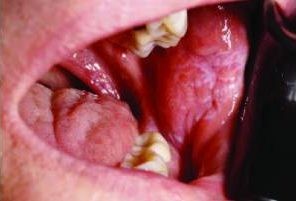
Desquamative gingivitis, while a frequently encountered clinical presentation in dental practice, is not a diagnosis in itself. Instead, this term describes the erythematous, erosive, and often ulcerated appearance of the gingiva. Patients experiencing desquamative gingivitis commonly report symptoms such as mucosal sloughing, spontaneous gingival bleeding, and significant oral discomfort, particularly when consuming certain foods or beverages. These subjective complaints and objective clinical findings, although seemingly uniform, necessitate a deeper understanding from clinicians. It’s crucial to recognize that desquamative gingivitis is a descriptive term that encompasses a range of underlying conditions. In a significant number of cases, patients presenting with desquamative gingivitis are eventually diagnosed with conditions such as erosive lichen planus, mucous membrane pemphigoid (MMP), or pemphigus vulgaris (PV). This article aims to delve into the clinical features, diagnostic methods, and contemporary treatment protocols for each of these key conditions associated with desquamative gingivitis.
Oral Lichen Planus: Unveiling the Complexities
Oral lichen planus (OLP) stands as a chronic mucocutaneous condition predominantly observed in middle-aged adults, exhibiting a slight predilection for females. While sometimes classified as an autoimmune disorder, the precise etiology of lichen planus remains elusive. Current theories suggest it arises from an aberrant activation of CD4+ T-lymphocytes, leading to the characteristic clinical manifestations observed in patients.
Oral lichen planus can manifest with varying degrees of symptomatic presentation. Asymptomatic OLP often takes on a reticular appearance, characterized by white, lace-like striations known as Wickham striae. The buccal mucosa is the most frequently affected site; however, lesions can develop in any area within the oral cavity. In some instances, the lesions may be exclusively confined to the gingiva. Bilateral and/or multifocal lesion presentations are common in oral lichen planus. Erosive lichen planus, while less frequent than its reticular counterpart, carries greater clinical significance due to its symptomatic and discomforting nature. Erosive lesions typically exhibit a mixed red and white appearance, although an erythematous, ulcerated presentation may dominate in some patients. Even in predominantly erosive cases, careful examination often reveals focal Wickham striae.
Mucous Membrane Pemphigoid: An Autoimmune Perspective
Mucous membrane pemphigoid (MMP), also known as cicatricial pemphigoid, is recognized as a chronic autoimmune disease with an etiology that is yet to be fully understood. The pathogenesis of MMP involves the production of autoantibodies that target hemidesmosomes, resulting in subepithelial separation. Clinically, this autoimmune reaction leads to the formation of vesicles or bullae that subsequently rupture, giving rise to ulceration and erythema. Similar to oral lichen planus, MMP predominantly affects middle-aged females and is considered a mucocutaneous disease.
The intraoral presentation of MMP shares notable similarities with erosive lichen planus. Desquamative gingivitis is a common clinical finding, and in many instances, MMP lesions may be primarily localized to the gingiva. While non-gingival site involvement can occur, it does not rule out a diagnosis of MMP. Although blister formation might be observed, intraoral bullae often rupture rapidly, leading to mucosal erythema and ulceration. A key diagnostic feature of MMP is a positive Nikolsky sign, where firm pressure applied to clinically normal tissue can induce blister formation.
Pemphigus Vulgaris: A Severe Autoimmune Condition
Pemphigus vulgaris (PV) represents a severe and progressive autoimmune disease driven by autoantibodies directed against intraepithelial desmosomes. This autoimmune attack results in intraepithelial clefting, which is the hallmark of PV and produces the characteristic clinical lesions, manifesting as vesicles and bullae affecting both the skin and mucous membranes. Due to the more superficial microscopic separation in PV compared to MMP, the resulting blisters are more fragile and prone to rupture and ulceration. PV is most frequently diagnosed in middle-aged adults, with no significant gender predilection reported. Notably, a higher incidence is observed in individuals of Mediterranean, Ashkenazi Jewish, or South Asian descent. The reported incidence is approximately 1 in 5 million, highlighting its relative rarity yet significant clinical importance.
Oral lesions in pemphigus vulgaris typically present as fragile vesicles that quickly rupture, leading to ulcerations surrounded by erythema. These ulcers can be substantial in size, often described as having irregular, ragged borders. Any oral site can be affected, and PV often presents bilaterally and/or multifocally. When the gingiva is involved, it results in desquamative gingivitis, clinically similar to lichen planus and MMP. Like MMP, pemphigus vulgaris exhibits a positive Nikolsky sign. Importantly, oral lesions of PV often precede the development of cutaneous manifestations, sometimes by almost a year. Oral lesions also tend to resolve more slowly than skin lesions. In severe, undiagnosed chronic PV cases, patients may experience weight loss due to lesion-induced difficulties in maintaining proper nutrition. While mortality from PV is infrequent with current treatments, prompt diagnosis and management are critical.
Differential Diagnosis: Expanding the Clinical Spectrum
While lichen planus, MMP, and PV constitute approximately 90% of cases presenting clinically as desquamative gingivitis, a comprehensive differential diagnosis must consider other conditions that can manifest with similar oral presentations. These include lichenoid mucositis, systemic lupus erythematosus, erythema multiforme, graft-versus-host disease, and paraneoplastic pemphigus, among others. Although a detailed discussion of these entities is beyond the scope of this article, their consideration is crucial in clinical practice when deemed appropriate.
The clinical similarities between lichen planus, MMP, and PV, along with other less common chronic oral vesiculo-erosive processes, emphasize the necessity of tissue biopsy as the gold standard for definitive diagnosis. Achieving an accurate diagnosis is paramount because, despite some treatment overlaps, these conditions differ significantly in long-term prognoses and potential extraoral manifestations. Furthermore, the expected therapeutic response to specific medications can vary depending on the precise diagnosis.
Diagnostic Pathways: Histopathology and Immunofluorescence
Histopathologic examination using routine hematoxylin and eosin staining reveals distinct features for lichen planus, MMP, and PV, reflecting their underlying etiologies. In lichen planus, a dense, linear band of lymphocytes is characteristically observed directly beneath the surface epithelium. Lymphocyte migration from the connective tissue into the epithelial layers, known as exocytosis, is frequently present, leading to liquefactive degeneration of the basal epithelial layer. This microscopic presentation aligns with the proposed cytotoxic T lymphocyte-mediated etiology of lichen planus. Mucous membrane pemphigoid is characterized by a clean subepithelial separation, resulting from autoantibodies targeting hemidesmosomes that connect the epithelium and underlying connective tissue. Conversely, pemphigus vulgaris shows an intraepithelial separation due to autoantibodies directed against desmosomal attachment proteins within the epithelium. With this type of separation, rounded acantholytic epithelial cells, termed Tzanck cells, may be identified.
Ideally, these classic microscopic findings should enable a definitive diagnosis upon biopsy. However, in practice, histologic features can overlap significantly. For instance, artifactual tissue separation during biopsy or specimen processing can mimic the clefting seen in MMP or PV. Similarly, an inflammatory infiltrate resembling lichen planus can occasionally occur in other conditions. Consequently, a biopsy report for a desquamative lesion may sometimes yield a nonspecific diagnosis, with recommendations for repeat biopsy or submission for direct immunofluorescence (DIF). Direct immunofluorescence utilizes fluorescent antibodies targeting specific proteins to refine the diagnosis. In oral vesiculo-erosive diseases, DIF can further confirm MMP or PV diagnoses, as these conditions exhibit unique immunofluorescence profiles. Unlike standard biopsies preserved in formalin, DIF requires tissue submission in Michel’s solution.
Selecting an optimal biopsy site is crucial for obtaining a diagnostically valuable sample. This can be particularly challenging in multifocal diseases like lichen planus, MMP, and PV. The most visually striking lesion is not always the most diagnostically informative, and overtly ulcerated areas should be avoided if possible for biopsy. When an ulcerated site must be biopsied, a perilesional sample from the ulcer’s edge, rather than the center, is necessary to obtain diagnostic tissue. This is because ulcers, by definition, lack surface epithelium, and the diagnosis of these conditions relies on analyzing the epithelium-connective tissue interface. Biopsies from ulcer centers are likely to result in nonspecific ulceration and inflammation diagnoses. The most informative biopsy sites are mixed red and white lesions; areas exhibiting a clinically visible Wickham striae-like pattern are of high diagnostic yield.
As previously noted, lichen planus, MMP, and PV constitute the majority of desquamative gingivitis cases. Among these, lichen planus is the most prevalent. In erosive lichen planus cases, identifying Wickham striae is a helpful, though not definitive, clinical finding. Clinically, MMP may be more frequently suspected in patients presenting with desquamative gingivitis without other oral mucosal lesions.
Therapeutic Strategies: Managing Desquamative Gingivitis
Topical corticosteroids are typically the first-line treatment for desquamative gingival lesions, administered as rinses, gels, or sprays. Due to the often multifocal nature of these conditions, corticosteroid rinses are advantageous for providing broad coverage of affected sites without demanding complex application techniques from the patient. If initial topical steroid therapy proves insufficient, stronger topical and/or systemic therapies can be considered. Once symptomatic relief is achieved, patients should be encouraged to use the corticosteroid rinse only as needed for flare-ups. For localized symptoms persisting after rinse therapy, a topical steroid gel can be prescribed for direct application to specific sites. Nonsteroidal therapies, including tacrolimus and apremilast, are emerging as potential treatment options for chronic oral ulcerative processes that are refractory to topical corticosteroids. In cases where pemphigus vulgaris is suspected, short courses of systemic steroids should be avoided initially, as they may lead to a rebound effect and lesion exacerbation upon therapy cessation.
Patient education is a cornerstone of effective management for desquamative gingivitis. Patients should understand that lichen planus, MMP, and PV are chronic conditions. Complete resolution of clinical lesions with medication is not always achievable, though significant reductions in erythema and ulceration are typically expected. Treatment success is primarily assessed by symptom improvement. Asymptomatic patients generally do not require treatment. Patients should be informed about the fluctuating nature of these conditions, with lesions potentially appearing and resolving in different oral sites over time, and that this is a typical disease course, not necessarily a cause for alarm. While various environmental triggers, such as physiological or psychological stress and certain foods or beverages, may exacerbate symptoms, there are no universally mandated dietary restrictions. Upon symptom worsening, patients should resume prescribed medications until relief is obtained. Prolonged, continuous use of topical steroid rinses should be discouraged due to potential side effects like oral dryness and increased risk of candidal infections.
A definitive diagnosis is critical due to the distinct long-term sequelae and extraoral manifestations associated with each condition. Patients with lichen planus should be informed about a slightly elevated risk of developing oral squamous cell carcinoma. They should also be educated about the importance of ongoing long-term follow-up with an oral and maxillofacial pathologist. Approximately 25% of MMP patients may develop ocular lesions that can lead to blindness if untreated, necessitating referral to both an ophthalmologist and a dermatologist for comprehensive management of potential eye and skin involvement. Patients with PV should be referred to a dermatologist, even in the absence of skin lesions, as oral manifestations can precede cutaneous signs by up to a year. Management of PV frequently requires strictly regimented systemic steroid dosing or other systemic immunosuppressive therapies.
Conclusion: Navigating the Diagnostic and Therapeutic Journey
The presentation of a patient with desquamative gingivitis marks the beginning of a potentially complex yet ultimately rewarding diagnostic process for both the patient and the clinician. Chronic oral vesiculo-erosive lesions can remain undiagnosed or improperly managed for extended periods, leading to patient frustration and diminished quality of life. The ability to accurately recognize this disease presentation and establish a specific diagnosis is essential for improving patient outcomes and enhancing the standard of care in managing desquamative gingivitis.
References
- Rogers RS, Sheridan PJ, Nightingale SH. Desquamative gingivitis: clinical, histopathologic, immunopathologic, and therapeutic observations. J Amer Acad Dermatol. 1982;7:729–735.
- Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: an update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–132.
- Cheng YL, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:332–354.
- Bagan J, Muzio LL, Scully C. Mucous membrane pemphigoid. Oral Dis. 2005;11:197–218.
- Black M, Mignogna MD, Scully C. Pemphigus vulgaris. Oral Dis. 2005;11:119–130.
- Muller S. Oral lichenoid lesions: distinguishing the benign from the deadly. Moder Pathol. 2017;30:S54–S67.
- Bettencourt M. Oral lichen planus treated with apremilast. J Drugs Dematol. 2016;15:1026–1028.
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45–46.