Introduction
Cerebral small vessel disease (CSVD) encompasses a spectrum of conditions affecting the brain’s arterioles, venules, and capillaries, arising from diverse etiologies (1, 2). Age and hypertension are recognized as the most prevalent contributing factors to CSVD (3, 4. Despite the varied pathological pathways, CSVD commonly manifests with similar neuroimaging markers, notably lacunes, white matter hyperintensities (WMH), enlarged perivascular spaces (EPVS), and cerebral microbleeds (CMB) (2). While small vessels themselves are typically beyond the resolution of computed tomography angiography (CTA) or magnetic resonance angiography (MRA), the resultant parenchymal lesions are readily detectable via magnetic resonance imaging (MRI). The STandards for Reporting Vascular changes on nEuroimaging (STRIVE) initiative provides an internationally recognized framework for classifying and defining these CSVD markers (5). In diagnosing CSVD, MRI offers superior specificity compared to clinical assessments. The characteristics of these neuroimaging markers are crucial in etiological evaluations (5). Arteriolosclerosis is strongly implicated in sporadic CSVD pathogenesis; however, the potential involvement of venules in CSVD has received less attention. Consequently, the role of venules in CSVD pathogenesis and the diagnostic utility of venule abnormalities as imaging markers remain underexplored.
The central vein sign (CVS), indicating a vein centrally located within white matter lesions (WMLs), has emerged as a significant imaging marker for multiple sclerosis (MS) (6–8), a chronic inflammatory demyelinating and neurodegenerative disease of the central nervous system (CNS). Research indicates that CVS aids in differentiating MS from conditions that mimic it (9, 10), including CSVD (11–16). Dawson’s fingers, characterized as elongated, flame-shaped lesions oriented perpendicularly to the lateral ventricle walls, are visible on fluid-attenuated inversion recovery (FLAIR) or T2-weighted MRI. T2-weighted/FLAIR MRI’s capability to visualize venules within WMLs suggests that Dawson’s fingers represent inflammatory activity surrounding these venules (17). This characteristic has firmly established Dawson’s fingers as a crucial imaging marker in the diagnosis and differential diagnosis of MS (18, 19). The diagnostic landscape is further complicated by the imaging similarities between CSVD and MS, such as white matter demyelination and brain atrophy. Periventricular demyelination is a valuable imaging feature for distinguishing MS from other conditions (18. Dawson’s fingers, indicative of perivascular inflammation around veins and venules, share a close pathological link with CVS. Prior studies have demonstrated the utility of Dawson’s fingers in differentiating MS from neuromyelitis optica spectrum disorder (18, 20) and MOG antibody disease (20). However, the manifestation and significance of Dawson’s fingers in CSVD remain largely unknown.
This study was designed to: (1) ascertain the prevalence of Dawson’s fingers in CSVD; (2) identify factors contributing to the development of Dawson’s fingers in CSVD patients; (3) characterize the imaging and clinical profiles of CSVD patients exhibiting Dawson’s fingers; and (4) evaluate the potential of Dawson’s fingers as a specific marker to distinguish MS from CSVD, thereby contributing to the differential diagnosis of these neurological conditions.
Methods
Patients and Population
This prospective observational study enrolled participants from Tianjin Medical University General Hospital and Beijing Tiantan Hospital through consecutive recruitment. Inclusion criteria were patients with clinically diagnosed CSVD and at least one of the following imaging features: lacunes, WMH, EPVS, or CMBs. MS diagnoses were based on the 2017 McDonald criteria. Exclusion criteria included patients with: (1) unidentified vascular risk factors for CSVD, such as a history of hypertension, hypercholesterolemia, diabetes mellitus (DM), smoking, and alcohol consumption; (2) evidence of other conditions that could potentially cause WMLs; and (3) contraindications to MRI scanning.
Standard Protocol Approvals, Registrations, and Patient Consents
The study received ethical approval from the Ethics Committees of Tianjin Medical University and Beijing Tiantan Hospital. Informed consent was obtained from all participants.
Image Acquisition Protocol
Brain MRI scans were performed using a 3.0-T scanner (Magnetom Trio Tim; Siemens). Standard sequences included T2, FLAIR, T1, diffusion-weighted imaging (DWI), and susceptibility-weighted imaging (SWI) (Supplemental Table 1). Two trained neurologists, blinded to clinical information, independently assessed all MRIs for the presence, location, and size of lesions (WMH, EPVS, lacunes, microbleeds) following the STandards for Reporting Vascular Changes on nEuroimaging (STRIVE) recommendations. The total SVD score (ranging from 0 to 4) was calculated by assigning points for individual imaging features: 1 point for any lacune, 1 for any microbleed, 1 for moderate-to-severe EPVS in the basal ganglia (EPVS >10), and 1 for WMHs (deep tissue: Fazekas score 2 or 3 and/or periventricular: Fazekas score 3). WMH lesion masks were manually delineated on T2 images using MRIcro software (http://www.cabiatl.com/mricro/mricro/), and WMH lesion volumes were subsequently calculated.
Dawson’s fingers were defined as elongated, flame-shaped WMH lesions perpendicular to the lateral ventricle wall, identified on T2/FLAIR images. Inter-rater reliability was high (interclass correlation coefficient = 0.86). Discrepancies were resolved by a third rater.
Clinical Assessment
Two neurologists conducted clinical assessments for each patient, collecting data on demographic factors, clinical factors, and vascular risk factors, including: age, sex, hypertension (systolic blood pressure (SBP) >140 mm Hg or diastolic blood pressure (DBP) >90 mmHg), DM (plasma glucose 2 h post-meal ≥11.1 mmol/L or fasting plasma glucose ≥ 7.0 mmol/L), hyperlipidemia (triglycerides >1.7 mmol/L or serum total cholesterol level >5.72 mmol/L), Modified Rankin Scale (mRS), disease duration, and current smoking (21). Laboratory results, including atrial fibrillation status, and cognitive assessments using the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) were also recorded.
Statistical Analysis
Continuous variables with normal distributions are presented as mean ± standard error of mean and compared using Student’s t-tests. Non-normally distributed continuous variables are presented as medians and interquartile ranges (IQR). Categorical variables were analyzed using χ2-tests, with Fisher’s exact test applied when expected values were low (p
Data Availability
The datasets generated during this study are available upon request from the corresponding author.
Results
Dawson’s Fingers Manifest in CSVD Patients
CSVD is predominantly considered an arterial disease based on conventional radiological markers like CMB, lacunes, WMH, and moderate to severe EPVS (5). However, the role of venous abnormalities in CSVD has been under-investigated. To explore the presence of venous involvement in CSVD, we evaluated the occurrence of Dawson’s fingers in 65 CSVD patients. Baseline characteristics of the CSVD and MS groups are detailed in Table 1. Our analysis revealed Dawson’s fingers, a venous abnormality, in the periventricular regions on MRI scans of CSVD patients (Figure 1). Dawson’s fingers were observed in 20 of the 65 CSVD patients (30.8%). As expected, Dawson’s fingers were more frequent in MS patients (32/46) compared to CSVD patients (20/65) (69.6% vs. 30.8%, P P = 0.001), yielding a sensitivity of 71% and specificity of 69% for Dawson’s fingers in distinguishing MS from CSVD.
Table 1. Demographic and clinical characteristics of patients with MS and CSVD.
| Characteristic | MS (N = 46) | CSVD (N = 65) | P-value |
|---|---|---|---|
| Age, year, median (IQR) | 31.5 (24.8–36.3) | 54 (47–61) | 0.000 |
| Male sex, n (%) | 15 (32.6) | 31 (47.7) | 0.112 |
| Hypertension, n (%) | 1 (2.2) | 43 (66.2) | 0.000 |
| SBP, mmHg, median (IQR) | 115 (108–120) | 134 (122–144) | 0.000 |
| DBP, mmHg, median (IQR) | 75 (70–80) | 80 (73.5–86.5) | 0.000 |
| Hyperlipidemia, n (%) | 11 (23.9) | 29 (44.6) | 0.025 |
| Diabetes, n (%) | 1 (2.2) | 10 (15.4) | 0.049& |
| Atrial fibrillation (%) | 0 (0) | 1 (1.6) | 1.000 |
| Current smoking, n (%) | 1 (2.2) | 22 (33.8) | 0.000 |
| Alcohol, n (%) | 1 (2.2) | 17 (26.2) | 0.001 |
| Disease duration, year, median (IQR) | 5 (1.9–7.2) | 2 (0.6–4.0) | 0.001 |
Hypertension = Systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg; Diabetes = Fasting plasma glucose ≥7.0 millimoles per liter and/or plasma glucose after a meal for 2 h ≥11.1 millimoles per liter; Hyperlipidemia = Serum total cholesterol levels >5.72 millimoles per liter; and/or triglycerides >1.7 millimoles per liter.
MS, multiple sclerosis; CSVD, cerebral small vessel disease; IQR, interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure; & P value .
Figure 1. Dawson’s fingers in a CSVD patient with diabetes mellitus.
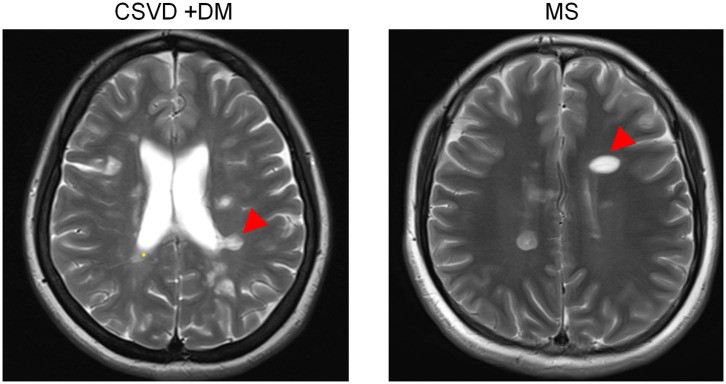 Dawson's fingers in CSVD patient
Dawson's fingers in CSVD patient
FLAIR image showing Dawson’s fingers (arrowheads) around the ventricles in a CSVD patient, highlighting the venous abnormality feature. While Dawson’s fingers are a known marker for MS, this image demonstrates their presence in CSVD, particularly in patients with diabetes mellitus, impacting their utility in differential diagnosis.
Demographic and Vascular Risk Factors Associated with Dawson’s Fingers in CSVD
To identify factors contributing to Dawson’s fingers in CSVD, we compared demographic and vascular risk factors between CSVD patients with and without Dawson’s fingers. Apart from diabetes mellitus (DM), no significant differences were found in demographic factors, number of attacks, disease duration, or other vascular risk factors (hypertension, hyperlipidemia) between the two groups. A statistically significant association was observed between DM and the presence of Dawson’s fingers in CSVD patients (30% in patients with Dawson’s fingers vs. 8.9% in those without, P Table 2). The diagnostic utility of Dawson’s fingers in differentiating CSVD from MS (32/46 MS patients with Dawson’s fingers) was reduced in CSVD patients with DM (6/10) (AUC = 0.454; P > 0.05), indicating that DM status affects the specificity of Dawson’s fingers in differential diagnosis.
Table 2. Demographic and clinical characteristics of CSVD patients with or without Dawson’s fingers.
| Characteristic | CSVD without Dawson’s fingers (N = 45) | CSVD with Dawson’s fingers (N = 20) | P-value |
|---|---|---|---|
| Age, year (mean, SE) | 54.0 ± 1.8 | 52.5 ± 2.6 | 0.378 |
| Male sex, n (%) | 18 (40.0) | 13 (65.0) | 0.063 |
| Hypertension, n (%) | 32 (71.1) | 11 (55.0) | 0.205 |
| SBP, mmHg, median (IQR) | 133 (122–144) | 137 (121–146) | 0.754 |
| DBP, mmHg, median (IQR) | 80 (74.5–85.0) | 80 (70.0-88.0) | 0.938 |
| Hyperlipidemia, n (%) | 29 (42.2) | 10 (50.0) | 0.560 |
| Diabetes mellitus, n (%) | 4 (8.9) | 6 (30.0) | 0.029 |
| Current smoking, n (%) | 14 (31.3) | 8 (40.0) | 0.485 |
| Alcohol, n (%) | 12 (26.7) | 5 (25.0) | 0.888 |
| Disease duration, year (median, range) | 2.0 (0.2–20.0) | 2.5 (0.2–8.0) | 0.209 |
| mRS (mean, median, range) | 1.2, 1 (1–4) | 1.3, 1 (1–3) | 0.244 |
| EDSS (median, range) | 2 (2–8) | 3 (2–6) | 0.147 |
| Number of attacks* (median, range) | 1 (0–4) | 1 (0–4) | 0.630 |
| Atrial fibrillation (%) | 1 (2.4) | 0 (0) | 1.000 |
Hypertension = Systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg; Diabetes = Fasting plasma glucose ≥7.0 millimoles per liter and/or plasma glucose after a meal for 2 h ≥11.1 millimoles per liter; Hyperlipidemia = Serum total cholesterol levels >5.72 millimoles per liter; and/or triglycerides >1.7 millimoles per liter.
*Only note ischemic attack in CSVD, but not include transient ischemic attack (TIA).
CSVD, cerebral small vessel disease; SE, standard error; SBP, systolic blood pressure; DBP, diastolic blood pressure; IQR, interquartile range; mRS, modified Rankin scale, range from 0 to 6; EDSS, Expanded Disability Status Scale, range from 0 to 10, higher scores indicate a greater degree of disability.
Cognitive and Imaging Impact of Dawson’s Fingers in CSVD
Given the presence of Dawson’s fingers in CSVD and their association with DM, we investigated their effects on clinical function and imaging markers in CSVD patients. Imaging analysis revealed statistically significant differences in WMH, lacunes, and CMB between CSVD patients with and without Dawson’s fingers, particularly in mixed lacunes (p Table 3). Functional analysis showed no significant difference in mRS scores (1.3 ± 0.1 vs. 1.2 ± 0.1, p = 0.244). However, MoCA scores were significantly lower in CSVD patients with Dawson’s fingers compared to those without (18.9 ± 1.8 vs. 24.0 ± 0.8, p p Figure 2), indicating that Dawson’s fingers in CSVD are associated with reduced cognitive performance.
Table 3. Cerebral small vessel disease markers of patients with or without Dawson’s fingers.
| Characteristic | CSVD without Dawson’s fingers (N = 45) | CSVD with Dawson’s fingers (N = 20) | P-value |
|---|---|---|---|
| Cerebral Microbleeds, n (%) | 19 (42.2) | 15 (75.0) | 0.018 |
| Lobar CMBs | 18 | 13 | 0.105 |
| Deep CMBs | 16 | 14 | 0.015 |
| Mixed CMBs | 12 | 12 | 0.014 |
| CMB burden | 0.011 | ||
| 0 (0 CMB) | 26 | 5 | 0.017 |
| 1 (1–5 CMB) | 5 | 3 | 0.693 |
| 2 (6–15 CMBs) | 6 | 10 | 0.004 |
| 3 (>15 CMBs) | 7 | 2 | 0.710 |
| Lacunes, n (%) | 30 (66.7) | 19 (95.0) | 0.014 |
| Lobar lacunes | 24 | 17 | 0.015 |
| Deep lacunes | 21 | 18 | 0.001 |
| Mixed lacunes | 14 | 16 | |
| Lacunes burden | 0.003 | ||
| 0 (0 lacunes) | 15 | 2 | 0.067 |
| 1 (1–3 lacunes) | 14 | 2 | 0.117 |
| 2 (4–10 lacunes) | 12 | 8 | 0.383 |
| 3 (>10 lacunes) | 4 | 8 | 0.005 |
| PVWMH (Fazekas score), n (%) | 0.050 | ||
| 0–1 | 21 | 3 | 0.025 |
| 2 | 8 | 6 | 0.332 |
| 3 | 16 | 11 | 0.178 |
| DWMH (Fazekas score), n (%) | 0.054 | ||
| 0–1 | 28 | 6 | 0.030 |
| 2 | 9 | 8 | 0.127 |
| 3 | 8 | 6 | 0.332 |
| Total WMH severity (Fazekas score), n (%) | 0.027 | ||
| 0–2 | 19 | 2 | 0.011 |
| 3–4 | 16 | 9 | 0.583 |
| 5–6 | 10 | 9 | 0.080 |
| WMH volume, ml (median, IQR) | 17 (10–65) | 38 (18–75) | 0.153 |
| Severe CSO-EPVS, n (%) | 9 | 6 | 0.524 |
| Severe BG-EPVS, n (%) | 14 | 10 | 0.171 |
| Total SVD score (median, IQR) | 3 (1.5–3) | 3 (3–4) | 0.005 |
CMBs, cerebral microbleeds; CSO, centrum semiovale; BG, basal ganglia; EPVS, enlarged perivascular spaces; PVWMH, periventricular white matter hyperintensities; DMWH, deep white matter hyperintensities; CSVD, cerebral small vessel disease; IQR, Inter Quartile Range.
The CMBs were divided into lobar CMBs group, deep CMBs group, and mixed CMBs group on the basement of locations (22). CMBs in cortices or subcortical and periventricular white matter were lobar CMBs. Deep CMBs were in areas including deep white matter (corpus callosum, internal, external, and extreme capsule), deep nuclei (basal ganglia and thalamus), and infratentorial structures (brain stem and cerebellum). Mixed CMBs included both lobar and deep CMB. The scoring standard of CMB burden is as follows: 0 points for 0 CMB, 1 point for 1–5 CMBs, 2 points for 6–15 CMBs, 3 points for over 15 CMBs.
Lacunes were defined as a subcortical cavity filled with fluid having a similar signal to CSF, shaped round or ovoid, ranging from 3 to 15 mm in diameter and they were classified based on T1, T2, FLAIR images (5). Lacunes were classified in three groups: lobar lacunes (in centrum semiovale, frontal, parietal, insular/subinsular, temporal, and occipital lobes), deep lacunes (in thalamus, basal ganglia, caudate, internal, and external capsule), and mixed lacunes (including both lobar and deep) (23). The scoring standard of lacunes burden is as follows: 0 points for 0 lacune, 1 point for 1–3 lacunes, 2 points for 4–10 lacunes, 3 points for over 10 lacunes.
WMH were defined as hyperintense of variable size in the demyelination of white matter on T2/FLAIR images on MRI. Fazekas grading scale was used to assess the level of WMH.
Severe BG-EPVS or CSO-EPVS were defined as >20 in the basal ganglia or centrum semiovale.
x2-test and Mann–Whitney test to test for differences between patient with Dawson’s fingers.
Figure 2. Cognitive impairment associated with Dawson’s fingers in CSVD patients.
(A,B) Graphs illustrating significantly lower Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) scores in CSVD patients with Dawson’s fingers compared to those without, demonstrating the association between Dawson’s fingers and cognitive decline in CSVD.
Discussion
While CSVD is traditionally viewed as primarily affecting arterioles, the involvement of venules has remained a point of debate. Dawson’s fingers are a well-established imaging marker for MS. This study investigated the presence of venous abnormalities in small arteriosclerotic CSVD and found Dawson’s fingers in 30.8% of patients, suggesting venous involvement in a subset of CSVD cases. A significant correlation between Dawson’s fingers and DM in CSVD patients was identified, and the presence of Dawson’s fingers was also linked to decreased cognitive function.
CSVD is commonly considered a disease of the small artery system (24, with arteriolosclerosis due to aging and vascular risk factors being the most prevalent etiology for sporadic CSVD (1, 25). Arteriosclerosis is characterized by lumen narrowing and vessel wall thickening and is associated with CSVD clinical manifestations like cognitive decline and neuroimaging features such as CMB (26). Hypertension is also a recognized independent risk factor for CSVD (27). However, venous involvement in CSVD has been relatively understudied. Central vein sign (CVS), a venous marker, is a common imaging feature in MS but is found in only 6–7% of CSVD patients. In contrast, Dawson’s fingers, another venous sign, were present in over 30% of CSVD patients in our study. This finding underscores the potential role of venous pathology in CSVD pathogenesis.
The pathophysiology of Dawson’s fingers is related to perivenous inflammation, occurring perpendicular to the lateral ventricles along subependymal veins (28). Dawson’s fingers are valuable for the differential diagnosis of MS from its mimics and other autoimmune demyelinating diseases (18, 29, 30). Distinguishing MS from CSVD based on MRI can be challenging, especially in older patients where CSVD-related leukoencephalopathy is common (31. In this study, while Dawson’s fingers were less prevalent in CSVD (30.8%) compared to MS (69.6%), the difference was statistically significant, suggesting their continued utility in differential diagnosis. However, the diagnostic value of Dawson’s fingers in distinguishing MS from CSVD is reduced in patients with DM. Therefore, when considering the differential diagnosis of MS and CSVD, especially in DM patients, a comprehensive assessment of imaging markers is crucial.
Diabetes mellitus is a significant contributor to CSVD development, accelerating arteriolosclerosis (26). CSVD patients with DM often exhibit a higher burden of CMB, WMH, and lacunes (32, and DM is known to cause venous damage (33). Our finding of a significantly higher prevalence of Dawson’s fingers in CSVD patients with DM suggests a potential link between DM and venous abnormalities in CSVD. This observation warrants further investigation in larger longitudinal studies to confirm the relationship and explore underlying mechanisms.
This study has limitations, including its exploratory nature and relatively small sample size. Therefore, caution is advised when generalizing these findings. Future research with larger cohorts is needed to validate these results. Furthermore, this study did not delve into the pathogenic mechanisms of Dawson’s fingers in CSVD. However, the detailed clinical data analysis highlights the importance of considering venous abnormalities and the impact of diabetes in the pathophysiology of CSVD.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Tianjin Medical University and Beijing Tiantan Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
YF conceptualized the study, acquired funding, and designed the research. YF, ZZ, and LY collected the data. LY, YY, WZ, and AL analyzed the data. YF, WZ, and AL wrote the manuscript. All authors contributed to and approved the final version.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
We express our gratitude to the patients who participated in this study.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (No. 81771279), the Professor Academic Development Fund of Fujian Medical University (No. JS15012), and the Shaanxi Province Natural Science Foundation Research Project (2018JM7016).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00669/full#supplementary-material
Click here for additional data file. (13.3KB, DOCX)
References
[References]
Associated Data
Supplementary Materials
Click here for additional data file. (13.3KB, DOCX)
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon request.
All datasets generated for this study are included in the article/Supplementary Material.