Introduction
Acute febrile illness (AFI), characterized by fever, malaise, and muscle pain, is a common and nonspecific presentation of numerous infectious diseases, particularly in tropical regions. In resource-limited settings, such as low-income countries like India, the absence of effective diagnostic tools for AFI significantly contributes to increased sickness and death. This review examines the range, nature, and quality of diagnostic methods employed for AFI evaluation in healthcare facilities across South India, specifically focusing on passive case detection. Understanding the current diagnostic landscape is crucial for identifying gaps and guiding improvements in AFI management.
What is Acute Febrile Illness (AFI)?
Fever in tropical environments presents a complex diagnostic challenge. Due to the diverse ways fever can manifest, it is often categorized by symptom duration into AFI and chronic fevers. While a universally accepted definition is lacking, chronic fevers are generally considered those lasting over 14–21 days, whereas AFI refers to fevers lasting less than 21 days. AFI is often used interchangeably with acute undifferentiated febrile illness (AUFI), defined as fever resolving within three weeks without specific organ-related signs or symptoms. These illnesses are frequently caused by infectious agents and pose a significant health burden in tropical, low-resource regions where febrile diseases are most prevalent.
AFI can be further categorized based on its cause: malaria-related fever and non-malarial acute febrile illness (NMAFI), caused by other pathogens. The historical focus on malaria as a primary cause of AFI in developing countries has spurred the development of high-quality point-of-care tests (POCT) and rapid diagnostic tests (RDTs). These advancements have improved early malaria diagnosis and treatment, but have also highlighted the previously underappreciated burden of NMAFI.
AFI remains a major cause of illness and death for both children and adults in low- and middle-income countries. In South Asia, common causes of AFI include scrub typhus, dengue, malaria, typhoid fever, and leptospirosis. India, a lower-middle-income country with a large rural population, faces particular challenges in AFI management. These challenges arise from geographical and seasonal variations in disease prevalence, a lack of comprehensive surveillance systems, syndrome-based fever management guidelines that are often non-specific, and the limited availability of high-quality diagnostic tests. Furthermore, lenient enforcement of antibiotic prescription policies leads to widespread and often inappropriate antibiotic use, contributing to the growing threat of antimicrobial resistance. Notably, while high-income countries have shown minimal increases in antibiotic consumption, middle-income countries like India, China, and Pakistan have experienced drastic increases. India, in particular, has seen a substantial rise in antibiotic use, further emphasizing the need for targeted diagnostic and treatment strategies for AFI.
To better understand the diagnostic tools available and utilized within the Indian healthcare system, this review aims to identify the diagnostic approaches employed for managing AFI in healthcare centers at all levels in India. The primary objectives are to identify commonly used tests for diagnosing and evaluating AFI in patients seeking medical care and to determine the number of diagnostic tests typically performed per patient with AFI. This scoping review seeks to highlight gaps in our understanding of AFI diagnosis and management based on existing literature, ultimately aiming to emphasize the need for diagnostic innovation, improved guidelines, and interventions to enhance AFI diagnosis and patient care.
Methods
This review followed a structured approach, beginning with a concept note outlining objectives, inclusion criteria, and expected outcomes. A detailed search strategy was developed, and articles were screened for eligibility. The screening, assessment, and data synthesis were primarily conducted by one reviewer, with a second reviewer involved in resolving complex cases. Both reviewers participated in data interpretation and analysis.
Key variables extracted from the studies included:
- Types of AFI investigated
- Diagnostic tests used for AFI evaluation
- Number of diagnostic tests per patient to reach an AFI diagnosis
- Study setting (public or private sector)
Selection Criteria
Publications were included based on the following criteria:
Case Definition
Studies describing patients presenting to healthcare facilities with acute onset fever (≤ 3 weeks duration).
Study Design
Peer-reviewed original research articles on diagnostic tests used for AFI evaluation in South India, including cross-sectional studies, case-control studies, case reports, and case series.
Publication Type
Original research papers focusing on AFI management in South India. Excluded were reviews, mathematical models, nosocomial infection/fever articles, letters, abstracts, and short communications.
Patient and Setting
Studies involving patients of all ages with AFI symptoms were included. The review focused on South India, encompassing the states of Karnataka (KA), Andhra Pradesh (AP), Telangana (TS), Kerala (KE), and Tamil Nadu (TN). Settings included outpatient departments (OPD), emergency departments, inpatient departments (IPD), and intensive care units (ICU) in both public and private healthcare facilities in rural and urban areas of South India.
Diagnostic Tests
The review considered both pathogen-specific and non-specific diagnostic tests. Pathogen-specific tests, which provide an etiological diagnosis, were further categorized as antigen/antibody detection assays, molecular tests for nucleic acid detection, and phenotypic tests (e.g., microscopy for malaria, blood culture). The review assessed publications documenting the types and number of pathogen-specific diagnostics used for AFI evaluation, monitoring, and prognosis.
Search Strategy
Electronic searches were conducted in Embase (1946-July 2018), Medline (1946-July 2018), PubMed (1996-July 2018), and IndMED (1985-July 2018) for English language publications. The search strategy combined terms for acute fever (e.g., pyrexia, febrile illness), diagnostic tests (e.g., point of care testing, bedside testing), and India. Synonyms and individual AFI causes (e.g., scrub typhus, dengue) were included. Keywords and MESH terms were used, combined with Boolean operators (OR and AND), and truncation was used for terms like diagnos* and test*. Manual searching of journals and snowballing techniques supplemented electronic searches.
Data Collection and Extraction
Search results were managed in Endnote, and duplicates were removed. Full-text articles of potentially relevant studies were retrieved and assessed against selection criteria. Data extraction was performed using a modified Cochrane Effective Practice and Organization of Care (EPOC) data extraction form.
Quality Assessment
Study quality was assessed using the AXIS tool for cross-sectional studies and the Joanna Briggs Institute Reviewer’s manual (JBI tool) for case-control studies, case series, and case reports.
Data Synthesis
A narrative synthesis of the data described the types and number of diagnostics used in AFI evaluation. Descriptive and analytical statistics on laboratory profiles and commonly suspected pathogens were reported.
Results
Study Characteristics
The search identified 7140 records, from which 7016 were excluded based on title. After abstract review of 124 articles, 54 were further excluded due to publication type or setting. Seven articles were irretrievable. From 63 eligible articles, 23 were excluded based on detailed characteristics (Additional file 1: Table S4). A total of 40 studies met the inclusion criteria (Fig. 1).
Fig. 1. Study Selection Flow Diagram
Figure 1: PRISMA flow diagram illustrating the study selection process. Adapted from Mohr et al., demonstrating the systematic approach to identifying and selecting relevant studies for the review.
Study Design, Location, and Setting
The 40 studies comprised 26 cross-sectional studies (65%), one case-control study (2.5%), four case series (10%), and nine case reports (22.5%). All studies were conducted in tertiary care settings and published between 2000 and 2018. Among cross-sectional studies, most were prospective. The majority of studies were conducted in Tamil Nadu (TN) and Karnataka (KA), with fewer in Kerala (KE) and Telangana (TS) (Fig. 2).
Fig. 2. Geographical Distribution of Studies in South India
Figure 2: Geographical distribution of the included studies across South India. The map highlights the states of Tamil Nadu and Karnataka as regions with a higher concentration of studies on AFI diagnosis.
Patient Populations
Case Series and Case Reports
Case series primarily focused on malaria and rickettsial infections in children, and scrub typhus in adults. Case reports covered a range of infections including scrub typhus, dengue, typhoid, hantavirus, and malaria, often highlighting coinfections or unusual presentations (Table 1).
Table 1. Patient Population in Case Series and Case Reports
| A |
|---|
| Name of study |
| Kumar et al., 2008 |
| Katoch et al., 2016 |
| Saifudheen et al., 2012 |
| Prasannan et al., 2017 |
| B |
| Name of study |
| Manickam et al., 2014 |
| Chandy et al., 2009 |
| Devarajan et al., 2012 |
| Thangaratham et al., 2006 |
| Bhat et al., 2015 |
| Jagdishkumar et al., 2016 |
| Kakarapathi et al., 2014 |
| Madi et al., 2014 |
| Sitalakshmi et al., 2005 |
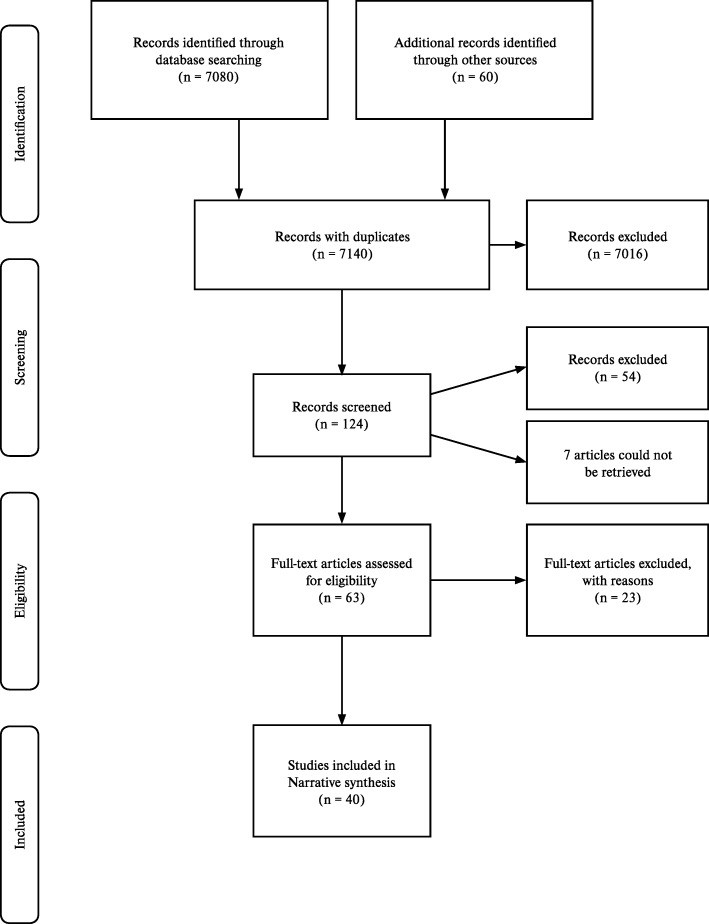
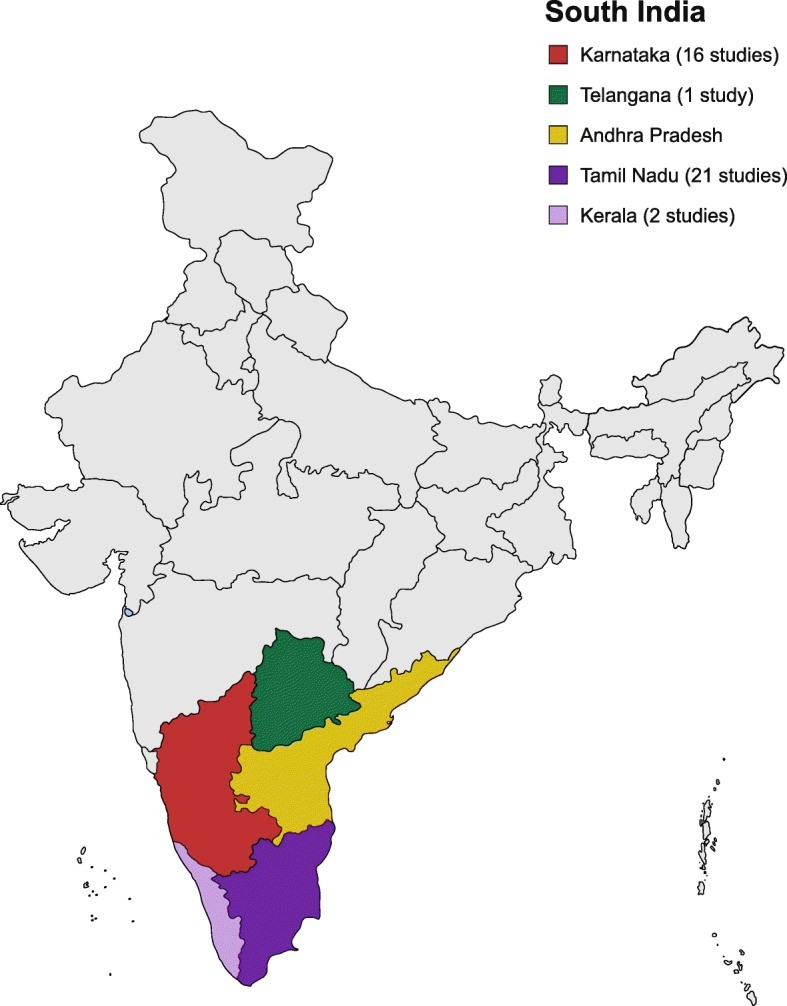
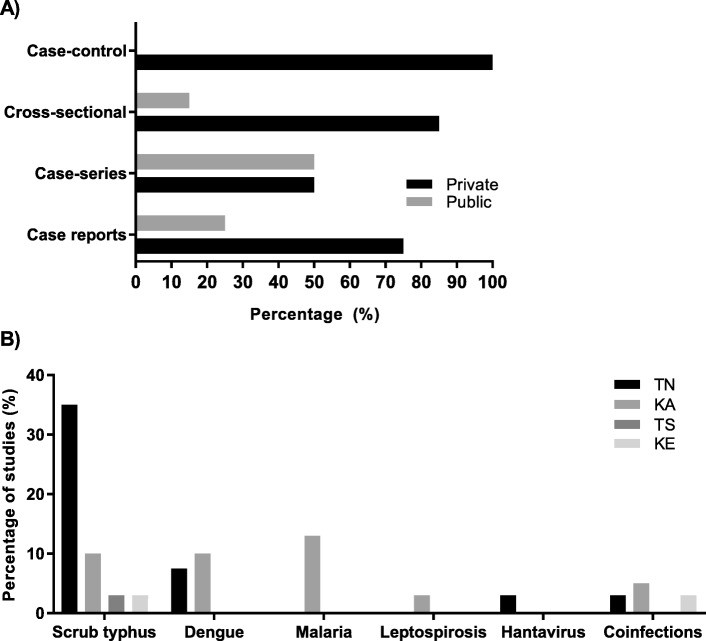
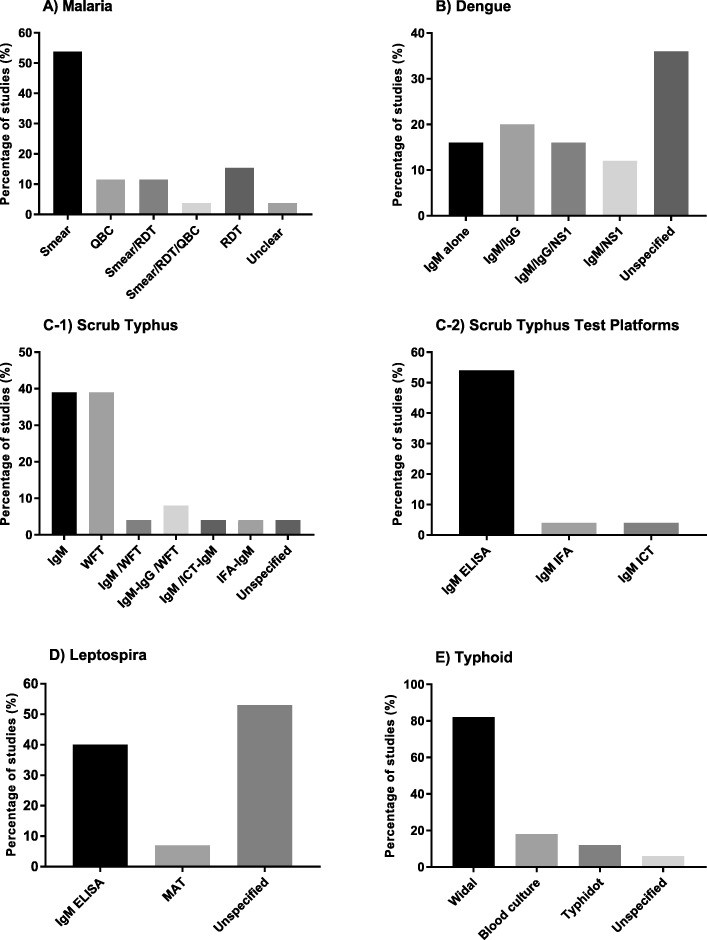
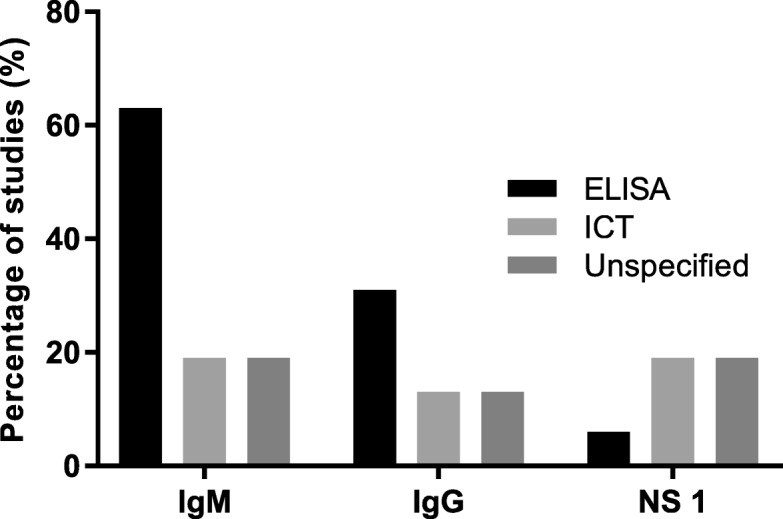
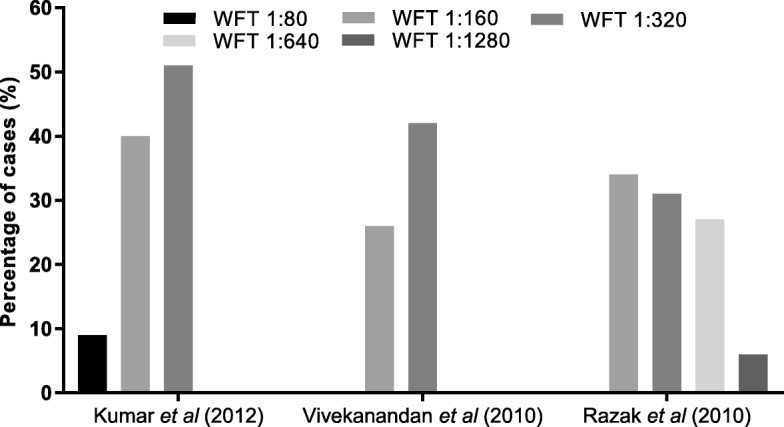
Table 1: Characteristics of patient populations in case series (A) and case reports (B). This table provides details on the specific patient demographics and inclusion criteria for each study type, offering a snapshot of the diverse AFI cases investigated.
Cross-sectional and Case-control Studies
Cross-sectional studies focused on AFI etiology, dengue in children, malaria in adults, leptospirosis in adults, and scrub typhus in both children and adults. The case-control study examined dengue and scrub typhus coinfection. A significant majority of studies were conducted in private healthcare settings (Fig. 3a). Scrub typhus was frequently evaluated in TN, while malaria and dengue were more studied in KA (Fig. 3b).
Fig. 3. Study Distribution by Setting and Pathogen
Figure 3: (a) Percentage distribution of study types across private and public healthcare settings, highlighting the predominant role of private settings in AFI research. (b) Distribution of studies investigating specific AFI causes/pathogens across different states in South India, showing regional variations in research focus.
Number of Diagnostics per Patient
Most studies (63%) used a combination of pathogen-specific and non-specific tests for AFI diagnosis. A considerable portion (28%) employed more than 10 pathogen-specific tests per patient, while 56% used between 5 and 9 tests, and 16% used 7-8 tests (Additional file 1: Table S11).
Specific Tests for AFI Etiology
The most frequently tested diseases in AFI evaluation were malaria (96%), dengue (80%), scrub typhus (76%), typhoid (68%), and leptospirosis (60%). HIV testing was less common (10%), and Epstein-Barr virus (EBV) testing was rare (4%). Hantavirus was investigated in 8% of studies.
Malaria Diagnostics
Malaria testing was performed in 26 studies. Smear microscopy was used in 54% of these studies, while the remainder used RDTs or quantitative buffy coat (QBC) methods, either alone or in combination (Fig. 4a).
Fig. 4. Diagnostic Tests for Specific AFI Causes
Figure 4: Types of diagnostic tests used for specific AFI causes: (a) Malaria, showing a mix of smear microscopy and rapid diagnostic tests. (b) Dengue, illustrating the use of IgM/IgG ELISA/ICT and NS1 antigen tests. (c1) Scrub typhus, highlighting the reliance on Weil-Felix Test (WFT) and IgM ELISA/ICT/IFA. (c2) Diagnostic platforms for IgM detection in Scrub Typhus. (d) Leptospirosis, indicating the use of IgM ELISA and MAT. (e) Typhoid, showing the use of Widal test, blood culture, and Typhidot test.
Dengue Diagnostics
Dengue was tested for in 25 studies. While 36% did not specify the tests used, 64% reported using dengue IgM and/or IgG enzyme-linked immunosorbent assays (ELISA) or immunochromatographic tests (ICT), and Non-structural protein 1 antigen (NS1) assays (ELISA or ICT) (Fig. 4b). ELISA was the most common platform for IgM and IgG detection (Fig. 5).
Fig. 5. Test Platforms for Dengue IgM, IgG, and NS1 Detection
Figure 5: Percentage distribution of studies using different test platforms for IgM, IgG, and NS1 detection in dengue diagnosis. ELISA is shown as the predominant platform, indicating its widespread use in dengue serology.
In studies with available data, NS1 tests detected 67–85% of dengue cases (Fig. 6). One study found that IgG-positive patients were more prone to complications than IgM-positive patients.
Fig. 6. Dengue Case Detection Rates by NS1, IgM, and IgG Tests
Figure 6: Distribution of dengue cases detected by NS1, IgM, and IgG tests across three studies with varying sample sizes. The graph illustrates the relative detection rates of each test type, providing insights into their diagnostic utility.
Scrub Typhus Diagnostics
Scrub typhus testing was reported in 65% of the studies. Most studies (63%) specified using the Weil-Felix test (WFT) with OX-K antigen, and IgM or IgG detection by ELISA, ICT, or immunofluorescence assay (IFA) (Fig. 4c1, c2). WFT thresholds for positivity varied across studies (Table 2), with different breakpoint titers used to define a significant immunogenic response (Fig. 7).
Table 2. Breakpoint Titre Thresholds for Positive WFT
| Study | Positive threshold | Threshold for convalescent sera |
|---|---|---|
| Kumar et al., 2012 | ≥ 1:80 | – |
| Mathai et al., 2003 | ≥ 1:80 | – |
| Razak et al., 2010 | ≥ 1:160 | 4-fold rise starting from 1:40 |
| Stephen et al., 2015 | ≥ 1:320 | 4-fold rise starting from 1:40 |
| Subbalaxmi et al., 2014 | ≥ 1:80 | – |
| Viswanathan et al., 2013 | ≥ 1:20 | – |
| Vivekanandan et al., 2010 | ≥ 1:80 | – |
| Katoch et al., 2016 | ≥ 1:320 | 4-fold increase in paired sera |
| Manickam et al., 2014 | ≥ 1:160 | – |
| Prasannan et al., 2017 | ≥ 1:320 | – |
Table 2: Breakpoint titre thresholds for a positive Weil-Felix Test (WFT) across various studies. This table shows the variability in WFT interpretation, with different studies using different titre cut-offs to define a positive result.
Fig. 7. Scrub Typhus Diagnosis at Different WFT Thresholds
Figure 7: Percentage of scrub typhus cases diagnosed using different antibody detection thresholds on the Weil-Felix Test (WFT) in three studies. The graph highlights how varying WFT thresholds can impact the diagnosis rate of scrub typhus.
Leptospirosis Diagnostics
Leptospirosis testing was conducted in 15 studies. IgM ELISA was used in 40% of these studies, and microscopic agglutination test (MAT) in one study (Fig. 4d).
Typhoid Diagnostics
Typhoid fever was investigated in 17 studies. Blood culture was used in three studies, Widal test in 14, and Typhidot test in two studies (Fig. 4e).
Etiology of AFI
Three studies specifically evaluated the causes of AFI. Scrub typhus, dengue, and malaria were identified as the most common causes. A significant proportion of AFI cases (17.5% to 8%) remained undiagnosed despite investigations.
Quality of Evidence
Quality assessment indicated varied risk of bias across study types. Case series and case reports generally showed low to moderate risk of bias. The case-control study had a high risk of bias. Cross-sectional studies presented a range of bias risks, from very low to high (Additional file 1: Tables S12-S20).
Discussion
The diagnostic practices for AFI in South India reveal a pattern of suspecting malaria most frequently, followed by dengue, scrub typhus, typhoid, and leptospirosis. This aligns with broader AFI etiology studies in India, where malaria, dengue, and scrub typhus are leading causes.
Microscopy remains a popular method for malaria diagnosis, consistent with national guidelines that recognize it as the gold standard, although RDTs are also recommended for rapid detection. In dengue diagnosis, a subset of studies used combined NS1 and IgM detection, which is supported by evidence of high diagnostic accuracy when used together and recommended by WHO and ICMR guidelines. The combined approach enhances diagnostic sensitivity, particularly after the initial days of illness, as NS1 is an early marker and IgM becomes more reliable later in the acute phase.
For scrub typhus, ICMR guidelines define a probable case based on WFT titres and positive IgM ELISA. Confirmed cases require PCR or IFA. While IgM ELISA offers early diagnosis, the WFT’s diagnostic accuracy is variable due to cross-reactivity and delayed positivity. The varying breakpoint titres used across studies and the inherent limitations of WFT raise concerns, especially given its widespread use (39% of studies) in diagnosing acute scrub typhus.
Typhoid diagnosis in the reviewed studies heavily relies on the Widal test, which is known to be unreliable due to variability, cross-reactivity, and poor reproducibility. More accurate tests like Typhidot RDTs, with better sensitivity and specificity, are available but less frequently used in the studies reviewed.
The prevalent use of WFT and Widal, along with smear microscopy’s limitations at low parasite densities, highlights the use of suboptimal pathogen-specific tests in AFI diagnosis in South India. Coinfections further complicate diagnosis, necessitating broader pathogen testing.
This review, while providing valuable insights, has limitations. The single reviewer assessment may introduce bias. The focus on English language peer-reviewed articles could result in publication and language bias. The tertiary care setting of all studies might skew the findings towards more complex AFI cases referred from peripheral centers, potentially exaggerating the number of diagnostics used. Finally, the South India focus limits generalizability to other parts of India due to regional variations in AFI epidemiology and healthcare practices. The teaching hospital/research settings of the studies may also represent best practices not consistently followed in other healthcare settings.
Conclusion
This review offers a comprehensive overview of AFI diagnostic practices in South India, revealing the extensive use of multiple tests and raising concerns about the reliance on suboptimal diagnostic tools. Timely and accurate AFI diagnosis is critical for reducing morbidity and mortality. Healthcare providers need clearer guidance on appropriate test utilization and their effectiveness at different disease stages, such as the differential use of IgM versus NS1 for dengue. National or sub-national guidelines could promote more standardized diagnostic approaches. Further research is needed to refine AFI and chronic fever definitions and develop algorithmic management strategies that incorporate optimal diagnostic test use. Improved diagnostic algorithms will facilitate more appropriate and timely antibiotic use, helping to combat unnecessary antibiotic overuse and antimicrobial resistance.
Supplementary Information
Additional file 1 provides complete search strategies, detailed statistics, tables on diagnostic tests, quality assessment, and characteristics of included and excluded studies.
Acknowledgements
The authors acknowledge Dr. Heidi Hopkins, Dr. Robin Bailey, and Dr. Tilak M Dhamgaye for their valuable inputs, and the LSHTM library staff for their assistance with the search strategy.
Abbreviations
AFI Acute febrile illness
AP Andhra Pradesh
AUFI Acute undifferentiated febrile illness
DDD Defined daily doses
EBV Epstein-Barr virus
ELISA Enzyme linked immunosorbent assay
EPOC Effective Practice and Organization of Care
HIC High income country
HIV Human immunodeficiency virus
ICMR Indian Council of Medical Research
ICT Immunochromatographic test
ICU Intensive care unit
IgM/IgG Immunoglobulin M/G
IPD Inpatient department
JBI Joanna Briggs Institute reviewer’s manual
KA Karnataka
KE Kerala
LMIC Lower middle-income country
MAT Microscopic agglutination test
NMAFI Non-malarial acute febrile illness
NSP 1 Non-structural protein 1
OPD Out-patient department
PCR Polymerase chain reaction
POCT Point-of-care test/testing
QBC Quantitative buffy coat
RDT Rapid diagnostic test
TN Tamil Nadu
TS Telangana State
WFT Weil-Felix test
WHO World Health Organization
Author’s Contributions
All authors contributed to the manuscript. DB and SD conceptualized the study, developed the search strategy, and conducted study assessments. DB and SD analyzed and interpreted the data. SC, SS, RS, and SA contributed to data analysis, interpretation, and manuscript revision.
Funding
This work was supported by FIND’s Malaria and Fever program, funded by UK aid and the Australian government.
Availability of Data and Materials
Supplementary data is available in the additional file.
Ethics Approval and Consent to Participate
Ethics approval was obtained from the London School of Hygiene Ethics Board. This secondary research used publicly available information, appropriately cited.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral regarding jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Divyalakshmi Bhaskaran, Phone: +44 0 7435575600, Email: [email protected].
Sarabjit Singh Chadha, Email: [email protected].
Sanjay Sarin, Email: [email protected].
Rajashree Sen, Email: [email protected].
Sonia Arafah, Email: [email protected].
Sabine Dittrich, Email: [email protected].
Supplementary Information
Supplementary information accompanies this paper at 10.1186/s12879-019-4589-8.