elevated after 2–3 months on maximum tolerated doses. If cortisol is reduced but not normalized, and/or clinical improvement occurs, combination therapy can be considered. Clear treatment resistance warrants switching to a different therapy. However, ensuring insufficient disease control is not due to under-dosing, rather than true resistance, is crucial. Cushing diagnosis guidelines provide guidance on adjusting medical therapy based on monitoring results.
Adrenal-targeting agents may raise tumor growth concerns due to ACTH-cortisol feedback interruption. However, differentiating tumor progression due to feedback loss from aggressive recurrent disease is challenging. ACTH level monitoring is suggested; significant elevations may indicate new tumor growth and MRI need, with caveats of ACTH’s short half-life and fluctuating levels not necessarily reflecting tumor growth. Progressive tumor size increase warrants treatment suspension and management reassessment. MRI is typically done 6–12 months post-treatment initiation, repeated every few years based on clinical scenario.
Combination therapies necessitate monitoring for potential overlapping toxicities, particularly QTc prolongation, and drug-drug interactions, as per cushing diagnosis guidelines.
Primary and Preoperative Medical Therapy for De Novo CD
Primary medical therapy is used when successful adenoma resection is unlikely due to unfavorable localization, significant invasiveness, or absent MRI visualization. Recent double-blind randomized phase 3 trials evaluating novel drugs included few de novo CD patients (0–28%). Further studies are needed to demonstrate different medical therapies’ utility in this setting, as monotherapy or combination, considering potential treatment effects on adenoma size. Cushing diagnosis guidelines acknowledge the limited data on primary medical therapy.
Published evidence on preoperative medical therapy in CD is sparse, and it is not routinely used, although regional variations exist. A meta-analysis showed no cortisol normalization rate differences between preoperative versus later adjuvant cortisol-lowering medications. Preoperative therapy may be an option in severely ill patients contraindicated for surgery or with long surgical wait times, or in patients with life-threatening hypercortisolism complications requiring rapid cortisol control. Physician surveys show preoperative therapy (mostly ketoconazole and/or metyrapone) use in up to 20% of CD patients, especially those with more severe clinical features or nonvisible adenoma. Cushing diagnosis guidelines suggest considering preoperative therapy in specific high-risk scenarios.
Retrospective studies show preoperative steroidogenesis inhibitor therapy for ≈4 months yields 50–72% cortisol normalization rates, although subjective symptom improvement was only in ≈one-third of cases. Lower postoperative hypoadrenalism rates from preoperative medical therapy could theoretically protect against proinflammatory and procoagulant states, but postsurgical complications, including VTE, are similar regardless of preoperative therapy use. If the HPA axis recovers during preoperative treatment, postoperative AI may not occur, potentially complicating remission determination.
Preoperative cabergoline likely has limited value; significant cortisol reduction was seen in only ≈one-fourth of patients in a prospective 6-week cohort.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines indicate no rigorous data supporting primary or preoperative medical therapy. Most experts would consider adrenal steroidogenesis inhibitors if surgery is delayed (scheduling or external factors like pandemics).
Patients with severe CD and life-threatening metabolic, psychiatric, infectious, or cardiovascular/thromboembolic complications may benefit from preoperative medical therapy in select cases. Although not definitively confirmed, some experts believe it may favorably affect glucose, cardiovascular, and coagulation parameters. Few use it to reduce postoperative cortisol withdrawal manifestations.
Monitoring and follow-up of preoperative therapy patients can be challenging, as postoperative cortisol assessments for surgical cure are unreliable. Patient perspectives on this approach would be valuable in future research.
RADIATION THERAPY
Background
RT is primarily used as adjuvant therapy for persistent or recurrent disease post-TSS, or for aggressive tumor growth, according to cushing diagnosis guidelines. Conventional external-beam RT (typically 45–50 Gy in fractions) achieves biochemical remission in ≈two-thirds of patients years post-treatment. More recent series with SRS, including whole sellar RT, show higher biochemical remission rates. A multicenter GammaKnife SRS study in 278 subjects followed for mean 5.6 years reported 80% biochemical control and 57% durable hypercortisolism control. Tumor control rates are typically higher, with ≈95% of SRS-treated patients showing decreased or stable tumor volume on MRI. A small proton beam RT study showed complete response (cortisol or ACTH normalization) in persistent corticotroph adenoma patients (CD or Nelson’s syndrome), with low morbidity after median 62 months follow-up.
SRS may also be primary therapy for patients with high surgical risk or refusing surgery. In this setting, endocrine remission was achieved in 81% of 46 patients at 5 years follow-up. Long-term follow-up is needed, as recurrence and tumor growth have been reported post-RT. Cushing diagnosis guidelines consider RT, especially SRS, for persistent/recurrent CD and inoperable cases.
Given the latency to post-RT remission, adjuvant medical therapy is needed to control hypercortisolism; periodic withdrawal allows cortisol secretion evaluation to assess treatment effect. Whether ketoconazole or cabergoline treatment at SRS time limits efficacy is debated, but they are often temporarily withheld at RT time.
Hypopituitarism is the most common side effect of conventional RT and SRS, seen in 25–50% of patients, generally increasing over time. Secondary malignancy, cranial nerve damage, and stroke risk are low with SRS. In SRS-treated patients, a ≥3–5 mm distance between tumor and optic chiasm and chiasm dose <10–12 Gy are recommended to minimize optic neuropathy risk. Longer-term data will clarify whether different SRS modalities (GammaKnife, LINAC, proton beam) confer lower stroke and hypopituitarism rates compared to conventional RT.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines recommend RT most commonly for persistent hypercortisolism after incomplete corticotroph tumor resection, particularly if the tumor is aggressive or invasive and/or unresectable. SRS is likely more convenient due to fewer treatment sessions, but avoiding optic chiasm exposure is critical. Lifelong monitoring for pituitary hormone deficiencies and recurrence is required in all RT patients. Imaging for secondary neoplasia in the radiation field should also be considered.
ADRENALECTOMY
Background
BLA offers immediate cortisol excess control in persistent/recurrent CD patients unresponsive to medical therapy, but is considered for select patients due to resultant AI and lifelong GC and mineralocorticoid replacement, according to cushing diagnosis guidelines. Laparoscopic BLA (transperitoneal or posterior retroperitoneal approach) has a 10–18% complication rate in the largest series, and mortality <1%. Long-term clinical hypercortisolism relapse due to adrenal rest stimulation by high ACTH is uncommon (<5%).
Corticotroph tumor progression post-BLA is a long-term concern in 25–40% of patients after 5–10 years. Most cases can be managed with surgery, RT, or medical therapy. However, aggressive tumors may continue to grow, necessitating long-term monitoring. A recent European consensus focused on managing these patients.
Corticotroph tumor progression post-BLA does not appear to be influenced by pregnancy. This may make BLA preferable in female patients with immediate pregnancy plans. However, BLA is rarely first-line treatment after initial pituitary surgery failure, and disease duration before adrenal surgery is typically 3–4+ years. Whether and how this impacts long-term outcomes is unknown. Cushing diagnosis guidelines position BLA as a later-line therapy option.
Clinical Considerations and Recommendations
Cushing diagnosis guidelines often consider BLA a last resort in most centers after all other options fail. However, BLA may be warranted earlier in severe hypercortisolism cases needing rapid, definitive cortisol reduction to avoid prolonged systemic disease effects. Many expert centers recommend BLA earlier for CD females desiring pregnancy.
Post-BLA, plasma ACTH and serial pituitary imaging are used for monitoring at intervals dictated by clinical scenario, usually starting 6 months post-surgery. More frequent evaluation may be needed if corticotroph tumor progression is clinically suspected.
ADDITIONAL CONSIDERATIONS
Genetics of CD
Corticotroph adenomas are predominantly sporadic, arising from monoclonal expansion of a single mutated cell. They abundantly express EGFR, signaling ACTH production. Somatic activating driver mutations in USP8 are present in 36–60% of corticotroph adenomas, leading to persistent EGFR overexpression and perpetuated ACTH hypersynthesis. Rarely, mutations in glucocorticoid receptor NR3C1, oncogene BRAF, deubiquitinase USP48, and TP53 are found. Familial tumor syndromes (MEN1, FIPA, DICER1) rarely lead to corticotroph adenomas. Corticotroph tumors may be sub-classified based on USP8 driver mutations and clinical behavior. USP8 mutational status may predict post-TSS recurrence. Genomic classifications may open new avenues for targeted, personalized treatments in future cushing diagnosis guidelines.
Diagnosis and Management of CS in Children
Endogenous CS is extremely rare before age 18. Germline mutations in MEN1, RET, AIP, PRKAR1A, CDKN1B, DICER1, SDHx, and CABLES1 may predispose children to CD, though screening is usually reserved for family history or other genetic syndrome indicators. Cushing diagnosis guidelines for children consider genetic predispositions.
Lack of height gain with weight gain is the most common CS presentation in children, making diagnosis somewhat easier than in post-pubertal adolescents or adults. Severe GHD prevalence in children (peak GH <7 μg/L) is 75% at CS diagnosis, and 25% in remission, using insulin tolerance or glucagon stimulation tests.
24-hour UFC, LNSC, or overnight 1 mg DST are used to confirm diagnosis. Diagnostic approaches and test performances differ slightly from adults, as recently reviewed. Dex-CRH test is unhelpful in children. In children >age 6, CD is the most common CS cause, while adrenal causes are more common in younger children. Algorithms exist for distinguishing ACTH-dependent from ACTH-independent CS. IPSS role in children is more limited than in adults, according to cushing diagnosis guidelines for pediatric CD.
As in adults, surgical resection of the ACTH-secreting tumor is first-line treatment. However, thromboprophylaxis should not be routinely used in children due to bleeding risk, reserved for selected pediatric patients. Adrenal function typically recovers within ≈12 months post-successful treatment. GHD evaluation should be done by 3–6 months postoperatively, with immediate GH replacement if needed for proper growth; GH replacement ensures adequate final height, but obesity is not fully reversible. For medical therapy, ketoconazole or metyrapone are typically used, with morning cortisol monitoring. Pasireotide is not recommended, and osilodrostat trials in children are underway. Block-and-replace regimens with metyrapone may also be considered. Cushing diagnosis guidelines for pediatric CD emphasize early diagnosis and expert multidisciplinary care.
Early diagnosis and expert management are critical given potential long-term adverse health outcomes from prolonged hypercortisolism and TSS/RT-associated morbidity. Children with CS should be referred to multidisciplinary centers of excellence with pediatric endocrinologists expert in pituitary disorders, and specialized neurosurgery units. Underlying genetic syndromes necessitate genetic counseling for the child and family, and investigation into other syndrome-associated disorders, as per cushing diagnosis guidelines.
Supplementary Material
1
NIHMS1752854-supplement-1.pdf (45.8KB, pdf)
Acknowledgments
The authors thank Professors Annamaria Colao, Richard Feelders, and A.J. van der Lely for their contributions to the meeting. The authors thank Shira Berman of Cedars-Sinai Medical Center for editorial assistance.
Declaration of Interests
MF has received grants to the institution from Novartis, Strongbridge, Novo Nordisk, Crinetics, Millendo, Ascendis, and Pfizer, and personal honoraria for consulting and advisory boards from Crinetics, HRA Pharma, Novartis, Recordati, Strongbridge, Sparrow, Ascendis, Novo Nordisk, and Pfizer, and has served on the Board for Pituitary Society.
RA has received grants to the institution from Strongbridge Biopharma, Novartis Pharmaceuticals, and Corcept Therapeutics, and personal honoraria for consulting and advisory boards from Strongbridge Biopharma, Recordati Rare Diseases, Corcept Therapeutics, and Novartis Pharmaceuticals.
IB has received grants and fees for consulting, advisory boards, and authorship to the institution from the National Institutes of Health, Strongbridge, Corcept, HRA Pharma, Sparrow Pharmaceutics, Adrenas Pharmaceutics, and Elsevier, and non-financial support to the institution from HRA Pharma.
AB-S has received personal honoraria for advisory boards from Recordati.
JB has received grants to the institution from Novartis, HRA Pharma, and Recordati, and personal honoraria for consulting, lectures, and meeting attendance from Novartis, HRA Pharma, Ipsen, and Recordati.
NB has served on the Board or as an advisor for European Neuroendocrine Association and the European Reference Network on Rare Endocrine Conditions.
CLB has received grants and personal honoraria for lectures from Novartis and served on the Board or as an advisor for Sociedade Brasileira de Endocrinologia e Metabologia, Endocrine Society, and European Society Of Endocrinology.
MDB has nothing to declare.
MB has nothing to declare.
JDC has received grants to the institution from Novartis, Strongbridge, and Crinetics, personal honoraria for consulting and authorship from Recordati, Novo Nordisk, Corcept, and Merck Manual, and served on the Board or as an advisor for Pituitary Society, Endocrine Society, and American Association of Clinical Endocrinologists.
FFC has served on the Board for Pituitary Society.
FC has received personal honoraria for consulting, lectures, and support for meeting attendance from Recordati Rare Diseases, Ipsen, and HRA Pharma.
PC has received grants and honoraria to the institution for consulting and lectures from Novartis and Recordati.
JF has received grants to the institution from Novartis and personal honoraria for consulting and advisory boards from Corcept, Recordati, and Novartis.
MG has received non-financial support from Novartis and Recordati and served on the Board or as an advisor for Pituitary Society and Brazilian Society of Endocrinology and Metabolism.
EBG has received grants and personal honoraria for consulting, lectures, and advisory boards from Novartis, Corcept, Strongbridge, Bristol-Myers Squibb, Recordati, and HRA Pharma, and has served as an advisor for Cushing’s Support & Research Foundation.
AG has received grants to the institution from Pfizer and personal honoraria for consulting and advisory boards from Abiogen, Novo Nordisk, and Recordati, and has served on the Board or as an advisor to European Society of Endocrinology and Glucocorticoid Induced Osteoporosis Skeletal Endocrinology Group.
AG has served as an advisor to Novartis and as an editor for Neuroendocrinology and Journal of Neuroendocrinology.
MG has received personal honoraria for consulting and lectures from Recordati Rare Diseases UK, HRA Pharma, and Ipsen, and is a Board member or advisor for UK Society for Endocrinology and European Society of Endocrinology.
KH has nothing to declare.
AGI has received grants to the institution from Recordati, Novartis, and Strongbridge, and personal honoraria for consulting from Recordati and Strongbridge.
UBK has received grants as co-investigator from Corcept, and personal honoraria for consulting, advisory board, and lectures from Acerus Pharmaceuticals and Novo Nordisk, and serves as editor for Journal of Clinical Endocrinology and Metabolism and as Board member for Endocrine Society.
NK has received personal honoraria for consulting from Recordati Rare Diseases and HRA Pharma, and has served as Board member or advisor for Pituitary Society, European Neuroendocrine Association, Endocrine Society, and European Society of Endocrinology.
LK has received personal honoraria for consulting from Strongbridge and Recordati.
DFK has received royalties from Mizuho, Inc..
AL has received grants from Recordati and Corcept, personal honoraria for lectures, support for meeting attendance, and advisory boards from Pfizer, Ipsen, Corcept, and the European Journal of Endocrinology, and royalties from UpToDate Endocrinology, and served as a Board member for International Society of Endocrinology.
AM has received grants and personal honoraria for lectures/presentations and support for meeting attendance from Pfizer, Ipsen, Novartis, and Novo Nordisk, and served as a Council member for Endocrine Society of Australia.
SM has received grants to the institution from US Food and Drug Administration and nonfinancial support from Cyclacel.
MM has received grants to the institution from Corcept, Novartis, and Strongbridge, fees for expert testimony from Janssen and Corcept, personal honoraria for advisory boards for Merck, Pfizer, Recordati, and Strongbridge.
PM has nothing to declare.
JN-P has received grants and honoraria to the institution for consulting and advisory boards from Diurnal Ltd and HRA Pharma, Recordati, and Novartis, and served as a Board member or advisor for Pituitary Foundation and Endocrine Society.
LN has received royalties from UpToDate and support for meeting attendance from the National Institutes of Health, and has served as a Board member for Endocrine Society.
AMP has received grants to the institution from HRA Pharma and served as advisor for European Reference Network on rare Endocrine Conditions and European Endocrine Society.
SP has received personal honoraria for lectures and advisory boards from HRA Pharma, Recordati Pharma, Novartis Pharma, and Crinetics Pharmaceuticals.
RP has received grants to the institution from Novartis, Pfizer, Ipsen, Shire, IBSA Farmaceutici, HRA Pharma, Cortendo AB, Corcept Therapeutics, and Merck Serono, personal honoraria for consulting, lectures, support for meeting attendance, and advisory boards from Novartis, Shire, HRA Pharma, Cortendo AB, Pfizer, Recordati, IBSA Farmaceutici, and Crinetics Pharmaceuticals.
HR has received personal honoraria for consulting from Cerium and non-financial support from Corcept.
MR has received personal honoraria for consulting, lectures, and advisory boards from Novartis, Recordati, HRA Pharma, and Ipsen.
RS has received grants to the institution from Corcept and Crinetics, and personal honoraria for consulting from Strongbridge, Corcept, HRA Pharma, and Sterotherapeutics.
CS has received grants and personal honoraria for lectures from Pfizer, Novartis, Recordati Rare Diseases, and HRA Pharma.
IL has received personal honoraria for consulting from Medison Pharma.
CAS has received grants from Pfizer, personal honoraria for consulting and support for meeting attendance from Lundbeck Pharma and the National Institutes of Health, hold patents on the genetics of PRKAR1A, PDE11A, and GPR101, and has served as a Board member or advisor for Society for Pediatric Research, Children’s Inn at NIH, and Cushing’s Foundation.
BS has nothing to declare.
AT has received grants to the institution from HRA Pharma, personal honoraria for consulting, lectures, and support for meeting attendance from HRA Pharma, Recordati Rare Diseases, and Ipsen.
YT has received personal honoraria for consulting and lectures from Novo Nordisk, Recordati Rare Diseases, Bohringer Ingelheim, and Sumitomo Dainippon Pharma.
MT has served as an advisor to European Society of Endocrinology.
ST has received grants to the institution from Strongbridge, Crinetics, and Novartis, and personal honoraria for lectures, advisory boards, and support for meeting attendance from Recordati, Pfizer, and Ipsen.
EV has received from grants through the European Society of Endocrinology from HRA Pharma, and from Novartis, Recordati, and Corcept, and received personal honoraria for consulting, lectures, and advisory boards from HRA Pharma, HRA Pharma Spain, and Recordati Rare Diseases.
EVV has nothing to declare.
GV has received grants to the institution from Novartis, Recordati, and Corcept, and personal honoraria and honoraria to the institution for consulting and lectures from HRA Pharma and Recordati, and served as an advisor for Cushing’s Hub.
JW has nothing to declare.
SMW has received from grants through the European Society of Endocrinology from HRA Pharma, and from Novartis, Recordati, and Corcept, and received personal honoraria for consulting and lectures from HRA Pharma and Recordati Rare Diseases.
MCZ has served as a Board member for European Neuroendocrine Association.
BMKB has received grants to the institution from Novartis, Opko, Strongbridge, and Millendo, personal honoraria for consulting from Aeterna Zentaris, Ascendis, Crinetics, Merck Serono, Novartis, Novo Nordisk, Recordati, Strongbridge, and Sparrow, and served as an advisor for Endocrine Society.
Funding Statement
Virtual meeting logistics were supported by unrestricted educational grants to the Pituitary Society from Ascendis Pharma, Crinetics Pharmaceuticals, HRA Pharma, Novo Nordisk, Recordati Rare Diseases, and Strongbridge Biopharma. Supporters were invited to observe the highlight summaries presented during the meeting, but did not participate in the small group discussions, had no role in the development of consensus recommendations or topics for future research, and did not review the manuscript prior to publication.
Footnotes
Search Strategy and Selection Criteria
References for this review were identified through searches of PubMed for articles published from January, 2015, to April, 2021, by use of the terms “diagnosis,” “urinary free cortisol,” “salivary cortisol,” “screening tests,” “confirmatory testing,” “differential diagnosis,” “localization testing,” “genetics,” “surgery,” “radiation therapy,” “medical therapy,” “biochemical treatment goals,” “tumor shrinkage,” “clinical outcomes,” “adrenal steroidogenesis inhibitors,” “glucocorticoid receptor blockers,” “somatostatin receptor ligands,” “dopamine agonists,” “mortality,” “comorbidities,” “quality of life,” “preoperative treatment,” “combination therapy,” and “guidelines” in combination with the terms “Cushing’s disease” and “ectopic Cushing’s”. English-language articles resulting from these searches and relevant references cited in those articles were reviewed.
Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed.
REFERENCES
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
NIHMS1752854-supplement-1.pdf (45.8KB, pdf)
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed.