INTRODUCTION
Decompensated heart failure (DHF) is a significant clinical syndrome arising from structural or functional cardiac abnormalities that impair the heart’s ability to effectively eject blood or accommodate it at physiological pressures. This leads to functional limitations and necessitates immediate medical intervention.1 Recognizing its substantial epidemiological impact and unique clinical features is crucial for effective management. This article aims to provide clinicians with a comprehensive guide to the diagnosis of DHF, emphasizing the pivotal role of clinical assessment and diagnostic studies.
EPIDEMIOLOGY
Heart failure (HF) is a global health concern with high incidence and prevalence rates. It is estimated that 1-2% of the population in developed nations suffer from HF, with this figure escalating to 10% among individuals aged 70 and above. Europe alone accounts for approximately 10 million HF patients with ventricular dysfunction and an additional 10 million with heart failure with preserved ejection fraction (HFPEF).2,3 Data from Brazil in 2012 revealed that HF accounted for 21.5% of over 1.1 million hospitalizations for circulatory system diseases, accompanied by a 9.5% in-hospital mortality rate, with 70% of cases occurring in those over 60 years old.4
Hospitalization costs for DHF constitute about 60% of the total expenditure on HF treatment.5 The 90-day mortality rate following discharge is approximately 10%, with a readmission rate of about 25% within the same period.5 Ischemic cardiomyopathy is frequently cited as the primary cause of HF.6 However, in regions like Brazil, hypertensive, Chagasic, and valvular cardiomyopathies are also significant contributors, especially in DHF-related hospitalizations.7,8 Accurate and timely diagnosis is paramount to address this widespread and costly condition.
CLASSIFICATION OF DECOMPENSATED HEART FAILURE
DHF can manifest acutely or as an exacerbation of chronic HF. Understanding these classifications is vital for appropriate diagnosis and management.8
New-Onset Acute Heart Failure
This refers to the initial presentation of HF in patients without prior HF diagnosis. It is often triggered by acute events such as myocardial infarction, hypertensive crises, or mitral chordae tendineae rupture. Typically, pulmonary congestion is present without systemic congestion, and blood volume is generally normal. Diagnosis in these cases focuses on identifying the acute trigger. Aggressive diuretic therapy is usually not the initial step; instead, treatment targets the underlying cause, such as vasodilators for hypertensive crises, revascularization for acute coronary syndrome (ACS), or surgical correction for mitral regurgitation.
Decompensated Chronic Heart Failure
This is the more common presentation of DHF, characterized by a gradual or sudden worsening of HF signs and symptoms in patients with a pre-existing HF diagnosis. This exacerbation necessitates immediate and intensified therapy. 8 Diagnosis involves recognizing the acute decompensation in the context of chronic HF. The most frequent cause is non-adherence to treatment regimens, including dietary restrictions and medication protocols. Other contributing factors include infections, pulmonary embolism, certain medications like NSAIDs, and arrhythmias. Clinical presentation often involves both pulmonary and systemic congestion with hypervolemia. Diagnostic efforts should focus on identifying the triggers for decompensation while initiating volume management with diuretics.
CLINICAL PRESENTATION
In diagnosing DHF, patient history and physical examination are invaluable. They not only confirm the syndrome but also provide critical insights into the onset of symptoms, etiology, decompensating factors (Chart 1), and prognosis.
Chart 1: Common Triggering Factors for Decompensation in Heart Failure
| Factor Category | Specific Triggers |
|---|---|
| Dietary & Lifestyle | Excessive water and salt intake, Excessive physical exertion, Excessive alcohol consumption |
| Medication Related | Non-adherence to treatment, Lack of access to medication, Inappropriate prescription/insufficient doses, Self-medication/alternative therapies, Digitalis intoxication, Water-retaining drugs (NSAIDs, steroids, etc.), Negative inotropic drugs, Myotoxic drugs |
| Cardiovascular Events | Acute atrial fibrillation or other tachyarrhythmias, Bradyarrhythmias, Systemic hypertension, Pulmonary thromboembolism, Myocardial ischemia |
| Systemic Conditions | Fever and infections, Elevated room temperature, Anemia, Nutritional deficiencies, AV fistulas, Thyroid dysfunction, Decompensated diabetes, Renal failure, Pregnancy, Depression |
| Social & Environmental | Social factors (abandonment, social isolation), IV fluid overload during hospitalization, Lack of training in HF management, Failure to provide adequate patient advice, Undetected volume overload |
Source: Bocchi et al.1
AV: arteriovenous; IV: intravenously; NSAIDs: non-steroidal anti-inflammatory drugs; HF: heart failure.
Dyspnea is the most prevalent symptom of DHF, although it lacks specificity as it occurs in various conditions. Similarly, nocturnal cough, leg edema, and pulmonary wheezes or rales are common but not definitive. However, orthopnea, paroxysmal nocturnal dyspnea, and a third heart sound are more specific indicators of HF, aiding in differential diagnosis.9 A thorough personal and family medical history, along with a review of systems, can provide crucial data for determining the underlying cause and presence of comorbidities. Identifying the etiology is essential as it informs specific treatments, predicts prognosis, and guides pharmacological management of decompensation.
Clinical-hemodynamic profiling, based on bedside physical examination, is crucial for guiding DHF treatment and risk stratification. Congestion, present in 70-80% of DHF cases, is indicated by tachypnea, pulmonary crackles, a third heart sound, elevated jugular venous pressure, peripheral edema, tender hepatomegaly, hepatojugular reflux, pleural effusion, and ascites. Poor perfusion is suggested by tachypnea, hypotension, pulsus alternans, prolonged capillary refill time, cyanosis, and altered mental status.
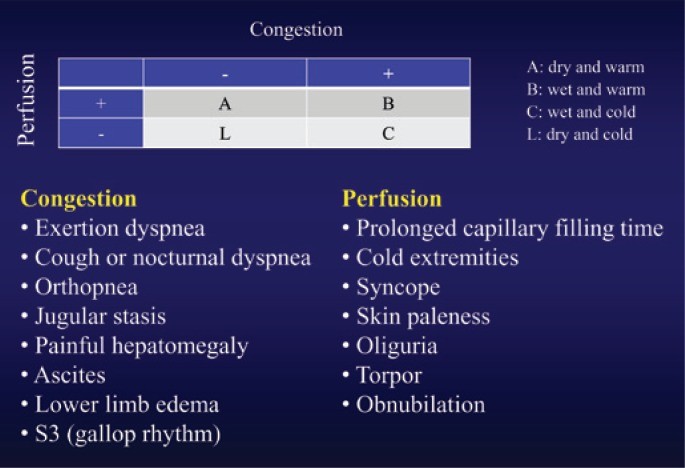
Stevenson’s algorithm10 classifies patients with congestion as “wet” and those without as “dry”. Patients with inadequate perfusion are “cold,” and those with good perfusion are “warm,” leading to four clinical-hemodynamic profiles (Figure 1):
- Profile A (“dry-warm”): Compensated.
- Profile B (“wet-warm”): Most common.
- Profile C (“wet-cold”): Poorest prognosis.
- Profile L (“dry-cold”): Less frequent.
Figure 1: Clinical Assessment of Decompensated Heart Failure
Alt text: Stevenson’s clinical-hemodynamic profiles for decompensated heart failure diagnosis, showing four quadrants: Warm & Dry (Profile A), Warm & Wet (Profile B), Cold & Wet (Profile C), and Cold & Dry (Profile L), based on congestion and perfusion status.
DIAGNOSTIC STUDIES IN DECOMPENSATED HEART FAILURE
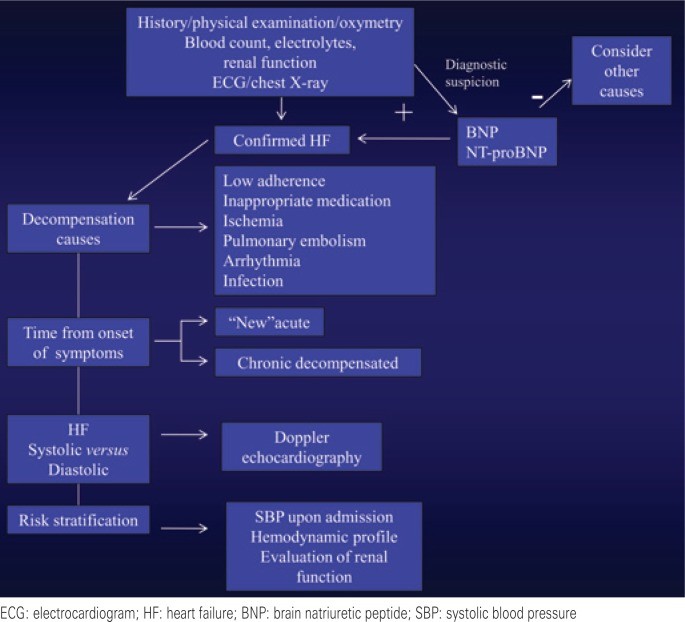
While DHF diagnosis initially relies on history and physical examination, diagnostic studies are critical for confirmation, assessing cardiac remodeling, identifying systolic and/or diastolic dysfunction, determining etiology and causes of decompensation, detecting comorbidities, and risk stratification (Figure 2).
Figure 2: Diagnostic Assessment of Decompensated Heart Failure
Alt text: Flowchart of diagnostic assessment for decompensated heart failure, including history, physical exam, ECG, chest X-ray, lab tests (BNP/NT-proBNP, electrolytes, renal function), echocardiography, and pulmonary artery catheterization.
Electrocardiography (ECG)
ECG is essential, particularly in suspected ACS. Specific ECG findings can suggest etiologies: Q waves, lack of R wave progression, and ST-segment abnormalities may indicate ischemia. Right bundle branch block with left anterior superior division block can suggest Chagas disease. Low voltage might indicate storage disease or pericardial effusion. Left bundle branch block can signify acute myocardial infarction or significant myocardial remodeling, both associated with poorer prognosis. Arrhythmias identified on ECG may be the cause of DHF and have therapeutic and prognostic implications. ECG is a crucial diagnostic tool for identifying underlying cardiac issues contributing to decompensation.
Laboratory Tests
Routine blood tests, including complete blood count, BUN, creatinine, blood glucose, electrolytes, and urinalysis, are valuable for identifying comorbidities, causes of decompensation, and guiding prognosis and treatment. In suspected ACS, myocardial necrosis markers are crucial for diagnosis; elevated levels even without obstructive coronary disease have prognostic significance. Arterial and central venous blood gases, lactate levels, and liver function tests are indicated in more severe cases. Thyroid function tests and serology for Chagas disease may also be considered based on clinical context. These tests aid in assessing the systemic impact of DHF and identifying contributing factors.
Biomarkers
Biomarkers play a significant role in both diagnosis and prognosis of DHF. Natriuretic peptides, specifically BNP and NT-ProBNP, are the most widely used and clinically established. Produced primarily in the ventricles in response to increased wall stress, their levels are elevated in both systolic and diastolic dysfunction (higher in systolic dysfunction). Measuring natriuretic peptides is particularly useful in differentiating dyspnea of cardiac origin from other causes in emergency settings.11,12 Elevated levels confirm cardiac involvement. While their value as markers of treatment response is debated,13,14 they remain valuable diagnostic and prognostic tools. Emerging biomarkers, like exhaled acetone, are also being investigated for their diagnostic and prognostic potential in DHF.15
Echocardiography
Echocardiography is the primary non-invasive imaging modality for HF diagnosis. In DHF, it helps determine etiology, prognosis, and the type of dysfunction (systolic, diastolic, or both). It provides information on chamber size and function, valvular abnormalities, regional wall motion abnormalities, and pericardial disease. Echocardiography can reveal the extent of cardiac remodeling and identify causes of decompensation such as pericardial effusion, pulmonary embolism, or acute ischemia. Hemodynamic echocardiography can further guide therapy by assessing cardiac pressures and volumes.16
Pulmonary Artery Catheterization
Pulmonary artery catheterization allows for direct measurement of intracardiac and intravascular pressures and microhemodynamic parameters. It is particularly useful in managing complex DHF cases, especially those with shock, and for assessing pulmonary vascular resistance in patients considered for cardiac transplantation. However, the ESCAPE trial showed no benefit of routine pulmonary artery catheter use in DHF management without cardiogenic shock.17 Its use is reserved for select, complicated diagnostic scenarios.
RISK STRATIFICATION IN DECOMPENSATED HEART FAILURE
Several factors are associated with a poorer prognosis in DHF (Chart 2). Systolic blood pressure (SBP) and renal function are particularly significant (ADHERE registry).18 Patients with BUN >90mg/dL and SBP <115mmHg on admission have a significantly elevated in-hospital mortality risk (21.9%) compared to those without these factors (2.14%). Cardiorenal syndrome in DHF involves renal hypoperfusion from myocardial dysfunction or hypovolemia and systemic congestion with renal venous hypertension.19 An increase in creatinine levels by 0.3mg/dL at admission is also linked to higher mortality.20 Risk stratification is crucial for guiding the intensity of diagnostic and therapeutic interventions.
Chart 2: Factors Associated with Worse Prognosis in Decompensated Heart Failure
| Prognostic Factor Category | Specific Factors |
|---|---|
| Patient Demographics & History | Age (above 65 years), Cachexia |
| Laboratory Findings | Hyponatremia (sodium <135mEq/L), Impaired renal function, Anemia (hemoglobin <12g/dL) |
| Clinical Signs | Signs of peripheral hypoperfusion, Persistent congestion, Persistent third heart sound |
| ECG Findings | Complete left bundle branch block, Atrial fibrillation, Sustained ventricular tachycardia or ventricular fibrillation |
| Echocardiographic Findings | Restrictive filling pattern on Doppler |
| Biomarker Levels | Persistent elevation of natriuretic peptide levels despite treatment |
Source: Bocchi et al.1
DHF: decompensated heart failure.
TREATMENT OF DECOMPENSATED HEART FAILURE
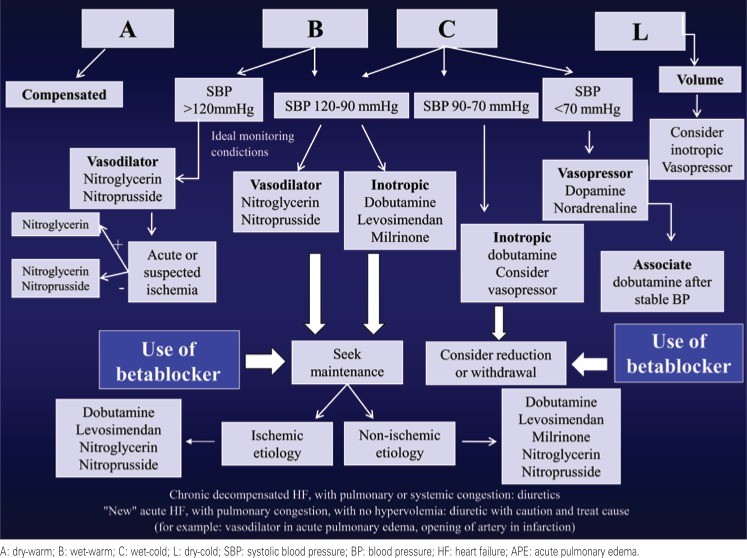
The primary goal in treating DHF is to achieve hemodynamic and symptomatic improvement. Secondary objectives include preserving or improving renal function, preventing further myocardial damage, modulating neurohormonal and inflammatory activation, and managing comorbidities that exacerbate the syndrome.[21](#B21] Diagnosis, particularly the clinical-hemodynamic profile, guides the treatment strategy (Figure 3).
Figure 3: Treatment Algorithm for Decompensated Heart Failure
Alt text: Algorithm for decompensated heart failure treatment based on clinical-hemodynamic profiles (Warm/Dry, Warm/Wet, Cold/Wet, Cold/Dry), guiding diuretic, vasodilator, and inotropic agent use.
Most DHF patients present with congestion and adequate perfusion (“wet-warm” – Profile B), requiring diuretics and vasodilators. In cases of worsening renal function, inotropes may be considered, especially if SBP is between 90-120mmHg. Patients with congestion and poor perfusion (“wet-cold” – Profile C) require inotropes and diuretics; vasodilators can be used with careful BP monitoring. Poor perfusion without congestion (“dry-cold” – Profile L) is less common and may respond to volume repletion; inotropes might be necessary. Hospitalization criteria are outlined in Chart 3.1
Chart 3: Criteria for Hospitalization in Decompensated Heart Failure
| Hospitalization Urgency | Criteria |
|---|---|
| Immediate | Pulmonary edema or respiratory distress at rest, Oxygen saturation <90%, Heart rate >120 bpm (excluding chronic atrial fibrillation), Systolic blood pressure <90 mmHg, Mental status changes due to hypoperfusion, DHF with ACS, New-onset acute HF |
| Urgent | Severe liver distension, massive ascites, or anasarca, DHF with acutely decompensated non-cardiac conditions (pulmonary disease, renal dysfunction), Rapidly progressing HF symptoms |
| Consider | Rapid serum sodium drop (<130 mEq/L), Rapid creatinine elevation (>2.5mg/dL), Persistent symptoms at rest despite optimized oral treatment, Comorbidities likely to worsen HF |
Source: Bocchi et al.1
HF: heart failure.
CLINICAL TREATMENT
Non-Pharmacological Measures
While evidence is limited, personalized water and sodium restriction is recommended, with daily weight monitoring to assess treatment response.
Monitoring and Ventilatory Support
Unstable patients require continuous ECG, non-invasive blood pressure, and oximetry monitoring. Hypoxia should be corrected to ensure adequate oxygenation and reduce respiratory effort. Non-invasive ventilation (CPAP or BiPAP) has shown to reduce intubation rates and mortality, particularly in acute pulmonary edema.22
Vasodilators
Vasodilators reduce preload and afterload, decreasing myocardial oxygen demand compared to inotropes. Studies suggest lower mortality in DHF with vasodilator use.23,24 They are indicated for pulmonary and systemic congestion (Profiles B and C) and in patients with poor perfusion and SBP >90mmHg (Profile C). Intensive SBP monitoring and dose titration are necessary. Oral vasodilators and diuretics are suitable for Profile B patients asymptomatic at rest with SBP >120mmHg. Intravenous vasodilators are indicated for dyspnea at rest and acute pulmonary edema. Avoid vasodilators in hypotension (SBP <90mmHg).
Nitroglycerin25
Intravenous nitroglycerin is short-acting. Low doses (30-40 µg/min) cause venodilation, while higher doses (250 µg/min) induce arteriolar dilation. Benefits include pulmonary congestion relief and increased coronary flow, making it useful in DHF with ACS. Common side effects are headache and nausea.
Sodium Nitroprusside25
This potent arterial and venous vasodilator reduces preload and afterload, improving biventricular function. Usual dose is 0.5-10 µg/kg/min. Avoid in ACS due to risk of reduced coronary perfusion pressure and “coronary steal”. Hypotension is a common side effect, potentially worsening renal function. Gradual withdrawal is recommended to prevent rebound effects, transitioning to oral vasodilators. Prolonged high-dose use, especially in renal or hepatic dysfunction, carries a risk of thiocyanate and cyanide toxicity.
Inotropic Agents26
In patients with low cardiac output (Profiles L and C), inotropes may improve tissue perfusion.5 While they improve hemodynamics, they haven’t consistently improved outcomes and are linked to ischemia. Short-term use is appropriate for hemodynamic deterioration, severe chronic HF, elevated nitrogenous waste, and diuretic/vasodilator resistance. They are also valuable for hemodynamic support pre-transplant or revascularization and in cardiogenic shock. Contraindicated in HFPEF.
Dobutamine
This beta-adrenergic agonist increases intracellular calcium, leading to inotropy and chronotropy. Ischemia and arrhythmias are common side effects due to increased oxygen demand. Despite mortality concerns, dobutamine is frequently used.27 It improves cardiac output, usually without hypotension. Dose range is 3-20 µg/kg/min for Profiles C and L. In hypotensive patients (SBP <70mmHg), norepinephrine may be added.
Milrinone
A phosphodiesterase-III inhibitor with inodilator properties, milrinone increases cardiac contractility and causes vasodilation by increasing intracellular cAMP and calcium. It increases cardiac output and reduces vascular resistance. Studies suggest increased mortality, especially in ischemic etiologies.28 It may be an option for patients on beta-blockers as its mechanism is independent of the adrenergic system. Arrhythmogenic potential is a notable side effect. Recommended dose is 0.3-0.75 µg/kg/min. Loading dose is not advised due to hypotension risk. Adjust dose in renal failure.
Levosimendan
A calcium sensitizer, levosimendan enhances troponin C’s calcium sensitivity without increasing intracellular calcium load. It provides hemodynamic benefits similar to other inotropes and has vasodilator effects via ATP-dependent potassium channel activation.29 It is effective and safe in various DHF etiologies, especially in patients on beta-blockers.30,31 Long half-life active metabolites extend its effect up to 7 days. Hypotension, headache, and arrhythmias are major side effects. The SURVIVE study32 showed no mortality difference between levosimendan and dobutamine. A maintenance dose of 0.1 µg/kg/min over 24 hours, without loading, may reduce side effects.
Vasopressors
Norepinephrine and dopamine are common vasopressors used for symptomatic hypotension despite volume correction. Norepinephrine, primarily alpha-adrenergic, causes significant vasoconstriction with mild heart rate increase and increased myocardial oxygen demand. In DHF, it is used with inotropes for cardiogenic shock refractory to other support. Dopamine has both beta and alpha effects (alpha at >10 µg/kg/min). It also increases heart rate, myocardial oxygen consumption, and arrhythmia risk.33
Beta-blocker discontinuation in DHF remains debated. In most “wet-warm” (Profile B) patients, stopping or reducing beta-blockers is unnecessary. Abrupt withdrawal may worsen sympathetic activation, potentially increasing apoptosis and arrhythmias, and reducing survival.34,35 When inotropes are needed in patients on beta-blockers, milrinone or levosimendan, which don’t rely on beta-adrenergic receptors, may be preferred. In cardiogenic shock with chronic high-dose beta-blocker use, dobutamine is crucial, and beta-blocker reduction or discontinuation should be considered.
Management of Hypervolemia
Diuretics36
Diuretics reduce extracellular fluid, filling pressures, and cardiac chamber size, improving cardiac performance and quickly relieving congestion symptoms. Adverse effects include hypotension, electrolyte imbalances, renal dysfunction, and neurohormonal activation due to hypovolemia. Rational diuretic use aims to preserve renal function using the lowest effective dose. Loop diuretics like furosemide and bumetanide are preferred. In diuretic-resistant patients, continuous intravenous infusion or combination with thiazides and aldosterone antagonists may be used. In new-onset acute HF, diuretics should be used cautiously due to potential normovolemia or hypovolemia.37
Hypertonic Saline Solution
In patients resistant to oral loop diuretics, hypertonic saline (150mL of 1.4-4.6% NaCl, adjusted to serum sodium) followed by high-dose intravenous furosemide improved renal function compared to furosemide alone.38 Brazilian studies also show renal function preservation and increased diuresis.39,40 Suitable patients include those with hyponatremia, systemic congestion, and diuretic-induced renal function worsening.
Ultrafiltration
Ultrafiltration via a peripheral IV line can be used in hypervolemic patients, those with frequent rehospitalizations, or in a day-hospital setting. One study showed weight reduction and fewer 90-day rehospitalizations compared to diuretics, despite initial creatinine elevation.41 However, a more recent trial showed no benefit.42
SURGICAL TREATMENT
In DHF secondary to ACS, coronary angiography is essential and may guide revascularization (percutaneous or surgical). Acute valvular heart disease may also require percutaneous or surgical intervention.7
For DHF patients, particularly those with cardiogenic shock unresponsive to medical therapy, ventricular assist devices (VADs) may be considered. Short-term devices (intra-aortic balloon pump, ECMO, Impella™, etc.) can serve as a bridge to recovery, decision, or longer-term VAD. Long-term VADs are bridges to transplantation or destination therapy when transplantation is contraindicated.43
Patients with refractory HF and frequent DHF hospitalizations may be candidates for VADs or cardiac transplantation.44
CONCLUSION
DHF is a common cause of hospitalization with high rehospitalization and mortality risks. Accurate and timely diagnosis, including clinical hemodynamic profiling, is crucial for guiding effective therapy, ranging from non-pharmacological and pharmacological approaches to advanced interventions like VADs and cardiac transplantation.