Introduction
Surgical resection remains a cornerstone treatment for early-stage lung cancer, yet global guidelines diverge on the necessity of preoperative histological confirmation. While some advocate for mandatory preoperative diagnosis to avoid unnecessary surgeries and refine treatment strategies like sublobar resections, others suggest that in cases of high clinical suspicion, surgery can proceed without prior biopsy, relying on intraoperative pathological diagnosis (IOD). This discrepancy leads to variations in clinical practice and raises questions about optimal patient care. This article delves into the critical concept of preoperative diagnosis in lung cancer surgery, exploring its definition, current practices, and implications for patient outcomes.
The European Society for Medical Oncology (ESMO) guidelines emphasize obtaining pathological diagnosis before curative treatment unless multidisciplinary consensus deems biopsy risks unacceptable. Conversely, the United States’ National Comprehensive Cancer Network (NCCN) guidelines suggest biopsies are not always needed for clinically suspected early-stage lung cancer, provided intraoperative tissue diagnosis is performed. Similarly, the American College of Chest Physicians (ACCP) and British Thoracic Society (BTS) endorse upfront surgery when malignancy probability exceeds 65% and 70%, respectively. These varied recommendations highlight the ongoing debate surrounding the necessity of preoperative diagnosis, and what it truly means in the context of lung cancer management.
Defining preoperative diagnosis is crucial in understanding these guideline variations. In essence, it refers to the histological or cytological confirmation of lung cancer before the primary surgical resection. This confirmation typically involves procedures like bronchoscopy, needle biopsies (CT-guided or ultrasound-guided), or less commonly, surgical biopsies performed prior to definitive resection. The aim is to establish a definitive cancer diagnosis, determine the histological subtype, and assess potential staging information to guide surgical planning and avoid unnecessary interventions for benign conditions.
The debate around Define Preoperative Diagnosis is fueled by several factors. Studies reveal a 5–20% risk of benign findings in surgeries performed without preoperative pathological confirmation, exposing patients to unnecessary procedures. Moreover, the increasing use of sublobar resections for early-stage lung cancer necessitates accurate preoperative pathological classification. Histological subtypes, such as solid or micropapillary adenocarcinoma, can influence recurrence risk after sublobar resection compared to lobectomy, making preoperative knowledge of these subtypes highly relevant. The accuracy of intraoperative frozen section (FS) in identifying these high-risk features remains a subject of discussion.
Given these conflicting guidelines, variations in surgical care are anticipated. Prior research has indicated quality disparities in treatment delivery and unwarranted care variation in lung cancer management. This study aims to quantify the proportion of lung cancer resections performed in the Netherlands without preoperative diagnosis, using the intraoperative pathological diagnosis (IOD) rate as a proxy. Furthermore, it seeks to identify factors influencing IOD frequency and explore variations across hospitals, particularly for small-sized early-stage tumors and sublobar resections. The hypothesis is that IOD rates are higher for small peripheral tumors undergoing limited resections due to diagnostic accessibility challenges and guideline ambiguities.
Methods
This retrospective cohort study analyzed data from the Netherlands National Cancer Registry (NNCR) spanning 2010 to 2015. The study included 10,226 patients undergoing surgical treatment for primary invasive lung cancer. IOD was defined when the diagnosis date coincided with the first surgical intervention date, suggesting the absence of preoperative diagnosis. Data extracted from the NNCR included demographics, diagnosis (pathology, stage), treatment (surgery type), and hospital details. Patients under 18, those with main bronchi cancers, surgeries performed abroad or in hospitals that ceased lung surgery during the study period, and those with synchronous or metachronous tumors were excluded to maintain population homogeneity. Wedge resections were also excluded due to unclear intent (diagnostic vs. curative) in the NNCR database.
Variables analyzed included age, gender, pathological subtype (adenocarcinoma, squamous cell carcinoma, small cell lung cancer, large cell carcinoma, and others), surgery type (segmentectomy, lobectomy, bilobectomy, pneumonectomy), clinical T and N stages, prior cancer history (excluding non-melanoma skin cancer), and referral status (diagnosis and surgery in different hospitals). Statistical analysis using Stata 14 involved tabulations, chi-square tests for subgroup differences, and hierarchical logistic regression to identify predictive parameters for IOD, accounting for hospital-level clustering.
Results
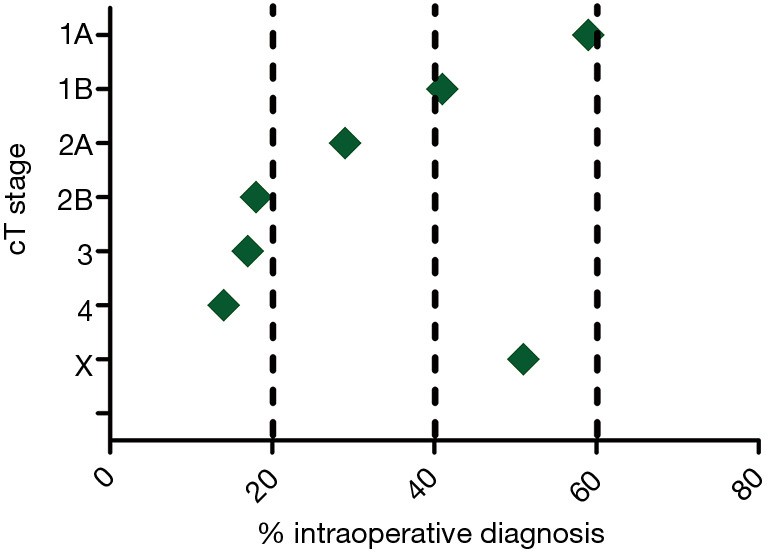
Of the 10,226 patients analyzed across 43 hospitals, 36% underwent surgery classified as IOD, indicating a lack of preoperative diagnosis. This proportion remained consistent over the study period and was not significantly influenced by age or gender. However, IOD rates showed a strong inverse correlation with tumor size, decreasing from 59% for cT1a tumors to 17% and 14% for cT3 and cT4 tumors, respectively. Adenocarcinoma exhibited the highest IOD rate (41%) among pathological subtypes, and IOD frequency varied significantly with surgery extent: 57% for segmentectomy, 39% for lobectomy, and 11% for pneumonectomy. Patients referred from a different diagnostic hospital had a lower IOD rate (27%) compared to those diagnosed and operated in the same hospital (39%).
Sub-analysis of segmentectomy patients (n=173) revealed adenocarcinoma as the most common pathology (65%) with a 57% IOD rate. Squamous cell carcinoma showed a higher IOD rate (72%) in this group. Multivariable analysis confirmed pathology, tumor location, diagnosis year, surgery type, TNM stage, and referral status as independent predictors of IOD. Significant residual variation in IOD rates between hospitals persisted even after adjusting for these factors.
Discussion
This study reveals that over one-third of lung cancer resections in the Netherlands are performed without preoperative diagnosis, highlighting a substantial reliance on IOD. This finding aligns with limited existing data suggesting approximately 30% of lung cancer cases lack preoperative pathological confirmation. This study provides crucial nationwide, population-based data on IOD rates in surgically treated lung cancer patients.
The absence of preoperative diagnosis, leading to IOD, presents potential challenges. Firstly, it raises the risk of unnecessary surgery for benign conditions. While this study, using cancer registry data, couldn’t quantify this risk directly, prior studies report benign findings in 5–20% of surgeries without preoperative diagnosis. Secondly, IOD necessitates intraoperative diagnostic procedures, typically frozen section (FS) analysis of wedge resections. However, not all nodules are amenable to wedge resection, and direct lobectomy without FS occurs in some cases, potentially leading to unnecessary resections of benign lesions. FS accuracy is also not absolute, with reported false-negative and indeterminate rates, and limitations in differentiating primary NSCLC from metastases intraoperatively.
Thirdly, the increasing application of sublobar resections for small tumors accentuates the importance of preoperative diagnosis. Diagnostic wedge resections can complicate subsequent segmentectomies, while upfront segmentectomy carries higher risks if the lesion is benign. Furthermore, FS has limited accuracy in identifying high-risk histological subtypes and features crucial for sublobar resection decisions. Paradoxically, this study found higher IOD rates associated with smaller tumors, adenocarcinoma, and segmentectomy, suggesting a potential disconnect between guideline recommendations and clinical practice, especially concerning the value of preoperative diagnosis in these scenarios.
The drawbacks of IOD must be weighed against the risks and accuracy of preoperative biopsies. While CT-guided biopsies carry pneumothorax risks, newer techniques like navigation bronchoscopy offer lower complication rates. However, navigation bronchoscopy was not widely adopted in the Netherlands during the study period. Improving the yield and accessibility of less invasive preoperative diagnostic methods is crucial to reducing reliance on IOD and enhancing preoperative diagnosis rates.
Study limitations include the use of cancer registry data, excluding benign cases and limiting analysis of preoperative workup and postoperative outcomes. Future research using broader surgical databases is planned to address these gaps. Despite these limitations, the study highlights the significant IOD rates and inter-hospital variation, underscoring the need for standardization in diagnostic approaches. With lung cancer screening programs increasing the detection of small nodules and sublobar resection becoming more prevalent, optimizing preoperative diagnosis and minimizing unwarranted variations in practice are crucial for improving patient-tailored surgical care. National and international consensus on guidelines regarding define preoperative diagnosis in lung cancer is warranted to reduce practice variability and ensure uniform high-quality care.
Conclusion
This nationwide study reveals that a significant proportion of lung cancer surgeries in the Netherlands are performed without preoperative diagnosis, as indicated by high IOD rates and substantial inter-hospital variation. These findings serve as a benchmark for quality improvement initiatives. Addressing the variation in IOD rates and promoting consistent approaches to preoperative diagnosis are essential steps towards achieving uniform, high-quality surgical care for lung cancer patients, particularly in the context of sublobar resection strategies and the increasing detection of early-stage disease. Further research should focus on optimizing preoperative diagnostic pathways and evaluating the impact of define preoperative diagnosis practices on patient outcomes.