I. Introduction
Cushing’s Syndrome (CS), encompassing both clinical and subclinical presentations, is estimated to affect 0.2–2% of the adult population. This suggests it is more prevalent than traditionally considered, moving beyond the classification of a rare or ‘orphan’ disease (previously estimated at 2–3 cases per million annually). Early recognition of Cushing’s Syndrome by healthcare providers is crucial for timely diagnosis and intervention. Effective screening tests such as late-night salivary cortisol, dexamethasone suppression tests, and 24-hour urinary free cortisol are valuable initial steps. Positive screening results necessitate a comprehensive, stepwise evaluation conducted by an endocrinologist. This article aims to underscore the significance of screening for Cushing’s Syndrome and proposes a structured diagnostic algorithm for suspected hypercortisolism.
While the diagnosis of Cushing’s Syndrome is frequently overlooked, often masked by symptoms overlapping with common metabolic disorders, it’s equally important to avoid overdiagnosis. This review details a stepwise diagnostic approach to hypercortisolism, designed to minimize both missed and incorrect diagnoses of Cushing’s Syndrome.
II. Step 1: Clinical Suspicion and Screening for Hypercortisolism
“There is no rule more invariable than that we are paid for our suspicions by finding what we suspect.”
Henry David Thoreau
The hypothalamic-pituitary-adrenal (HPA) axis operates under a normal circadian rhythm. Hypercortisolism arises from disruptions in this rhythm and an excess of cortisol. The clinical manifestations of hypercortisolism are diverse. While overt Cushing’s Syndrome is typically easily identifiable, distinguishing subclinical CS can be challenging, particularly in patients presenting with obesity, diabetes, and hypertension. Symptoms like visceral obesity, glucose intolerance, hypertension, and menstrual irregularities, although common in CS, are not specific indicators.
More discriminatory signs of hypercortisolism include skin changes such as livid striae and ecchymoses, muscle weakness (proximal myopathy), and bone issues like reduced height in obese children and vertebral compression fractures. These catabolic manifestations may be subtle in the early stages and become more pronounced with prolonged or severe hypercortisolism. Hypertrichosis on the forehead can also be a valuable clinical indicator.
Table 1. Clinical Features of Cushing’s Syndrome
| Feature Category | Specific Features | Prevalence (%) |
|---|---|---|
| General | Weight gain, Fatigue, Decreased libido | >80 |
| Skin | Facial plethora, Acne, Hirsutism, Striae (purple >1cm) | 60-80 |
| Cardiovascular | Hypertension, Edema | 70-90 |
| Metabolic | Glucose intolerance, Diabetes mellitus, Dyslipidemia | 50-70 |
| Musculoskeletal | Proximal myopathy, Osteoporosis, Back pain | 50-70 |
| Psychiatric | Depression, Irritability, Emotional lability | 50-80 |
| Reproductive (Women) | Menstrual irregularities, Amenorrhea, Infertility | 60-80 |
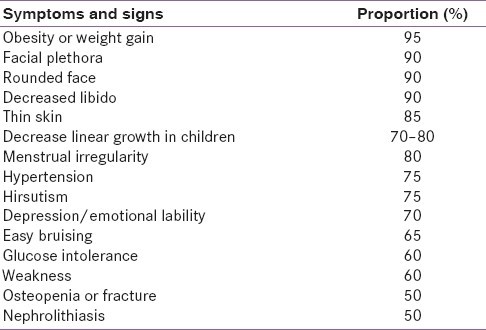 Clinical features of Cushing's syndrome detailed in a table, highlighting prevalence of various symptoms such as weight gain, skin changes, cardiovascular and metabolic issues.
Clinical features of Cushing's syndrome detailed in a table, highlighting prevalence of various symptoms such as weight gain, skin changes, cardiovascular and metabolic issues.
Clinical judgment combined with a systematic approach is essential for diagnosing or ruling out subclinical Cushing’s Syndrome. The Endocrine Society recommends screening for CS in the following scenarios:
- Patients exhibiting multiple, progressive, and specific signs suggestive of Cushing’s Syndrome.
- Individuals with atypical presentations such as hypertension or osteoporosis at a young age.
- Patients diagnosed with adrenal incidentalomas.
- Children showing a deceleration in height percentile alongside increasing weight.
Subclinical CS is observed in 5–30% of adrenal incidentaloma cases, which themselves are found in 4–7% of adults. Based on this data, the estimated prevalence of subclinical CS in adults ranges from 0.2–2.0%. Cushing’s Syndrome is also considered in the differential diagnosis of polycystic ovary syndrome (PCOS), which is essentially a diagnosis of exclusion.
Routine screening for CS in all diabetic patients is not generally recommended, even though occult CS prevalence in this population can range from 1–10% in some studies. This is largely due to the high rate of false-positive results in this group. For example, a study involving 171 obese diabetic patients found that 31 had abnormal cortisol levels in overnight dexamethasone suppression tests. However, further testing revealed that only three of these patients had elevated 24-hour urinary free cortisol, with two cases attributed to alcoholic pseudo-Cushing’s. Ultimately, only one patient out of the initial 31 was diagnosed with true Cushing’s Syndrome.
In contexts where clinical discrimination is challenging, the pretest probability of CS in a diabetic patient is equivalent to the prevalence of CS within the diabetic population. With such a low pretest probability, even highly specific tests may not significantly increase the posttest probability beyond 20%, offering limited diagnostic clarity. Consequently, universal screening for Cushing’s Syndrome in patients with isolated components of metabolic syndrome is not currently advised.
III. Step 2: Establishing Endogenous Hypercortisolism
“When you have mastered numbers, you will in fact no longer be reading numbers, any more than you read words when reading books. You will be reading meanings.”
W. E. B. Du Bois
Before proceeding with hormonal evaluations, it is critical to ascertain the patient’s history of exogenous glucocorticoid use to exclude exogenous Cushing’s Syndrome. In some regions, exogenous CS is frequently encountered due to unregulated use of powdered or injectable medications for pain relief, often dispensed without prescription. Certain features such as increased intraocular pressure, cataracts, benign intracranial hypertension, aseptic necrosis of the femoral head, osteoporosis, and pancreatitis are more commonly associated with iatrogenic Cushing’s Syndrome compared to endogenous forms. Diagnosis is usually straightforward and can be confirmed by drug analysis and suppressed basal serum cortisol and plasma adrenocorticotropic hormone (ACTH) levels.
A history of depression, severe obesity, or chronic alcoholism may suggest pseudo-Cushing’s Syndrome, a condition characterized by reversible HPA axis hyperactivity. These cases are typically clinically milder, may exhibit cortisol suppression with dexamethasone tests, and often resolve with treatment of the underlying condition. Insulin tolerance tests or loperamide tests are rarely necessary in these situations.
For quantitative assessment of cortisol load, the 24-hour urinary free cortisol (UFC) test is available. To evaluate the circadian rhythm and function of the HPA axis, midnight serum cortisol and late-night salivary cortisol tests are useful. The suppressibility of the HPA axis is assessed using the overnight dexamethasone suppression test (ODST), the standard 2-day low-dose dexamethasone suppression test (LDDST), and the LDDST with corticotropin-releasing hormone (CRH) stimulation test. Methodologies for these tests are summarized in Table 2.
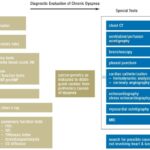
Initial diagnostic evaluation typically involves either UFC, late-night salivary cortisol, ODST, or LDDST. In patients with strong clinical suspicion but inconclusive, discordant, or negative initial test results, subsequent evaluation with midnight serum cortisol levels or LDDST-CRH stimulation test may be required. Figure 1 outlines a diagnostic algorithm for patients suspected of having Cushing’s Syndrome. This approach is predicated on the high sensitivity of initial tests for ruling out CS. In cases with positive initial results, repeat testing and additional tests enhance specificity, thereby reducing the likelihood of misdiagnosis.
Table 2. Tests for Diagnosis of Endogenous Hypercortisolism
| Test | Methodology