Introduction
Cushing’s disease (CD), a prevalent cause of endogenous Cushing’s syndrome (CS), arises from adrenocorticotropin (ACTH)-secreting pituitary tumors. Achieving the best possible outcomes for patients necessitates accurate diagnosis, thoughtful treatment strategies, and diligent long-term management of both the disease and its associated health issues. Compared to Cushing’s syndrome stemming from adrenal issues, Cushing’s disease often leads to a diminished long-term quality of life. Recognizing the advancements in diagnostic tools and treatment options since the last comprehensive clinical guidelines in 2015, The Pituitary Society convened a virtual workshop in October 2020. This workshop brought together over 50 academic researchers and clinical experts to critically evaluate the latest evidence and formulate updated Cushing Disease Diagnosis Guidelines and management recommendations.
This article presents a concise overview of these updated guidelines, focusing on key considerations for laboratory testing, imaging techniques, and treatment modalities. It also addresses the management of complications related to Cushing’s disease and its treatments. These Cushing disease diagnosis guidelines, grounded in expert consensus and current literature, aim to provide clinicians with a practical framework for the diagnosis and management of this challenging endocrine disorder, ultimately improving patient outcomes. Algorithms for both Cushing’s syndrome diagnosis and Cushing’s disease management, along with crucial considerations for medical therapies and complication management, are highlighted throughout this document, offering a comprehensive and accessible resource for healthcare professionals. Areas requiring further research are also identified to guide future investigations in Cushing’s disease.
Diagnostic Approach to Cushing’s Syndrome and Cushing’s Disease
Laboratory Tests for Diagnosis and Monitoring
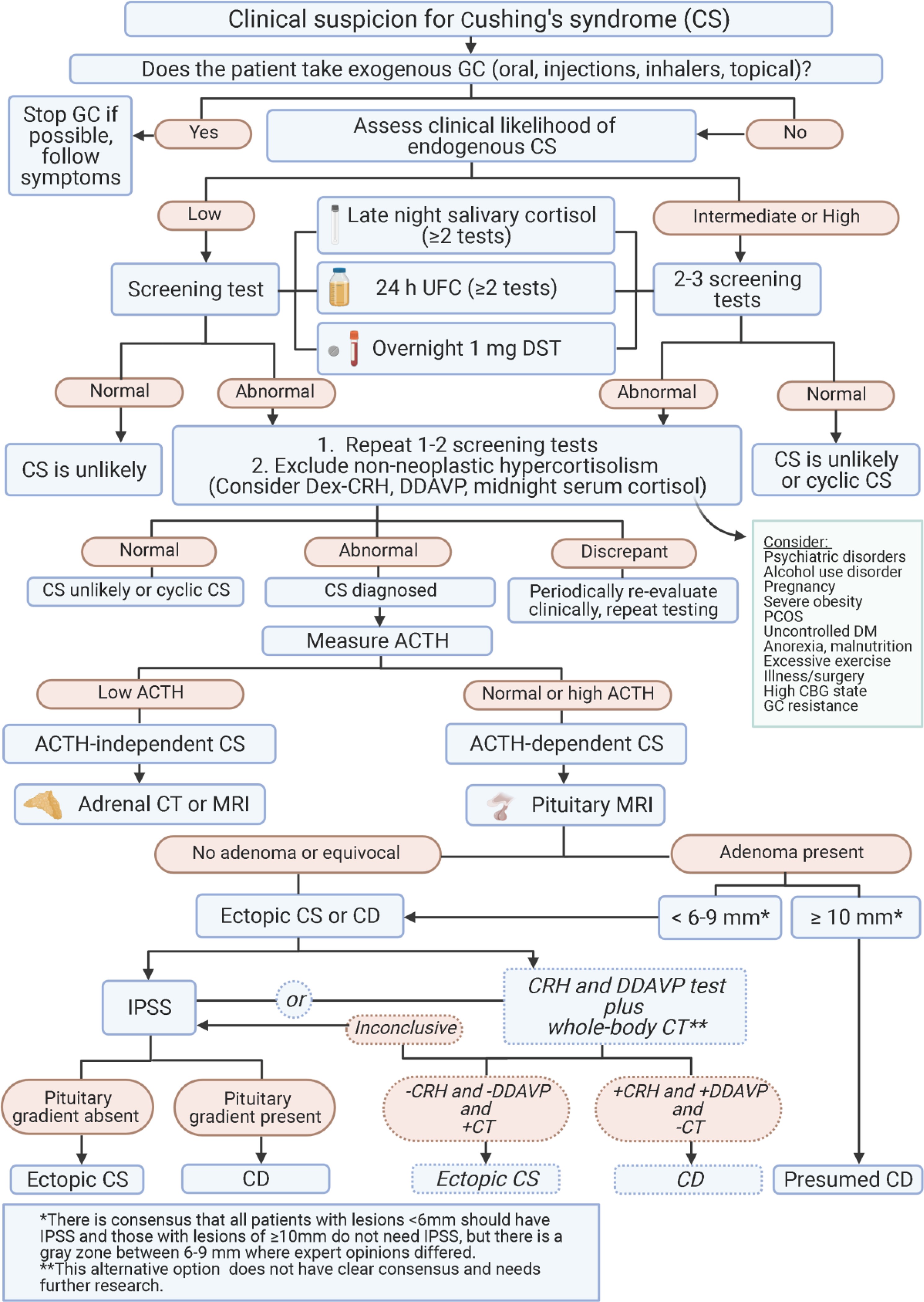
Accurate diagnosis is the cornerstone of effective management of Cushing’s disease. Cushing disease diagnosis guidelines emphasize a multi-faceted approach using laboratory tests, imaging, and differential diagnostic procedures. Laboratory tests play a crucial role in both the initial diagnosis of Cushing’s syndrome and the subsequent confirmation and monitoring of Cushing’s disease.
Screening and Confirmatory Tests for Cushing’s Syndrome
The initial step in Cushing disease diagnosis guidelines involves screening for Cushing’s syndrome in patients with suggestive clinical features. Common screening tests include:
-
Late-Night Salivary Cortisol (LNSC): This test leverages the disrupted circadian rhythm of cortisol secretion in CS patients. Elevated LNSC levels, typically measured on two or more consecutive nights, are highly sensitive for CS. Patients should be advised to avoid eating, drinking, smoking, or teeth brushing for 15 minutes before sample collection to prevent contamination. While LNSC boasts high sensitivity and ease of patient collection, it can exhibit intra-patient variability, and cut-offs may vary across laboratories. It is not recommended for shift workers or individuals with irregular sleep patterns.
-
Overnight 1-mg Dexamethasone Suppression Test (DST): This test assesses glucocorticoid feedback mechanisms. In healthy individuals, dexamethasone suppresses cortisol production. A serum cortisol level above 1.8 μg/dL (50 nmol/L) after dexamethasone administration suggests Cushing’s syndrome. DST is easy to administer and has a high negative predictive value. However, false positives are common due to factors like variable dexamethasone metabolism, medication interference, and estrogen use. Measuring dexamethasone levels alongside cortisol can enhance test interpretation.
-
24-Hour Urinary Free Cortisol (UFC): This test measures the total amount of cortisol excreted in urine over 24 hours, reflecting bioavailable cortisol levels. Elevated UFC levels, exceeding assay-specific reference ranges, indicate cortisol excess. Cushing disease diagnosis guidelines recommend collecting 2-3 samples due to intra-patient variability. UFC is independent of CBG changes and dexamethasone compliance, but accurate urine collection is crucial, and results can be influenced by factors like BMI, age, and urine volume.
Clinical Considerations for Screening and Confirmatory Testing:
- Initial Testing: For suspected CD, Cushing disease diagnosis guidelines recommend initiating screening with either UFC and/or LNSC. DST can be an alternative if LNSC is not feasible. Multiple LNSC tests are often easier for patients.
- Confirmation: Any of the tests (UFC, LNSC, DST) can be used to confirm CS. For UFC, average 2-3 collections. For LNSC, aim for ≥2 consecutive days. DST is useful in shift workers but less reliable in women on estrogen-containing oral contraceptives.
- Suspected Adrenal Tumor: Start with DST if adrenal tumor is suspected. LNSC has lower specificity in these cases unless cortisone levels are also measured.
Differentiating Pseudo-Cushing’s Syndrome
Cushing disease diagnosis guidelines recognize the importance of differentiating true Cushing’s syndrome from pseudo-Cushing’s states, which are conditions mimicking CS clinically and biochemically but without true hypercortisolism. Conditions like depression, alcoholism, obesity, and polycystic ovary syndrome can lead to pseudo-CS. Abnormal results in screening tests in pseudo-CS are usually milder, with UFC typically less than 3-fold above normal.
Tests to aid in differentiation:
- Low-Dose Dexamethasone Suppression Test with CRH stimulation (Dex-CRH test): This test assesses the pituitary-adrenal axis response to CRH after dexamethasone suppression. Patients with ACTH-dependent CS often show a cortisol response to CRH, while those with pseudo-CS do not. However, test reliability can vary due to protocol differences and assay characteristics.
- Desmopressin Test: This test utilizes the expression of vasopressin V1b (V3) receptors on ACTH-secreting adenomas. Desmopressin injection typically leads to an ACTH rise in CD patients but not in pseudo-CS. The desmopressin test is simpler and less expensive than the Dex-CRH test and shows good diagnostic performance for differentiating CS from pseudo-CS.
Clinical Recommendations for Ruling Out Pseudo-CS:
- Consider clinical history, symptom duration, and repeat testing to avoid misdiagnosis.
- In most pseudo-CS cases, hypercortisolism is mild and can be monitored for 3-6 months. Treating the underlying condition (e.g., depression) may normalize HPA axis function.
- LDDT or serial LNSC over time can correlate with the clinical picture in pseudo-CS.
- Desmopressin test is easily administered outpatient.
- Dex-CRH test can be valuable but requires expertise and dexamethasone level measurements.
Imaging and Tumor Localization in Cushing’s Disease
Once Cushing’s syndrome is confirmed, Cushing disease diagnosis guidelines emphasize imaging to locate the source of excess cortisol production, particularly to differentiate between pituitary (Cushing’s disease) and ectopic ACTH-secreting tumors.
MRI of the Pituitary Gland
Magnetic Resonance Imaging (MRI) is the preferred imaging modality for detecting pituitary adenomas in Cushing’s disease. However, due to the small size of most adenomas, standard 1.5T MRI detects only about 50% of microadenomas.
Advanced MRI Techniques to Enhance Detection:
- High-resolution MRI: Techniques like spoiled gradient-recalled (SPGR) acquisition echo, fluid attenuation inversion recovery (FLAIR), and constructive interference in the steady state (CISS) with 1mm slice intervals improve microadenoma detection.
- 3T and 7T MRI: Ultra-high field magnets (3T and 7T) offer superior resolution for better localization of microadenomas. However, they may also increase the detection of incidentalomas unrelated to CD.
Despite advancements, approximately one-third of MRI scans in CD patients remain negative. Tumor size does not always correlate with hypercortisolism severity; larger adenomas can sometimes present with milder hypercortisolism.
PET Imaging
Positron Emission Tomography (PET) is being explored as a complementary or alternative imaging modality to MRI.
- 18F-Fluoro-deoxy-glucose (18F-FDG) PET/CT: In some studies, 18F-FDG PET/CT has shown comparable detection rates to standard MRI. CRH stimulation prior to PET can increase 18F-FDG uptake and improve detection.
- PET Coregistration with Volumetric MRI (PET/MRCR): This technique combines functional and anatomical imaging, potentially improving localization accuracy.
- 11C-Methionine PET: This tracer may offer more precise localization of radiotracer uptake sites. In some studies, it has successfully localized corticotroph adenomas in de novo and recurrent CD cases, even with negative or equivocal standard MRI. However, this technique is not widely available.
Clinical Recommendations for Imaging:
- MRI remains the primary imaging modality for ACTH-secreting pituitary adenomas.
- Cushing disease diagnosis guidelines suggest 3T MRI over 1.5T MRI when available for improved sensitivity.
- 7T MRI is not routinely recommended if 1.5T/3T MRI is negative due to limited availability and uncertain added benefit.
- Functional imaging (PET) holds promise but requires further research to define optimal ligands and clinical applications. Referral to centers of excellence for advanced imaging may be considered.
Differentiating Cushing’s Disease from Ectopic ACTH-Dependent Cushing’s Syndrome
In cases of ACTH-dependent Cushing’s syndrome, it is crucial to differentiate between Cushing’s disease (pituitary source) and ectopic ACTH-secreting tumors (non-pituitary source).
Dynamic Tests
Dynamic tests help distinguish between pituitary and ectopic sources of ACTH.
- CRH Stimulation Test: ACTH and cortisol levels typically rise in CD patients after CRH administration, but less consistently in ectopic ACTH syndrome.
- Desmopressin Stimulation Test: Similar to the CRH test, desmopressin often stimulates ACTH and cortisol release in CD but less reliably in ectopic cases.
- High-Dose Dexamethasone Suppression Test (HDDST): While less accurate overall, HDDST is still used in some regions. Cortisol suppression with high-dose dexamethasone is more suggestive of CD, but ectopic tumors may also show some suppression.
No single dynamic test has 100% specificity, and results can be discordant in up to one-third of patients. The type of ectopic tumor, patient characteristics, and hypercortisolism severity can influence test outcomes.
Inferior Petrosal Sinus Sampling (IPSS)
IPSS remains the gold standard for differentiating pituitary from ectopic ACTH production. It involves measuring ACTH levels in pituitary venous drainage (inferior petrosal sinuses) and peripheral blood simultaneously.
- Central-to-Peripheral ACTH Gradient: A significant ACTH gradient (typically >2:1 at baseline or >3:1 after CRH stimulation) confirms a pituitary source (CD).
IPSS is an invasive procedure and should be performed in specialized centers due to potential risks.
Non-invasive Approaches
Non-invasive approaches combining dynamic tests and imaging are being explored to potentially reduce the need for IPSS.
- Combined CRH/Desmopressin Stimulation + MRI + Whole-body CT: A strategy combining CRH and desmopressin stimulation tests, pituitary MRI, and whole-body CT (if initial tests are inconclusive) has shown promise in diagnosing CD in some centers, potentially avoiding IPSS in select patients. A positive CT scan despite negative dynamic tests and MRI may have a high negative predictive value for CD.
- 68Ga-DOTATATE PET/CT: This PET tracer targets somatostatin receptors, which are often expressed in ectopic ACTH-secreting neuroendocrine tumors (NETs). 68Ga-DOTATATE PET/CT can localize approximately 65% of ectopic ACTH-secreting tumors, including those missed by conventional imaging. However, false positives can occur due to inflammation, and a positive scan does not definitively confirm the source of ACTH. 68Ga-DOTATATE PET/CT can guide clinical management in complex cases.
Clinical Recommendations for Differentiation:
- No single test or combination of tests perfectly differentiates pituitary and ectopic ACTH-secreting tumors. Clinical context and test results should guide management.
- If brain MRI is not immediately available and ectopic ACTH syndrome is highly suspected (e.g., male with very high UFC and hypokalemia), neck-to-pelvis thin-slice CT scan can be useful.
- IPSS may not be necessary if:
- Pituitary tumor ≥10 mm is detected on MRI and dynamic testing is consistent with CD.
- Some centers use a lower cutoff (e.g., >6mm) for lesions, but clinical presentation should always be considered as pituitary lesions can be incidental.
- IPSS is necessary if:
- MRI is negative or shows a lesion <6-10mm.
- Dynamic test results are discordant or inconclusive.
- Emerging data suggest CRH/desmopressin testing with pituitary MRI followed by whole-body CT scan might be a reliable noninvasive alternative in specialized centers.
Management of Complications in Cushing’s Disease
Cushing disease diagnosis guidelines recognize that managing complications is integral to optimizing patient outcomes in CD. Comorbidities associated with hypercortisolism often persist even after successful treatment and should be addressed proactively, sometimes even before CD-specific therapies.
Hypercoagulability
Hypercortisolism increases the risk of thromboembolic events (VTE) due to hypercoagulability, paradoxically coupled with increased bleeding tendency due to skin atrophy. Patients with CS exhibit an activated coagulation cascade. VTE incidence in endogenous CS is significantly elevated compared to patients with nonfunctioning adenomas and the general population. VTE risk persists post-surgery, indicating hypercoagulability is not immediately reversed by cortisol normalization.
Recommendations for Hypercoagulability Management:
- Thromboprophylaxis: No standard practice exists for pre- or post-operative thromboprophylaxis in CD. Consider estrogen therapy cessation in women awaiting surgery, but with caution if used for contraception.
- Risk Stratification: Prophylactic anticoagulation should be considered for patients with VTE risk factors: prior embolism, abnormal coagulation, severe hypercortisolism, estrogen/oral contraceptive use, immobility, prolonged hospitalization, high post-operative cortisol, or cortisol over-replacement in AI.
- Early Ambulation and Compression: Encourage early post-operative ambulation and compression stockings for all patients.
- Anticoagulant Preference: Low molecular weight heparin is preferred over oral anticoagulants due to shorter half-life and reversibility.
- Anticoagulation Timing: Anticoagulants may be discontinued pre-surgery to minimize bleeding risk, but optimal timing of cessation and re-initiation is unclear. Recommended durations vary widely among experts (pre-op: 2-4 days to 1-2 weeks; post-op: 1-2 days to 2-4 weeks or longer).
- Pediatric Considerations: Thromboprophylaxis is not routine in children due to bleeding risk, reserved for selected cases.
Cardiovascular Disease
CD patients have an adverse cardiovascular risk profile that may persist even after successful treatment. While fat mass may decrease post-remission, most patients remain overweight or obese. Type 2 diabetes mellitus (T2DM) and dyslipidemia are common at diagnosis. Structural cardiovascular changes like left ventricular hypertrophy and hypertension improve with remission but may not fully resolve. Myocardial infarction, stroke, and other vascular events are major contributors to increased mortality in active/persistent CD.
Recommendations for Cardiovascular Disease Management:
- Risk Evaluation and Management: Evaluate, monitor, and treat cardiovascular risk factors according to current guidelines for high-risk patients.
- Individualized Approach: Management should be individualized based on specific complications (hypertension, hyperlipidemia) and coordinated with primary care and cardiology physicians.
Bone Disease
Skeletal fragility is a frequent early complication of hypercortisolism. Vertebral fractures are common. Hypercortisolism suppresses GH/IGF-I and the hypothalamic-pituitary-gonadal axes, leading to decreased osteoblast function and bone formation. DXA may show low BMD, but fractures can occur even with normal BMD.
Recommendations for Bone Disease Management:
- Risk Assessment: Bone loss and fracture risk assessment is recommended for all patients.
- Beyond DXA: Standard DXA alone may be insufficient due to fracture risk even with normal BMD. Bone quality assessments (microscanner or trabecular bone score) or morphometric vertebral assessment are recommended where available to detect subclinical fractures. FRAX tool is not validated for CD.
- Monitoring and Follow-up: Monitor and follow-up as for all adult high-risk populations for osteoporosis.
- Osteoporosis Treatment: Consider conventional osteoporosis treatments (e.g., bisphosphonates) even with normal BMD in persistent CD due to increased fracture risk from cortisol excess.
Growth Hormone Deficiency (GHD)
Hypercortisolism inhibits GH secretion. While GH production can recover post-treatment, GHD persistence can worsen complications like bone loss and myopathy. GHD prevalence varies depending on testing timing post-surgery. IGF-I alone is not a reliable GHD screening test in adults.
Recommendations for GHD Management:
- GHD Testing Timing: No standard practice exists for GHD testing in CD adults. Delay assessment at least 6-12 months post-surgery due to delayed HPA axis recovery.
- Risk Factors: Macroadenomas and aggressive resection increase hypopituitarism risk. Patients with ≥3 pituitary hormone deficiencies are more likely to have GHD and may not need dynamic testing.
- IGF-I Insensitivity: Serum IGF-I alone is not a reliable GHD indicator.
- GH Replacement Accessibility: GH replacement access influences testing and treatment decisions. If GH replacement starts <2 years post-surgery, retest periodically to assess GH normalization upon HPA axis recovery.
- Physical Rehabilitation: CS-associated myopathy does not resolve spontaneously; physical rehabilitation is recommended for all patients.
- Pediatric GHD: Evaluate for GHD 3-6 months post-surgery in children and initiate GH replacement immediately if needed for growth.
Initial Treatment of Cushing’s Disease and Recurrence Monitoring
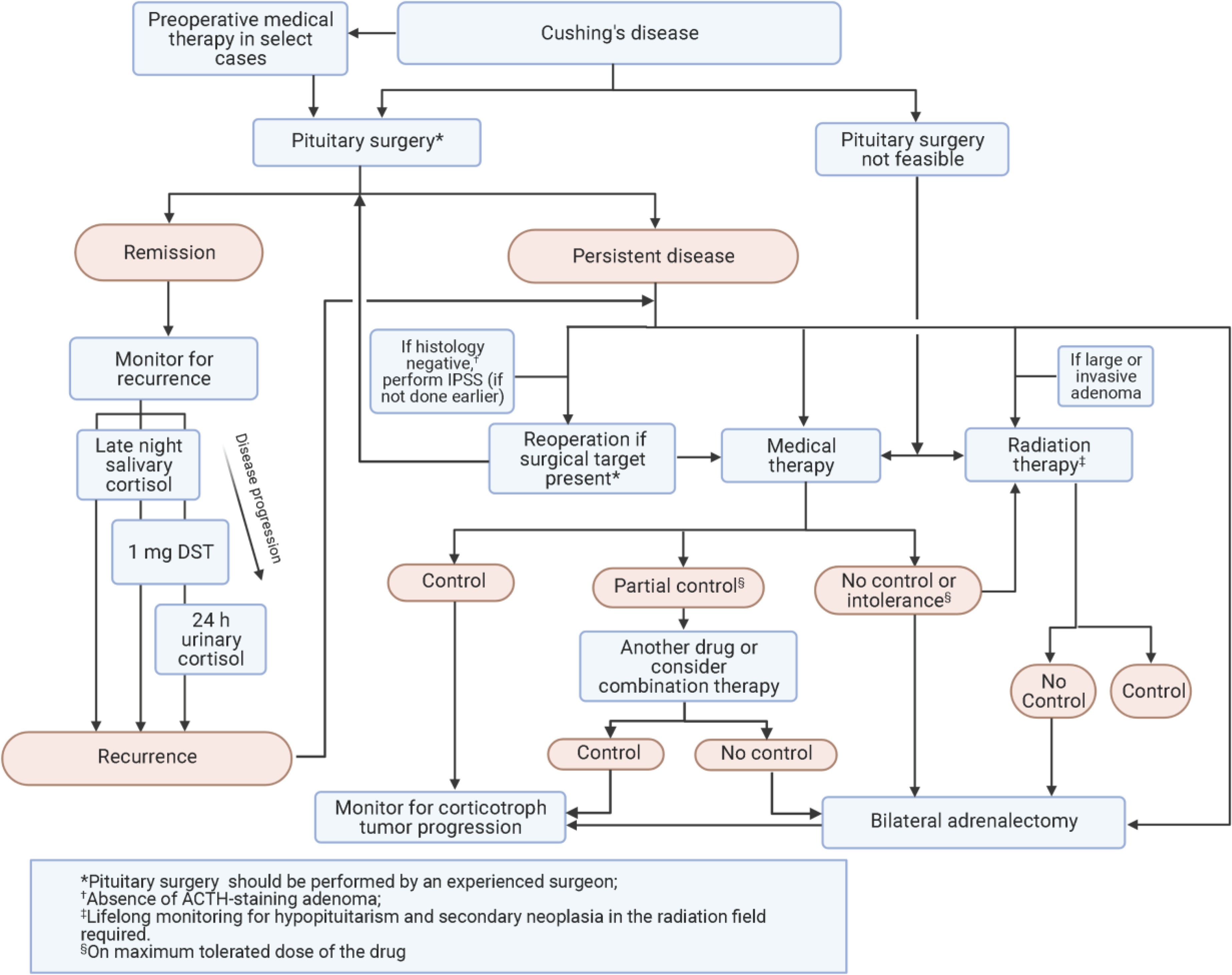
Pituitary Surgery (Transsphenoidal Surgery – TSS)
Cushing disease diagnosis guidelines recommend TSS as the first-line therapy for Cushing’s disease. Remission rates vary (60-90%) and are higher in microadenomas. Post-operative hypocortisolism indicates likely remission. Glucocorticoid replacement is needed until HPA axis recovery. Delayed remission can occur, requiring monitoring. Treatment at high-volume centers by experienced surgeons improves outcomes. Complication rates are generally low.
Recommendations for Pituitary Surgery:
- PTCOE Referral: Patients with CD should undergo surgery in specialized Pituitary Tumor Centers of Excellence (PTCOE) whenever possible.
- Experienced Team: Surgery should be performed by an experienced pituitary neurosurgeon, and follow-up managed by a multidisciplinary team including a pituitary endocrinologist.
- Outcome Reporting: Outcomes and cost-effectiveness of pituitary surgery should be reported and publicly available.
Monitoring for Recurrence
Lifelong monitoring for recurrence is essential in Cushing disease diagnosis guidelines. Recurrence rates vary (5-35%), with half occurring within 5 years and half later. Early post-operative low cortisol does not guarantee no recurrence.
Monitoring Tests for Recurrence:
- Late-Night Salivary Cortisol (LNSC): Most sensitive test for recurrence, should be done annually after HPA axis recovery.
- 1-mg Dexamethasone Suppression Test (DST) and 24-Hour Urinary Free Cortisol (UFC): Usually become abnormal after LNSC in recurrence.
Clinical Recommendations for Recurrence Monitoring:
- Lifelong Monitoring: Lifelong monitoring for CD recurrence is recommended.
- Post-operative Dynamic Testing: Post-operative dynamic testing may predict recurrence risk but is not consistently used.
- Annual LNSC: LNSC is the most sensitive test and should be done annually after HPA axis recovery.
- Consider Initial Diagnostic Tests: Monitoring should consider which tests were initially abnormal for each patient.
- Mild Biochemical Abnormalities: If only slight biochemical abnormalities without clinical hypercortisolism are present, close monitoring and comorbidity management can be considered before re-treatment of CD itself.
Repeat Pituitary Surgery
Repeat TSS can be considered for recurrent CD with visible tumor on MRI. Remission rates vary widely (37-88%) after reoperation. Complication rates may be higher with repeat surgery but are lower in experienced hands.
Recommendations for Repeat Pituitary Surgery:
- MRI-Visible Tumor: Repeat TSS is suggested for recurrent CD with MRI-evident tumor, especially if the initial surgery was not at a PTCOE.
- No MRI-Visible Tumor: Reoperation may be appropriate even without MRI-visible tumor if an experienced surgeon at a high-volume center deems it feasible and prior pathology or IPSS showed a central gradient.
Medical Therapy for Cushing’s Disease
Medical therapies play a crucial role in managing Cushing’s disease, particularly for persistent or recurrent cases, patients not suitable for surgery, and those awaiting radiation therapy effects. Drugs target adrenal steroidogenesis, pituitary receptors (somatostatin and dopamine), and glucocorticoid receptors.
Medical Therapy: Targeting Adrenal Steroidogenesis
Adrenal steroidogenesis inhibitors (ketoconazole, metyrapone, mitotane, etomidate, and osilodrostat) block adrenal enzymes, reducing cortisol synthesis. They effectively control cortisol excess but do not target the pituitary tumor or restore HPA axis rhythm. Risk of adrenal insufficiency (AI) exists with overtreatment. Adverse events can relate to ACTH increase and adrenal hormone precursor buildup. Drug-drug interactions are a key consideration.
Ketoconazole
Ketoconazole inhibits multiple adrenal enzymes. UFC normalization is seen in ~65% of patients initially, but escape occurs in 15-25%. It can cause hypogonadism in men and hepatotoxicity (requiring LFT monitoring). Gastrointestinal issues and AI are also common. Numerous drug-drug interactions exist.
Metyrapone
Metyrapone (11β-hydroxylase inhibitor) normalizes UFC in ~70% of patients. Rapid UFC and LNSC decrease is seen. Hyperandrogenism is a common side effect, especially in women. Dizziness, arthralgia, and nausea are also reported. 11-deoxycortisol cross-reactivity in cortisol assays can occur.
Osilodrostat
Osilodrostat (11β-hydroxylase and aldosterone synthase inhibitor) demonstrated 86% UFC normalization in a phase 3 randomized withdrawal study. Clinical improvements are seen. Adverse events include hypocortisolism, hyperandrogenism (hirsutism), hypokalemia, and QTc prolongation.
Mitotane
Mitotane has adrenolytic action and suppresses hypercortisolism in ~80% of cases, but with slow onset and variable bioavailability. It is rarely used for CD, primarily for adrenal carcinoma.
Etomidate
Etomidate (anesthetic) rapidly normalizes cortisol levels, used for acute severe hypercortisolism in hospitalized patients. Low-dose for partial blockade, high-dose for complete blockade (with IV hydrocortisone to avoid AI). Propylene glycol formulation has more adverse effects.
Medical Therapy: Targeting Pituitary Somatostatin and Dopamine Receptors
Cabergoline (dopamine agonist) and pasireotide (somatostatin receptor ligand) are used for persistent or recurrent CD. Pasireotide is approved for CD. Tumor shrinkage is clinically relevant, especially for residual or progressive tumors.
Pasireotide
Pasireotide (SC) normalized UFC in 15-26% of patients in a phase 3 study. Pasireotide LAR (IM monthly) normalized UFC in 40% in a phase 3 study. Clinical improvements are seen with both formulations. Pasireotide LAR can decrease tumor volume. High risk of hyperglycemia exists, requiring careful patient selection and management.
Cabergoline
Cabergoline normalizes UFC in 25-40% of patients in retrospective studies, with escape in 20-40%. Tumor shrinkage in ~50% of patients. Impulse-control disorder is a potential side effect. Cardiac valvulopathy risk is debated.
Medical Therapy: Targeting the Peripheral Tissue Glucocorticoid Receptor
Mifepristone
Mifepristone (glucocorticoid receptor blocker) controls hypercortisolism effects regardless of etiology. Improves hyperglycemia and hypertension. No biochemical markers for efficacy monitoring. Risk of hypokalemia and AI. Challenging to use outside specialized centers.
Clinical Recommendations for Medical Therapy:
- Individualized Therapy: Medical therapy should be individualized based on clinical scenario and hypercortisolism severity. Regulatory approvals and costs vary by region.
- Severe Disease: Aggressive treatment to normalize cortisol (or cortisol action with mifepristone) is crucial in severe disease. Use multiple UFC and LNSC tests for monitoring.
- Initial Treatment Selection:
- Adrenal steroidogenesis inhibitors (ketoconazole, osilodrostat, metyrapone) are usually first-line.
- Mild disease, no visible tumor: Ketoconazole, osilodrostat, or metyrapone preferred. Cabergoline also an option.
- Mild-moderate disease, residual tumor: Cabergoline or pasireotide (careful patient selection due to hyperglycemia risk).
- Severe disease: Rapid cortisol normalization is key. Osilodrostat and metyrapone act quickly, ketoconazole within days, etomidate for hospitalized patients. Combination steroidogenesis inhibitors may be needed. Consider BLA if medical therapy fails in severe cases.
- Pregnancy: Cabergoline or metyrapone may be considered (not approved for pregnancy).
- Bipolar/impulse control disorder: Avoid cabergoline.
- Mifepristone: Use cautiously by experts due to monitoring challenges.
- Adrenal Steroidogenesis Inhibitor Selection:
- Ketoconazole, metyrapone, osilodrostat are options (availability varies).
- Ketoconazole: Easy titration, but hepatotoxicity risk (LFT monitoring).
- Osilodrostat and metyrapone: Rapid control, no LFT monitoring concern, no hypogonadism in men. Osilodrostat expected to be used more widely due to efficacy and twice-daily dosing.
- Mitotane: Rarely used for CD.
- Block-and-replace regimen: Consider for severe, cyclical CS, or infrequent monitoring access, but caution against GC over-replacement.
- Monitoring Treatment Response:
- Regular monitoring of cortisol (except mifepristone), symptoms, and comorbidities (weight, glycemia, BP). QoL assessment.
- UFC often used for cortisol monitoring (not for AI diagnosis). Morning cortisol or LNSC can be alternatives.
- Change treatment if cortisol remains elevated after 2-3 months at maximum tolerated doses.
- Consider combination therapy if cortisol reduced but not normalized and some clinical improvement seen.
- Switch therapy if clear resistance to treatment.
- Tumor Growth Monitoring:
- Monitor ACTH levels (elevations may suggest tumor growth, but levels fluctuate).
- MRI typically at 6-12 months and then every few years.
- Suspend medical treatment and reassess management if progressive tumor growth is seen.
- Combination Therapy:
- Ketoconazole + metyrapone is a common combination for maximizing adrenal blockade.
- Ketoconazole + cabergoline or pasireotide, or pasireotide + cabergoline are options if tumor is visible.
- Triple combinations (cabergoline + pasireotide + ketoconazole; ketoconazole + metyrapone + mitotane) can be considered. Monitor for overlapping toxicities and drug interactions.
Primary and Preoperative Medical Therapy for De Novo CD
Primary medical therapy is used when surgery is unlikely to be successful. Preoperative medical therapy is not routinely used.
Recommendations for Primary and Preoperative Medical Therapy:
- Limited Data for Preoperative Therapy: No rigorous data support routine preoperative medical therapy.
- Consider for Delayed Surgery: Adrenal steroidogenesis inhibitors can be considered if surgery is delayed.
- Severe CD: Patients with severe, life-threatening complications may benefit from preoperative medical therapy in select cases.
- Monitoring Challenges: Post-operative cortisol assessments for surgical cure are less reliable after preoperative therapy.
Radiation Therapy for Cushing’s Disease
Radiation therapy (RT) is used as adjuvant therapy for persistent/recurrent CD post-TSS or for aggressive tumors. Conventional external-beam RT achieves remission in ~two-thirds of patients over years. Stereotactic radiosurgery (SRS) shows higher remission rates. Adjuvant medical therapy is needed during the latency period until RT effect. Hypopituitarism is a common long-term side effect.
Recommendations for Radiation Therapy:
- Persistent/Recurrent CD: RT is used for persistent hypercortisolism after incomplete resection, aggressive tumors, or unresectable tumors.
- SRS Preference: SRS is more convenient but optic chiasm avoidance is crucial.
- Lifelong Monitoring: Lifelong monitoring for pituitary hormone deficiencies and recurrence is required post-RT.
- Secondary Neoplasia: Consider imaging for secondary neoplasia in the radiation field.
Adrenalectomy for Cushing’s Disease
Bilateral adrenalectomy (BLA) offers immediate cortisol control for persistent/recurrent CD unresponsive to medical therapy. It is considered for select patients due to lifelong glucocorticoid and mineralocorticoid replacement. Laparoscopic BLA has a complication rate of 10-18%. Corticotroph tumor progression is a long-term concern in 25-40% of patients post-BLA.
Recommendations for Adrenalectomy:
- Last Resort: BLA is often considered a last resort after other options fail.
- Severe Hypercortisolism: BLA may be warranted earlier in severe hypercortisolism cases needing rapid cortisol control.
- Pregnancy Desire: BLA may be considered earlier in women with CD desiring pregnancy.
- Post-BLA Monitoring: Monitor ACTH and pituitary imaging post-BLA for corticotroph tumor progression.
Additional Considerations
Genetics of Cushing’s Disease
Corticotroph adenomas are mostly sporadic. Somatic USP8 mutations are common. Familial tumor syndromes rarely cause corticotroph adenomas. USP8 mutational status may predict recurrence and guide personalized treatment.
Diagnosis and Management of Cushing’s Syndrome in Children
Endogenous CS is rare in children. Lack of height gain with weight gain is a common presentation. Diagnostic approach is slightly different from adults. Surgical resection is first-line treatment. Thromboprophylaxis is not routine in children. GH replacement is often needed post-treatment in children. Ketoconazole or metyrapone are typically used for medical therapy in children. Early diagnosis and expert management are crucial in children.
Conclusion
These updated Cushing disease diagnosis guidelines, derived from expert consensus and current evidence, provide a comprehensive framework for the diagnosis and management of Cushing’s disease. By adhering to these guidelines, clinicians can optimize patient care, improve diagnostic accuracy, and tailor treatment strategies to individual patient needs, ultimately leading to better outcomes and enhanced quality of life for individuals affected by this challenging condition. Further research is crucial to refine diagnostic algorithms, optimize treatment approaches, and address the long-term complications of Cushing’s disease, as highlighted in the areas for future research identified by The Pituitary Society workshop.
Panel 3. Future Research Topics Ranked of Highest Importance
| Screening and diagnosis of CS |
|---|
| • Optimize pituitary MR and PET imaging using improved data acquisition and processing to improve microadenoma detection |
| • Compare diagnostic algorithms for the differential diagnosis using invasive versus non-invasive strategies |
| • Identify additional corticotroph adenoma mutations and development of a comprehensive panel of genomic/proteomic tests for CD diagnosis |
| Complications of CD |
| • Define use of anticoagulant prophylaxis and therapy in different populations and settings |
| • Optimize the approach in managing long-term complications |
| Treatment of CD |
| • Determine clinical benefit of restoring the circadian rhythm, potentially with a higher nighttime medication dose |
| • Identify better markers of disease activity and control |
| • Develop new, better tolerated, more effective medical therapies |
| • Define populations that might benefit from preoperative medical treatment |
Open in a new tab
Panel 1: Future research topics ranked of highest importance for Cushing’s Disease diagnosis, complications, and treatment.