1. Introduction
Dermatophytes represent a globally prevalent group of fungal pathogens, predominantly causing superficial infections of the skin, hair, and nails in both humans and animals [1,2,3]. With an estimated lifetime risk of 10–20% for developing dermatophytosis, and foot infections being the most common manifestation [4,5], these infections pose a significant global health concern. The economic burden of dermatophyte infections is substantial, with treatment costs reaching approximately $500 million annually worldwide [6]. Accurate and timely Dermatophytosis Diagnosis is paramount for effective patient management and the implementation of targeted therapeutic strategies.
Clinical differentiation of dermatophytosis from other dermatological conditions can be challenging due to overlapping clinical presentations [7]. This diagnostic complexity is further amplified in resource-limited settings, where access to healthcare and advanced diagnostic tools is often restricted [8,9]. Misdiagnosis can lead to severe consequences, particularly in immunocompromised individuals, where infections can progress to invasive and disseminated forms [10,11,12,13]. Furthermore, recent taxonomic reclassifications within medical mycology, driven by genetic analysis, have introduced name changes for numerous dermatophyte species [14,15]. These changes necessitate updates in clinical databases and may affect the specificity of certain diagnostic techniques, emphasizing the importance of robust and adaptable dermatophytosis diagnosis protocols.
This review aims to provide a comprehensive overview of dermatophytosis diagnosis, encompassing: (1) a discussion of dermatophyte classification relevant to human and animal infections, highlighting recent taxonomic changes; (2) an in-depth analysis of current diagnostic techniques employed in clinical settings for dermatophyte detection; (3) an exploration of promising future directions for dermatophytosis diagnosis, including novel metabolite-based assays; and (4) a focused clinical review of Microsporum canis, a prevalent zoophilic dermatophyte frequently encountered in clinical practice due to its zoonotic potential [16,17]. The emphasis throughout will be on enhancing the understanding and application of effective dermatophytosis diagnosis in diverse clinical scenarios.
2. Understanding Dermatophytes: Biology and Classification
Dermatophytes are a specialized group of fungi characterized by their ability to invade and degrade keratinized tissues such as hair, skin, nails, and feathers [18]. Phylogenetically, they belong to the Ascomycota phylum, Eurotiomycetes class, Onygenales order, and Arthrodermataceae family [15,19]. Currently, seven genera of dermatophytes are recognized: Trichophyton, Epidermophyton, Nannizzia, Paraphyton, Lophophyton, Microsporum, and Arthroderma [15]. The taxonomy of dermatophytes has undergone significant revisions as molecular approaches have complemented traditional morphology-based classification [14,15]. The shift towards a “One Fungus = One Name” system has consolidated teleomorph (sexual form) and anamorph (asexual form) names, simplifying species identification and impacting dermatophytosis diagnosis nomenclature [20,21].
Dermatophytes are further categorized based on their primary habitat: anthropophilic (human-associated), zoophilic (animal-associated), and geophilic (soil-associated) [14,15,22]. This ecological classification is clinically relevant as it can influence the clinical presentation and transmission patterns of dermatophytosis. Accurate dermatophytosis diagnosis often necessitates species-level identification, particularly given the recent reclassifications and shifts in habitat associations within dermatophyte genera [22]. Molecular characterization plays an increasingly crucial role in refining dermatophyte taxonomy and improving the precision of dermatophytosis diagnosis.
2.1. The Role of Molecular Characterization in Dermatophytosis Diagnosis
Molecular techniques have become indispensable in dermatophyte research, significantly advancing our understanding of their classification, epidemiology, and genetics. Dermatophyte genomes, ranging from 2.25 Mb to 24.1 Mb, have been subject to extensive analysis, with complete genomes of key species like Microsporum canis now annotated [23]. These haploid genomes, characterized by low repetitive DNA content [24], harbor a conserved core genome shared across different ecological groups, alongside species-specific genetic heterogeneity [23]. M. canis, for instance, exhibits the highest degree of genetic uniqueness among dermatophytes, possessing 943 unique genes [23].
Phylogenetic analyses based on gene regions like the internal transcribed spacer (ITS) and partial β-tubulin have revolutionized dermatophyte classification [22]. This molecular re-evaluation has led to the expansion of genera like Nannizzia, Paraphyton, Lophophyton, and Trichophyton, while condensing Arthroderma and Microsporum [22]. Clinically significant species, such as Nannizzia persicolor (formerly Arthroderma persicolor), Nannizzia nana (formerly Microsporum nanum), Trichophyton mentagrophytes (formerly Arthroderma vanbreuseghemii), and Nannizzia gypsea (formerly Microsporum gypseum), have been renamed [22]. These taxonomic revisions underscore the necessity for molecular methods in accurate dermatophytosis diagnosis, especially at the species level, to guide appropriate treatment strategies.
Epidemiological studies leveraging mitochondrial DNA sequences [26], rDNA non-transcribed spacer regions [27], RAPD [28,29], microsatellites [30,31,32], and RNA sequencing [33] have further elucidated dermatophyte genetic diversity and population structures. Microsatellite polymorphism analysis, in particular, offers a rapid and cost-effective approach for strain differentiation, facilitating genotypic comparisons in large sample sets. This technique has been instrumental in studying M. canis populations globally, revealing intraspecies genetic variations [30,31,32,34,35,36,37]. The enhanced genetic understanding of dermatophytes, propelled by molecular diagnostics, is crucial for improving dermatophytosis diagnosis and developing targeted interventions.
2.2. Initiation of Dermatophyte Infections: Implications for Diagnosis
Dermatophyte infections begin with the adhesion of arthroconidia, the infectious propagules, to keratinized tissues [42,43,44] (Figure 1). Within hours of contact, arthroconidia adhere to the epidermis and initiate germination within the stratum corneum [42,43,44] (Figure 1). Germ tubes penetrate the stratum corneum, and as keratin degradation progresses, the local pH becomes more alkaline, facilitating fungal protease activity [45]. Hyphal growth and tissue invasion ensue, with arthroconidia production commencing within approximately 7 days, enabling fungal spread [46] (Figure 1).
Figure 1. Dermatophytosis infection initiation process.
Intact, healthy tissue is generally resistant to dermatophyte invasion due to host immune defenses [2,18,47,48,49]. Predisposing factors, such as young age, immunosuppression, nutritional deficiencies, skin trauma, and warm, humid environments, are often necessary for infection establishment [2,16,18,48,49]. Understanding the early stages of dermatophyte infection is crucial for effective dermatophytosis diagnosis and intervention, particularly in identifying targets for early diagnostic assays.
2.3. Dermatophyte Viability and Environmental Persistence: Implications for Transmission and Diagnosis
Dermatophytosis can arise from direct contact with infected animals or humans, as well as from exposure to viable environmental conidia. Arthroconidia contamination is common in environments frequented by infected individuals, such as animal shelters and livestock facilities [51,52,53]. Public spaces like swimming pools, nail salons, and wrestling mats also pose risks due to environmental contamination [54,55,56]. Arthroconidia can remain viable in the environment for extended periods, up to 4.5 years under laboratory conditions, depending on the dermatophyte species [57,58,59,60]. This prolonged viability underscores the importance of environmental control and hygiene in preventing dermatophytosis transmission and complicates dermatophytosis diagnosis interpretation, as environmental contamination may lead to false positives in certain assays.
Long-term preservation techniques, such as lyophilization, cryopreservation, and specialized cryopreservation kits (e.g., Microbank), are crucial for maintaining dermatophyte isolates for research and diagnostic reference purposes [61,63,64,65,66,67]. Understanding dermatophyte viability in both clinical and environmental contexts is essential for improving decontamination protocols, managing outbreaks, and ensuring the reliability of dermatophytosis diagnosis through culture-based and molecular methods.
3. Dermatophyte Classification and Clinical Relevance for Diagnosis
Dermatophytes are broadly classified into anthropophilic, zoophilic, and geophilic groups based on their primary habitat [14,15,22]. While these categories are helpful, the lines can blur as some species adapt to new hosts, shifting their ecological preference [14,16,68,69,70]. This classification is clinically significant because the type of dermatophyte can influence the clinical presentation and severity of infection [15,24]. Over 40 dermatophyte species across these categories are known to cause infections in humans [14,24]. Accurate dermatophytosis diagnosis often requires considering the likely etiological agent based on clinical presentation and epidemiological context.
3.1. Anthropophilic Dermatophytes: Diagnostic Considerations
Anthropophilic dermatophytes, adapted to humans, are believed to have evolved from geophilic ancestors [14,68,69]. While primarily human pathogens, they can occasionally infect animals through anthropo-zoonotic transmission [71,72]. Approximately 10 species fall into this group, predominantly within the Trichophyton and Epidermophyton genera [15]. Trichophyton rubrum, Trichophyton interdigitale, and Epidermophyton floccosum are the most frequent causes of anthropophilic dermatophytosis, with T. rubrum being the most globally widespread human dermatophyte [73,74]. These species are highly adapted to the human host, often eliciting a subdued immune response and chronic, less inflammatory infections [24]. They typically reproduce asexually, with only one mating type documented per species [15,75,76, suggesting a loss of sexual reproduction due to adaptation to the stable human niche [77].
Anthropophilic dermatophytes exhibit tropism for specific body sites, leading to various clinical presentations. Tinea pedis (“athlete’s foot”) is a common foot infection [24,78], while other localized forms include tinea capitis (scalp), tinea unguium (nails), tinea barbae (beard), tinea faciei (face), tinea corporis (body), tinea manuum (hands), and tinea cruris (groin) [18] (Figure 2). T. rubrum is the major cause of tinea pedis [1,24,25], and T. interdigitale is also a frequent agent [15,74]. T. tonsurans is a primary cause of tinea capitis globally [24,79] (Figure 2, Table 1). Tinea capitis favosa, caused by Trichophyton schoenleinii, is a rarer form seen mainly in children and adolescents [74,80,81]. Geographic and socioeconomic factors influence infection distribution, with tinea pedis more common in developed countries and tinea capitis in developing regions [23]. Age also plays a role, with tinea unguium prevalent in older adults and tinea capitis in children [82]. Tinea unguium, or onychomycosis, encompasses all fungal nail infections, often caused by T. rubrum and zoophilic Trichophyton mentagrophytes [18]. Tinea barbae is mainly caused by zoophilic dermatophytes (T. mentagrophytes, T. verrucosum) and anthropophilic T. rubrum [68,84,85]. Tinea corporis etiology depends on transmission route; human-to-human cases are often due to T. rubrum and T. tonsurans, while animal contact can lead to M. canis infection [86]. Tinea faciei is a variant of tinea corporis, commonly caused by T. rubrum, T. mentagrophytes, T. tonsurans, or M. canis [87,88,89] (Table 1). T. rubrum and E. floccosum are primary agents of tinea cruris (“jock itch”) [18]. Tinea manuum is usually caused by T. rubrum or M. canis and is frequently associated with tinea pedis [18,90,91] (Figure 2, Table 1). While anthropophiles are the major cause of human dermatophytosis, zoonotic infections from zoophilic dermatophytes are also significant.
Figure 2. Human dermatophytosis classification based on anatomical location.
| Classification | Species | Primary Host/Habitat | Main Types of Infection | Geographical Distribution | Reference |
|---|---|---|---|---|---|
| Anthropophilic | Trichophyton rubrum | Humans | Tinea pedis, tinea unguium,tinea cruris, tinea faciei, tinea corporis, tinea manuum, tinea barbae | Worldwide | [1,18,24,25,74,86,87,89] |
| Trichophyton tonsurans | Humans | Tinea capitis, tinea corporis, tinea faciei | Worldwide | [24,74,79,86] | |
| Epidermophyton floccosum | Humans | Tinea cruris | Worldwide | [74] | |
| Trichophyton digitale | Humans | Tinea pedis | Worldwide | [74,92] | |
| Trichophyton schoenleinii | Humans | Tinea capitis favosa | Asia, Europe, Africa | [74,81] | |
| Zoophilic | Microsporum canis | Cats | Ringworm | Worldwide | [16,74,93] |
| Nannizzia persicolor (former name Arthroderma persicolor) | Voles, bats | Ringworm | Africa, Australia, Europe, North America | [74,94,95] | |
| Nannizzia nana (former name Microsporum nanum) | Pigs | Ringworm | Worldwide | [18,74,96] | |
| Trichophyton equinum | Horses | Ringworm | Worldwide | [74,97] | |
| Trichophyton mentagrophytes (former name Arthroderma vanbreuseghemii) | Mice, guinea pigs | Ringworm | Worldwide | [74,93] | |
| Trichophyton verrucosum | Cattle | Ringworm | Worldwide | [74,98,99] | |
| Geophilic | Nannizzia gypsea (former name Microsporum gypseum) | Soil | Ringworm (animals), tinea capitis/tinea corporis (humans) | Worldwide | [16,19,74,100] |
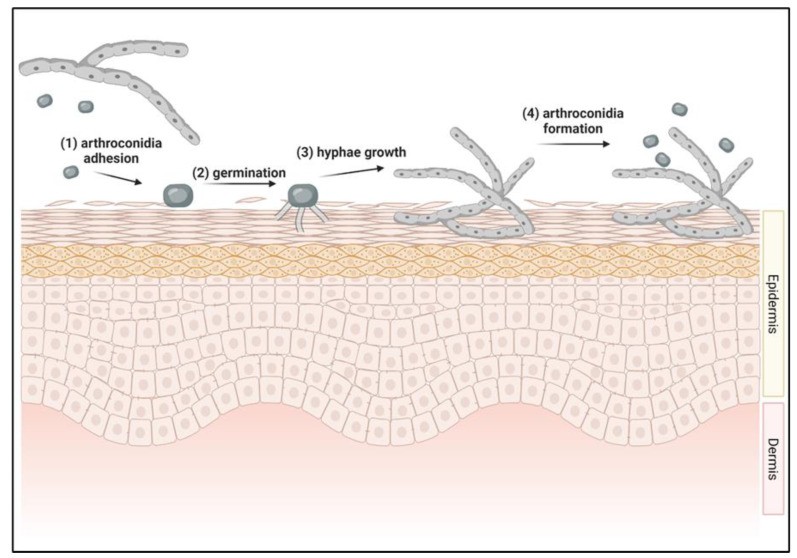
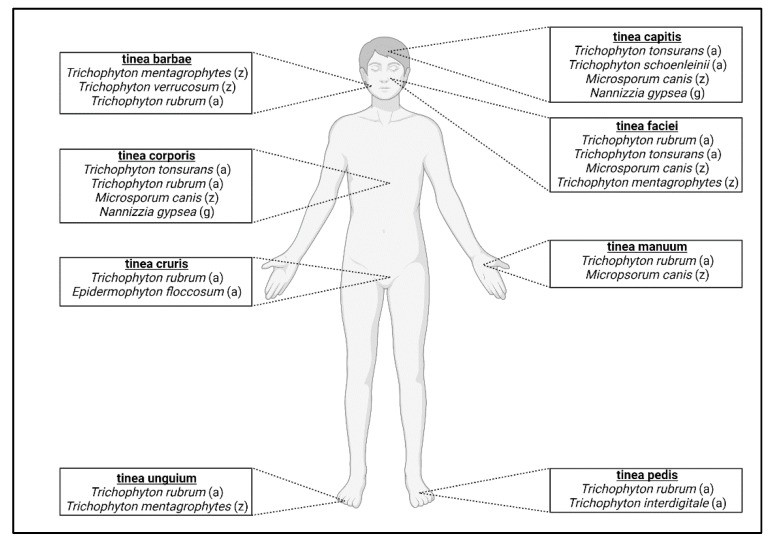
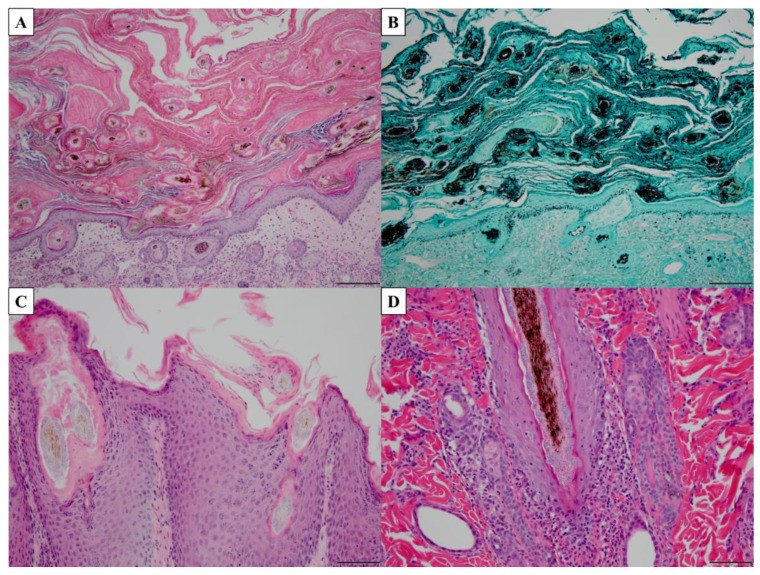
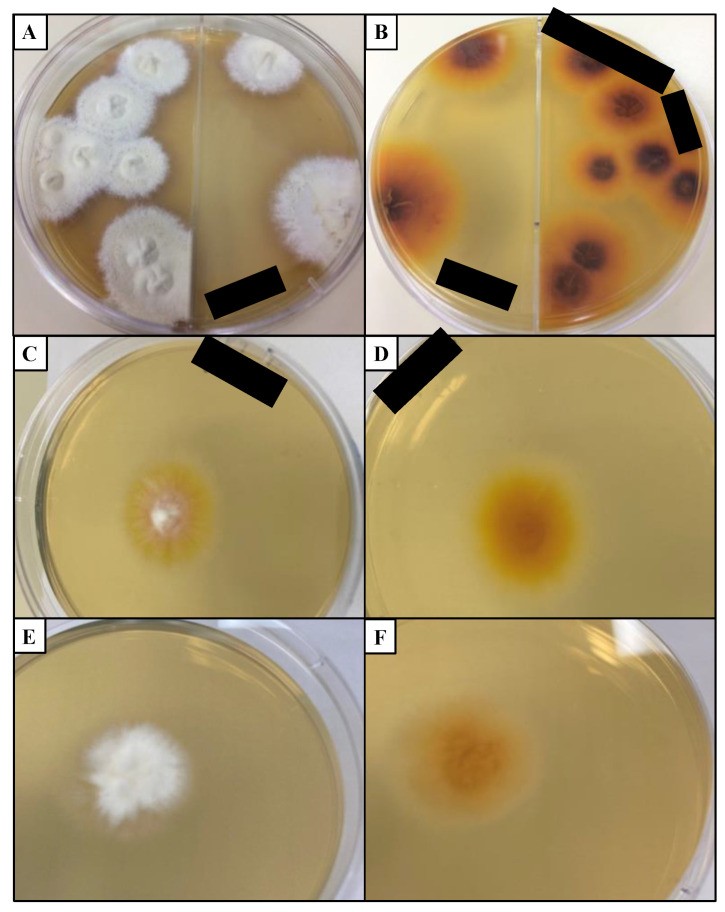
Table 1. Common dermatophytes causing human and animal infections.
A newly emerged anthropophilic species, Trichophyton indotineae, is becoming increasingly prevalent, causing recalcitrant infections with some isolates exhibiting terbinafine resistance [101,102]. This species can cause various dermatophytoses, including tinea pedis, unguium, cruris, corporis, and faciei [102,103]. Current treatment options involve alternative antifungals like itraconazole, but reduced sensitivity has been reported in some strains [102,104]. The spread of T. indotineae highlights the evolving landscape of dermatophytosis and the need for continuous surveillance and adaptation of dermatophytosis diagnosis and treatment strategies.
3.2. Zoophilic Dermatophytes: Diagnostic and Clinical Features
Zoophilic dermatophytes are adapted to non-human animal hosts [16]. Key species infecting animals include Microsporum canis, Nannizzia persicolor, Nannizzia nana, Trichophyton equinum, Trichophyton mentagrophytes, and Trichophyton verrucosum [16]. In humans, zoonotic infections are primarily caused by M. canis, T. mentagrophytes, and T. verrucosum [96]. Zoophilic dermatophytosis in humans typically presents with more pronounced inflammation and a shorter infection duration compared to anthropophilic infections [15]. This heightened inflammatory response is likely due to a lack of host-pathogen adaptation. In contrast to anthropophiles, zoophilic dermatophytes often retain the capacity for sexual reproduction [75,105], with two mating types present in nature [15,75,76]. However, an imbalance in mating type distribution is increasingly observed in some species, leading to a greater reliance on asexual reproduction [75]. Zoophiles associated with soil-dwelling animals are more likely to exhibit both mating types and undergo sexual reproduction compared to those from non-soil-associated animals [70]. Dermatophytosis is more common in mammals than in reptiles and birds [106,107,108].
In animals, dermatophytosis, commonly known as ringworm, is typically characterized by circular alopecic lesions with erythematous borders. Pruritus is not always a prominent feature [99]. Asymptomatic carriers are common, facilitating occult transmission to other animals and humans [99].
Cats are the primary host for M. canis, discussed in detail later. Other zoophiles affect livestock, such as Trichophyton verrucosum in ruminants [98] (Table 1), posing zoonotic risks to farmers and handlers. Increased prevalence in cattle is linked to high-density beef cattle farming [98,99]. Nannizzia nana is a common cause of dermatophytosis in pigs [18] (Table 1), and can also infect dogs and goats [96]. Trichophyton equinum primarily infects horses and rarely humans [97]. Trichophyton mentagrophytes is globally distributed, mainly isolated from rodents [74,93] (Table 1). Nannizzia persicolor (formerly Trichophyton persicolor), initially isolated from voles and bats, occasionally infects humans [94,95] (Table 1). The ecological overlap of some zoophiles with soil habitats blurs the distinction between zoophilic and geophilic dermatophytes [70]. Dermatophytosis diagnosis in zoonotic cases requires careful consideration of animal contact history and potential species involved.
3.3. Geophilic Dermatophytes: Diagnostic and Epidemiological Aspects
Geophilic dermatophytes primarily reside in soil and keratinous debris and are less frequent causes of infection in humans and animals [15,16]. They play a crucial ecological role in keratin degradation and nutrient cycling in soil [109]. Infections are typically acquired from environmental exposure, with limited host-to-host transmission [18,109]. If a geophile establishes sustained populations on hosts and causes frequent infections, it may be reclassified as a zoophile [70]. Nannizzia gypsea (formerly Microsporum gypseum) is the most common geophilic species causing infections in humans and animals [16,19]. Outdoor activities involving soil contact, especially without protection, are risk factors for geophilic dermatophytosis [99]. Certain occupations, like farming, also increase risk [109].
Geophilic dermatophytosis often exhibits a more intense inflammatory response and shorter infection duration compared to anthropophilic and zoophilic infections [24. This reflects a lack of host-fungus adaptation, contrasting with the dampened immune response seen in anthropophilic infections [24]. Clinical presentations can resemble other dermatological conditions, making dermatophytosis diagnosis challenging without culture or molecular identification [109]. N. gypsea can cause tinea corporis and, less frequently, tinea capitis in humans [100] (Table 1). Geophilic species retain both mating types and are more prone to sexual reproduction than host-adapted species [15,75,76]. The soil environment may favor fruiting body formation, associated with sexual reproduction, although these structures are not typically found in infected animals [70]. Despite ecological and reproductive differences, the fundamental diagnostic approaches for dermatophytosis diagnosis are applicable across all dermatophyte classifications.
4. Diagnostic Approaches for Dermatophytosis: A Comparative Analysis
Accurate dermatophytosis diagnosis is crucial for prompt treatment, preventing transmission, and differentiating dermatophytosis from other skin conditions. In animals, even asymptomatic carriers can harbor and transmit dermatophytes, necessitating consideration of fomite carriage in dermatophytosis diagnosis [16]. Prior antifungal treatment can interfere with diagnostic assays, underscoring the importance of pre-treatment sampling [16]. Proper sample collection is critical for diagnostic accuracy. Samples should be taken from the lesion periphery, as the center may contain non-viable fungal material [18]. Skin scrapings are suitable for poorly defined lesions [18]. Hair samples can be collected by plucking or using the Mackenzie brush technique, especially useful in animals for broad-area sampling [111,16]. Nail clippings or scrapings are collected for nail infections [18]. The choice of diagnostic method is often influenced by the sample type and the need for rapid versus definitive dermatophytosis diagnosis.
4.1. Direct Examination: Dermoscopy
Dermoscopy, using a handheld magnification device, is a non-invasive method for examining skin, hair, and nail lesions [112]. It is widely used in human medicine for dermatophytosis diagnosis and monitoring treatment progress [112,113,114]. Advances include polarized light sources and smartphone-integrated dermoscopy [115]. In veterinary medicine, dermoscopy aids in examining hair follicles and skin [116,117]. A limitation in veterinary use is patient compliance, as stillness is needed for image capture [118,Table 2]. Dermoscopy’s accuracy is highly examiner-dependent [118,Table 2], and while it can suggest dermatophytosis, it cannot identify the specific dermatophyte species. Dermoscopy is a valuable initial step in dermatophytosis diagnosis but often requires complementary methods for confirmation and species identification.
4.2. Wood’s Lamp Examination: Rapid Screening for Dermatophytosis
Wood’s lamp, emitting UV light (320-400 nm), is a common screening tool for dermatophytosis diagnosis, particularly in animal shelters [117]. It detects fluorescence characteristic of certain dermatophyte infections [119,120]. Dermatophytes reported to fluoresce include M. canis, M. audouinii, M. ferrugineum, M. distortum phenotype, N. gypsea, and Trichophyton schoenleinii [119]. Fluorescence rates in M. canis isolates vary from 30 to 100% [135,136,137,138,139,140]. A negative Wood’s lamp test does not rule out dermatophytosis, as not all species fluoresce [120,Table 2]. Fluorescence may persist even after topical treatments like lime sulfur and shampoos [141,142,143,144,145]. False positives can occur with other conditions, such as bacterial and yeast infections, and pigmentary disorders, that also fluoresce under Wood’s lamp [120,Table 2]. Wood’s lamp is a rapid, inexpensive screening tool in dermatophytosis diagnosis, but requires confirmation by more specific methods.
4.3. Microscopy and Histopathology: Direct Fungal Detection
Microscopy of clinical samples is a direct method for detecting fungal elements. Potassium hydroxide (KOH) preparations of hair or skin scrapings are used to visualize fungi [146,147]. While sensitive for fungal presence, KOH microscopy cannot differentiate between live and dead fungi or identify species [148,Table 2]. It also requires specialized equipment and trained personnel [114,Table 2]. Lactophenol cotton blue stain, targeting chitin in fungal cell walls, enhances fungal structure visualization and kills fungi, reducing contamination risk [149]. Mineral oil is another mounting medium that does not interfere with fluorescence [16,121,123]. Microscopy is relatively rapid, providing results within an hour [137], with a reported false negative rate of 5-15% [18,Table 2].
Microscopic examination of hair samples can differentiate ectothrix (hyphae and conidia on hair cuticle, common in geophiles and zoophiles) from endothrix (fungi within hair shaft, common in anthropophiles) infections [121,148,3]. Hyphal morphology can also aid in species differentiation. T. mentagrophytes shows spiral, nodular, or racquet hyphae [38]. M. audouinii is identified by pectinate bodies [38], and T. schoenleinii and T. violaceum can produce favic chandeliers [38].
Histopathology, while less common for routine dermatophytosis diagnosis, is valuable for deep infections. Histological features of dermatophytosis include parakeratosis, basket-weave keratin layer, epidermal neutrophils, spongiosis, dermal eosinophils, acanthosis/hyperkeratosis, and hyphal visualization [150,151] (Figure 3). Stains like periodic acid-Schiff (PAS), Gomori’s methenamine silver (GMS), and calcofluor white enhance fungal visualization [146,150,151] (Figure 3). Histopathology is less frequently used due to specialized stains and technical expertise [146].
Figure 3. Histopathological features of dermatophytosis.
4.4. Fungal Culture: The Gold Standard for Dermatophytosis Diagnosis
Fungal culture remains the “gold standard” for dermatophytosis diagnosis, allowing for dermatophyte isolation and identification [122,123,124]. Dermatophyte test medium (DTM) contains phenol red, which changes color to red in alkaline conditions due to dermatophyte metabolism [18]. However, DTM can alter colony morphology, complicating species identification [147]. Sabouraud dextrose agar (SDA) is often used in conjunction with DTM as it less alters colony morphology [152] (Figure 4). Both media typically contain cycloheximide to inhibit non-dermatophyte fungal growth [18]. For differentiating Trichophyton species, specialized media like SDA with 5% salt, vitamin-free agar, Bromocresol purple milk solid glucose agar, lactritmel agar, Littman oxgall agar, and 1% peptone agar can be used, as soil-associated Trichophyton species are often nutrient-independent [74]. Rice grain slopes can distinguish Microsporum species, inducing sporulation in M. canis but not M. audouinii [74]. Dermatophytes produce macroconidia, microconidia, and arthroconidia in culture [70].
Figure 4. Dermatophyte colonies on Sabouraud dextrose agar (SDA).
Culture is essential for tinea unguium dermatophytosis diagnosis where direct examination is often less effective [18]. False negatives can arise from non-dermatophyte overgrowth, insufficient sample, or improper inoculation [16,118,153,Table 2]. False positives can occur from environmental contamination [118,Table 2].
Dermatophyte cultures are incubated at room temperature (25 °C ± 5 °C), with no enhanced growth at 37 °C [154]. Cultures are typically grown in darkness [155], although light conditions may not significantly affect growth [156]. Cultures should be monitored for up to 4 weeks due to slow growth of some species [157,Table 2]. Species identification requires expertise due to pleomorphism [17,158,Table 2]. Culture remains critical for definitive dermatophytosis diagnosis and antifungal susceptibility testing, despite its longer turnaround time.
4.5. DNA-Based Assays: PCR for Rapid and Sensitive Dermatophytosis Diagnosis
Polymerase chain reaction (PCR) is increasingly used for dermatophytosis diagnosis due to its higher sensitivity compared to culture [125,126,Table 2]. PCR can detect fungal DNA even in culture-negative samples but, like microscopy, cannot distinguish between live and dead cells [16]. False negatives can occur due to sampling errors, and false positives from non-viable fungi on the host [16,Table 2]. DNA extraction methods are crucial for PCR accuracy, requiring specialized protocols to lyse fungal cells [159]. These may involve freeze-thaw cycles, heat, or mechanical/chemical lysis [160,161].
Qualitative PCR for dermatophyte detection and identification commonly targets the internal transcribed spacer (ITS) region, which can often identify species [134]. Primers targeting conserved dermatophyte ITS regions aid in identifying dermatophyte-positive samples [162]. Quantitative PCR (RT-PCR) assays using dermatophyte-specific ITS primers have been developed to differentiate dermatophyte species in clinical samples (hair, skin, nail) [162]. PCR offers rapid and sensitive dermatophytosis diagnosis and species identification, complementing culture-based methods.
4.6. Antibody-Based Assays: ELISA for Dermatophytosis Detection
Enzyme-linked immunosorbent assays (ELISA) are antibody-antigen based assays applicable to dermatophytosis diagnosis [162,163,164,165,166]. Various ELISA formats (direct, indirect, sandwich, competitive) offer different advantages [129]. Colorimetric detection is common due to standard plate reader compatibility [167]. Sensitivity can be enhanced by optimizing reagents, ELISA format, incubation times, or temperatures [129,168].
ELISAs have been developed for dermatophyte detection using serum samples and M. canis antigen [127,128]. M. canis-specific IgG antibody detection in cat and dog sera using ELISA showed sensitivity comparable to fungal culture [127,128,Table 2]. False positives are possible due to antibody persistence post-infection [127,128,Table 2]. Serum requirement makes sample collection more invasive than other methods [Table 2]. ELISA offers a specific dermatophytosis diagnosis tool but has limitations related to sample invasiveness and potential false positives.
4.7. Mass Spectrometry: MALDI-ToF MS for Rapid Dermatophyte Identification
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF MS) is gaining traction for fungal dermatophytosis diagnosis and identification. It can detect phenotypic variations through protein spectra changes [130]. Faster than culture, MALDI-ToF MS is limited by library availability, hindering identification of novel or rare species [130,Table 2]. Other limitations include sample quantity needs, unreliable results with mixed fungal samples, sample cross-contamination, equipment costs, specialized personnel training, and cleaning protocols [130].
Dermatophyte-specific libraries, including over 20 species, have been developed for MALDI-ToF MS [131,132,133]. While rapid, MALDI-ToF MS requires specialized equipment and ongoing library updates to reflect dermatophyte taxonomic changes [130,Table 2]. MALDI-ToF MS provides rapid and accurate dermatophytosis diagnosis and species identification when library resources are comprehensive.
| Diagnostic Method | Advantages | Disadvantages | Time to Results | Reference |
|---|---|---|---|---|
| Direct examination | – Non-invasive – Low cost | – Unable to determine species | Minutes | [116,117] |
| Wood’s lamp | – Non-invasive – Low cost | – Not all species fluoresce | Minutes | [119,120] |
| Microscopy | – Can detect unique features of species – Low cost | – Unable to distinguish dead and alive fungi | Minutes | [38,121] |
| Culture | – Low cost – Easy to perform – Can distinguish between species | – Requires expertise to determine species – Can be contaminated by saprophytes | Days–Weeks | [122,123,124] |
| PCR | – Highly sensitive – Can distinguish between species | – Unable to distinguish dead and alive fungi | Hours–Days | [16,125,126] |
| ELISA | – Highly specific | – False positives due to past infections | Hours–Days | [127,128,129] |
| MALDI-ToF | – Highly sensitive – Can distinguish between species | – Only detect species in library | Minutes–Hours | [130,131,132,133] |
| Genetic analysis | – Can distinguish between species – Highly sensitive | – Unable to distinguish dead and alive fungi | Hours–Days | [30,31,134] |
Table 2. Comparison of dermatophyte diagnostic methods.
5. Future Directions in Dermatophytosis Diagnosis: Targeting Fungal Metabolism
Current dermatophytosis diagnosis methods have drawbacks, especially in clinical settings. Differentiating between live and dead fungi, particularly post-treatment, remains a challenge. Targeting dermatophyte-specific metabolic products offers a potential solution, as their presence would indicate metabolically active fungi. Dermatophytes produce unique metabolites, from simple chemicals to complex proteins, providing diverse diagnostic targets [23,119,169]. Compared to other fungi, dermatophytes encode a greater number of secondary metabolites, including proteases [23,24]. Unique metabolic pathways include keratin degradation and fluorescent metabolite production [23,119,169]. These pathways and metabolites are promising targets for novel dermatophytosis diagnosis assays.
5.1. Targeting Unique Keratin Metabolism: Sulfite Efflux Pump (SSU1)
Dermatophytes uniquely metabolize keratin, a highly resistant protein [169]. They secrete sulfite and keratinases for keratinolysis [169]. The sulfite efflux pump (SSU1) secretes sulfite to break keratin disulfide bonds, releasing cysteine and S-sulfocysteine (SSC), further digesting keratin for fungal nutrition [169,170,171]. Cysteine, abundant in hair, is converted to sulfite within the fungal cell via cysteine dioxygenase (Cdo1) and subsequent reactions [45,169,171,172].
The SSU1 gene in T. mentagrophytes is essential for keratin degradation and infection virulence [169], making SSU1 and its metabolic products diagnostic targets. Detecting sulfite or SSC would indicate metabolically active dermatophytes, as skin bacteria and yeast do not actively degrade keratin for nutrition [173]. Crucially, persistence of these metabolites post-fungal death needs to be determined to avoid false positives. Further research into these metabolites is warranted for developing novel dermatophytosis diagnosis assays.
5.2. UV Fluorescent Metabolites: Enhancing Wood’s Lamp Specificity
Certain dermatophytes, including M. canis, M. audouinii, M. ferrugineum, N. gypsea, and Trichophyton schoenleinii, fluoresce under UV light [119]. Pteridine is the fluorescent compound in M. canis and N. gypsea [174,175], while pteridine and xanthurenic acid derivatives contribute to T. schoenleinii fluorescence [176]. These compounds produce blue-green and yellow fluorescence, respectively [120]. Other skin conditions fluoresce in different colors (e.g., coral-red for bacterial infections, bluish-white for vitiligo) [120]. Detecting these fluorescent metabolites using mass spectrometry, fluorometric assays, or antibody-based assays could improve dermatophytosis diagnosis sensitivity and specificity compared to Wood’s lamp. Investigating the metabolic pathways of these metabolites could also clarify strain-level variability in fluorescence production.
5.3. Dermatophyte-Specific Proteases: Targeting Virulence Factors for Diagnosis
Proteases, enzymes degrading proteins into amino acids/peptides [177], are highly expressed by dermatophytes, especially in the presence of keratin [23,178]. Endo- and exoproteases are produced for keratin and degradation product hydrolysis [24]. Endoprotease classes include fungalysins, subtilisins, and neutral proteases [171,179]. Protease production is linked to disease severity [180]. Subtilisin proteases are key for keratin degradation, with dermatophytes having expanded subtilisin protease families [171,23].
Subtilisin 3 (Sub3) from M. canis is crucial for adherence to keratinized tissues in early infection [177,181,182,183,184]. Sub3 detection indicates metabolically active M. canis [169]. Immunohistochemistry has detected M. canis Sub3 in cat hair follicles from clinical biopsies [183], suggesting antibody-based assays targeting Sub3 are feasible. Exploring these protease targets could improve dermatophytosis diagnosis and potentially enable species-level identification.
6. Microsporum canis: A Clinically Significant Dermatophyte and Diagnostic Focus
The Microsporum genus, comprising Microsporum canis, Microsporum audouinii, and Microsporum ferrugineum, is clinically important, causing significant human and animal disease [14,70]. M. canis, a zoophilic, soil-associated dermatophyte [14,70], is the earliest diverging dermatophyte species [15,17,23,185]. M. audouinii and M. ferrugineum are anthropophiles evolved from M. canis [14,70]. M. canis is the most common cause of human infections within Microsporum, typically transmitted from infected cats [16]. The name M. canis now encompasses both anamorph and teleomorph (Arthroderma otae or Nannizzia otae) forms [15,185]. Understanding M. canis is crucial for effective dermatophytosis diagnosis and management, especially in zoonotic contexts.
6.1. M. canis Morphology and Laboratory Diagnosis
M. canis colonies in culture are white to cream-colored, with a golden-yellow to brownish-yellow reverse pigment [74]. Colony topography is usually flat and spreading with radial grooves, and texture is cottony to woolly [17,74]. M. canis produces spindle-shaped macroconidia and microconidia [38,74]. Macroconidia have thick walls, potentially deterring arthropod grazing [70]. Sporulation can be induced on lactrimel agar or rice grains [74]. Unlike some Trichophyton species, M. canis does not require specialized nutrients for growth [70].
M. canis is hair perforation test positive by day 14 and urease positive in approximately 80% of isolates [17,74,186]. It exhibits ectothrix hair invasion [187]. M. canis typically fluoresces yellow-green under UV light [16,119]. These laboratory characteristics are essential for accurate dermatophytosis diagnosis and M. canis identification.
6.2. M. canis Habitat, Transmission, and Epidemiology
Cats are the primary M. canis reservoir, with infection rates reaching 100% in some populations [137,189,190,191]. Dogs are the second most common animal reservoir, with M. canis causing 40–90% of canine dermatophytosis cases [108,192]. M. canis is not considered part of the normal skin microbiota of these hosts [16]. It is less frequently isolated from other animals like horses, cattle, goats, sheep, rabbits, and pigs [93,108,193,194,195]. Transmission is mainly via direct contact with infected animals or fomites [124,196,197]. Outbreaks are common in high-density animal populations like shelters and catteries [52,123,144]. Human-to-human M. canis transmission is possible but limited after a few events [196,198,199]. M. canis‘ high transmissibility has led to its global distribution [1,74]. Epidemiological understanding is crucial for targeted dermatophytosis diagnosis and control.
6.3. Global Distribution of M. canis
M. canis has a worldwide distribution, with varying prevalence across countries [1,74]. It is a leading cause of tinea capitis in regions including Great Britain, Ireland, Western Europe, Spain, Greece, Kuwait, Hong Kong, Malaysia, Australia, New Zealand, USA, Canada, Venezuela, Brazil, Uruguay, Argentina, Chile, Algeria, Sudan, and South Africa [200,201,202,203,204,205,206,207,208,209,210,211,212,213]. M. canis is also a primary agent of tinea corporis in Australia, New Zealand, Brazil, Uruguay, and South Africa [203,204,206,212]. M. canis infections have decreased in recent decades, possibly due to stray animal management [51,214]. Understanding geographic distribution aids in dermatophytosis diagnosis and epidemiological surveillance.
6.4. M. canis Mating Types and Reproductive Biology
Dermatophytes can reproduce sexually or asexually, depending on mating partner availability [75,215]. Mating types are designated MAT1-1 (alpha-box gene) and MAT1-2 (HMG gene) [75,215,216]. Two M. canis mating types have been reported, but the MAT1-2 type is mainly isolated from Japan [185,217,218]. A hypothesis suggests M. canis is increasingly relying on asexual reproduction due to limited MAT1-2 identification outside Japan [37]. Reproductive biology insights are less directly relevant to routine dermatophytosis diagnosis but inform broader understanding of M. canis evolution and adaptation.
6.5. Clinical Disease Associated with M. canis Infection
M. canis infection lesions typically appear 1-3 weeks post-arthroconidia exposure [50]. Human M. canis infections are more inflammatory than M. audouinii infections, suggesting less human host adaptation [17]. Clinical signs range from mild scaling and alopecia to severe inflammation, pustules, and dermal invasion (Majocchi’s granuloma) [32,219,220]. M. canis mainly causes tinea corporis and tinea capitis in humans [18]. Tinea unguium due to M. canis is rare in humans [74].
Cats with dermatophytosis often present with mild circular alopecia and scaling [124]. Pruritus and miliary dermatitis are variable [16]. M. canis causes 90-100% of feline dermatophytosis cases [108,153,218]. Cats exhibit a weaker immune response to M. canis compared to other dermatophytes, suggesting host adaptation [99,221]. M. canis infections in cats are less treatment-responsive than other dermatophytoses [158]. Treatment aims to reduce fungal spread and infection duration [222]. Clinical resolution typically occurs within 7-17 weeks post-exposure [145,223]. Clinical understanding of M. canis infections informs appropriate dermatophytosis diagnosis and management strategies.
7. Conclusions and Future Perspectives in Dermatophytosis Diagnosis
Recent taxonomic revisions have renamed numerous dermatophyte species, impacting dermatophytosis diagnosis and treatment protocols. While fungal culture remains the gold standard for dermatophytosis diagnosis, diverse diagnostic methods are available. Novel diagnostic approaches focusing on fungal metabolites (sulfite metabolism, UV fluorescent metabolites, and proteases) hold promise for improved detection and differentiation of active infections.
M. canis is a clinically significant zoonotic dermatophyte, commonly affecting cats and causing tinea capitis and corporis in humans. It appears to be adapting to feline hosts, evidenced by the limited global distribution of the MAT1-2 mating type. Current diagnostic assays effectively identify M. canis and differentiate it from other Microsporum species. Further research into M. canis and other dermatophyte genetics and metabolism is crucial for developing rapid, inexpensive diagnostic tests and novel therapies for dermatophytosis in humans and animals. The future of dermatophytosis diagnosis lies in integrating advanced molecular and metabolic assays for rapid, accurate, and clinically relevant detection and species identification, ultimately improving patient outcomes and public health.
Acknowledgments
Chad Frank for providing histopathology pictures and Christopher P. Kozakiewicz for editing the manuscript.
Author Contributions
Conceptualization, A.E.M. and S.V.; methodology, A.E.M.; software, A.E.M.; validation, A.E.M.; formal analysis, A.E.M.; investigation, A.E.M.; resources, S.V.; data curation, A.E.M.; writing—original draft preparation, A.E.M.; writing—review and editing, A.E.M. and S.V.; visualization, A.E.M.; supervision, S.V.; project administration, A.E.M. and S.V.; funding acquisition, A.E.M. and S.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Morris Animal Foundation, grant numbers D21FE-024 and D21FE-402.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
[References from original article are included here and are not repeated for brevity]
Associated Data
Data Availability Statement
Not applicable.