Psoriatic arthritis (PsA) is a chronic inflammatory condition affecting a significant portion, approximately 20–30%, of individuals with psoriasis. This debilitating disease leads to joint deformities and damage, substantially diminishing quality of life and often resulting in long-term functional disability. Recent research underscores the importance of early Diagnosis And Intervention in PsA to prevent irreversible impairment. However, the diagnostic process is often complicated by the diverse clinical presentations of PsA, which overlap with other conditions such as reactive arthritis, osteoarthritis, and ankylosing spondylitis. These shared characteristics can significantly delay accurate diagnosis and timely intervention. This review aims to provide a comprehensive overview of the current understanding of early PsA diagnosis and to emphasize the critical implications of early intervention in managing this progressive disease.
1. Understanding the Onset of Psoriatic Arthritis
Psoriatic arthritis is a prevalent comorbidity in patients with psoriasis, affecting a considerable percentage, ranging from 14.0% to 22.7% across different global populations [1,2,3]. Geographical variations in PsA incidence have been observed, with higher rates in European (22.7%) and South American (21.5%) psoriasis patients compared to North American (19.5%), African (15.5%), and Asian (14.0%) populations [3]. A survey conducted by the Japanese Society for Psoriatic Research indicated a 10.5% prevalence of PsA among new psoriasis patients [4]. Overall prevalence of PsA ranges from 0.19% to 0.25% in the general population [5,6]. The disease manifests through a variety of musculoskeletal symptoms, including peripheral arthritis, spinal spondylitis, asymmetrical synovitis, enthesitis, and dactylitis [7]. In 1973, Moll and Wright categorized PsA into five distinct subgroups based on clinical presentation: asymmetric oligoarthritis, predominant distal interphalangeal joint involvement, symmetric polyarthritis, predominant axial involvement, and arthritis mutilans [8].
The heterogeneous nature of PsA presents diagnostic challenges, as its clinical features are diverse and often mimic other rheumatologic conditions [9]. Diseases such as reactive arthritis, osteoarthritis, and ankylosing spondylitis share overlapping symptoms with PsA [10], making accurate and timely diagnosis difficult. Delayed diagnosis of PsA is strongly linked to poorer physical function and the development of permanent disability [11,12]. There is a growing consensus within the medical community that early diagnosis and rapid therapeutic intervention, particularly with biologics before structural damage occurs, can significantly mitigate joint damage and prevent long-term disability [13]. Notably, even psoriasis patients without clinically evident PsA can exhibit signs of enthesophyte formation [14]. Early psoriatic arthritis (ePsA), defined by inflammatory joint symptoms consistent with PsA of less than 24 months duration [13], often manifests as enthesoarthritis and carries a substantial risk of progressing to erosive and deforming arthritis within the first year of disease onset [15,16]. Multiple recent studies have highlighted the critical role of early diagnosis and intervention in preventing permanent disability in PsA.
This review will concentrate on the current understanding of diagnosing early PsA and discuss the significance of initiating early intervention strategies to improve patient outcomes.
2. Contemporary Perspectives on PsA Onset
Pinpointing the exact onset of PsA in individual patients remains a complex task. Typically, PsA is diagnosed when individuals present with psoriasis skin lesions alongside rheumatoid factor (RF)-negative inflammatory arthritis. However, contemporary research suggests that the pathophysiological processes underlying PsA may begin considerably earlier, potentially years before a clinical diagnosis is established. The Delphi consensus study proposed a staging system to better understand the progression to PsA, identifying three key stages: (1) individuals with psoriasis at increased risk for PsA; (2) individuals with psoriasis and asymptomatic synovio-entheseal imaging abnormalities; and (3) individuals with psoriasis and musculoskeletal symptoms not explained by other diagnoses [17].
2.1. Identifying Individuals with Psoriasis at Increased Risk for PsA
Psoriasis patients face a significantly elevated risk of developing PsA compared to both healthy individuals and patients with other conditions [6,18,19]. Therefore, it is crucial to identify psoriasis patients at higher risk of progressing to PsA to implement preventative strategies. However, current knowledge in this area is still evolving.
Certain clinical features in psoriasis patients have been identified as predictors of PsA development, including nail pitting and involvement of the scalp and genital areas [20]. Additional risk factors include obesity, the presence of arthralgia, severe psoriasis, a history of uveitis, nail psoriasis, scalp psoriasis, having a first-degree relative with PsA, and specific genetic markers such as human leukocyte antigens (HLA)-B*08, HLA-B*27, HLA-B*38, and HLA-B*39 [6,21].
2.2. Subclinical Synovio-Entheseal Abnormalities in Psoriasis Patients
Recent imaging studies have revealed that a subset of psoriasis patients, even those without overt arthritis symptoms like joint swelling or pain, exhibit abnormalities detectable through magnetic resonance imaging (MRI) or ultrasonography (US) [22,23]. These imaging modalities encompass MRI for assessing axial disease and peripheral arthritis, US for peripheral arthritis and enthesitis, and plain radiography for peripheral arthritis [24]. The detection of these subclinical abnormalities highlights the potential for earlier identification of individuals progressing towards PsA.
2.3. Musculoskeletal Symptoms in Psoriasis Patients without Clear Diagnosis
Some psoriasis patients report musculoskeletal symptoms such as heel pain, stiffness, and arthralgia that cannot be attributed to other diagnoses and are not accompanied by imaging abnormalities [25]. These patients have been described in prior research using various terms including “prodromal PsA,” “subclinical PsA,” “psoriasis with arthralgia,” “psoriasis with musculoskeletal symptoms,” or “psoriasis with musculoskeletal symptoms without musculoskeletal signs.” Understanding this stage is crucial for early intervention strategies.
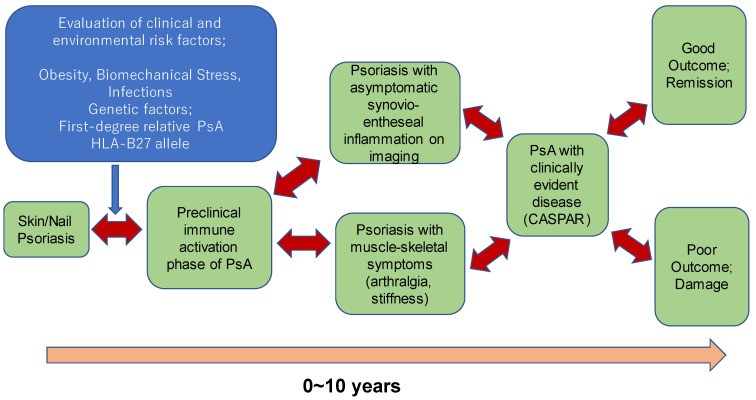 Progression of Psoriatic Arthritis
Progression of Psoriatic Arthritis
Figure 1. Clinical Stages of Psoriatic Arthritis. Illustration depicting the natural clinical progression of PsA, including preclinical stages. Note the potential reversibility at each stage, indicated by two-way arrows. Adapted from Pennington and Fitzgerald [26], Frontiers in Medicine 2021.
The progression through these stages to clinically defined PsA is illustrated in Figure 1. Given that PsA develops after a psoriasis diagnosis in over 80% of cases, identifying psoriasis patients at increased risk is paramount. Risk factors such as nail psoriasis, scalp and genital skin involvement are significant predictors of PsA in psoriasis patients. Psoriasis patients experiencing arthralgia (PsOAr) are also at a heightened risk. Importantly, approximately 50% of psoriasis patients without articular symptoms exhibit subclinical arthritis detectable through imaging. Among these, individuals with active enthesitis identified by ultrasonography have a greater likelihood of progressing to PsA [27].
3. Questionnaires as Screening Tools for Early PsA Diagnosis
Questionnaires designed to capture key symptoms such as joint pain, morning stiffness, and functional limitations can be valuable tools in the early diagnosis of PsA. Several questionnaires have been developed to screen psoriasis patients for potential PsA. The Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire is an effective initial screening tool with a reported sensitivity of 82% and specificity of 73% for detecting PsA [28]. The Psoriasis and Arthritis Questionnaire (PAQ), introduced in 1997, demonstrates a sensitivity of 85% and specificity of 88% in predicting PsA in psoriasis patients [29]. However, validation studies of the PAQ have shown variable results, with one study reporting a sensitivity of 60% and specificity of 62% [29]. A modified version of the PAQ has shown improved performance, with a sensitivity of 68.7% and specificity of 77.8% [29].
The Toronto PsA screening questionnaire (ToPAS) stands out by incorporating visual aids, including pictures of skin and nail lesions, to assess clinical features of PsA [30]. Unlike PASE and PAQ, ToPAS can screen for PsA regardless of whether a patient has a known psoriasis diagnosis [30], achieving a reported sensitivity of 94% and specificity of 92% [30]. This higher sensitivity and specificity make ToPAS a potentially valuable tool across various clinical settings.
The psoriasis epidemiology screening tool (PEST), developed in 2009 by Ibrahim et al., was designed for primary care settings and comprises five simple questions [31]. PEST showed a sensitivity of 92% and specificity of 78% in its primary care-based population [31]. The Early Arthritis for Psoriatic Patients (EARP) questionnaire, developed by Tinazzi et al. in 2012, uses ten straightforward questions and exhibits a sensitivity of 91% and specificity of 85% [32].
More recently, the Screening Tool for Rheumatologic Investigation in Psoriatic Patients (STRIPP) was developed by Burlando et al. [33]. STRIPP is a comprehensive tool with six sections covering demographic data, psoriasis assessment (PASI and specific locations like nails, scalp, genitalia), current treatments, a modified PASE (six questions), uveitis and inflammatory bowel disease history, and rheumatological evaluation including imaging and diagnosis. STRIPP has demonstrated a sensitivity of 91.5% and specificity of 93.3% [33].
Comparative studies of these PsA screening tools have yielded mixed results, with some indicating similar efficacy across different tools, while others, particularly comparing EARP, PEST, PASE, and ToPAS II, suggest that EARP has the highest sensitivity and ToPAS II the highest specificity. However, a common limitation of these questionnaires is their relatively low specificity, which can lead to the inclusion of other musculoskeletal pain conditions in PsA screenings. This is inherent to the heterogeneous nature of PsA and the challenge of developing a screening tool that effectively differentiates PsA from other causes of musculoskeletal pain. Therefore, clinicians must be aware of the possibility of encountering patients with musculoskeletal diseases other than PsA, even after initial screening with these questionnaires [34,35,36].
3.1. Classification Criteria for Psoriatic Arthritis
The absence of a universally accepted case definition for PsA has historically posed challenges for scientific and clinical research. However, in 2006, an international group of rheumatologists introduced the Classification of Psoriatic Arthritis (CASPAR) criteria, which remain the most widely recognized and utilized criteria, based on a large-scale prospective study [37]. Developed for clinical research purposes, the CASPAR criteria have demonstrated a high sensitivity (91.4%) and specificity (98.7%) in differentiating PsA from other forms of inflammatory arthritis. These robust metrics suggest potential utility as diagnostic criteria for PsA as well. Several studies have evaluated the sensitivity of CASPAR criteria specifically in detecting early PsA. While classification criteria like CASPAR are not primarily designed for diagnosis, their application in the context of ePsA detection continues to be explored [37]. Since their initial publication, the CASPAR criteria have been extensively studied to assess their effectiveness both as classification criteria and as a diagnostic aid, leading to their widespread adoption in various clinical studies on PsA [38,39,40].
4. Biomarkers in Early Psoriatic Arthritis
Biomarkers, defined as objective measures of physiological or pathological processes, hold significant promise for improving diagnosis and monitoring disease progression in PsA [41]. Currently, there are no disease-specific biomarkers uniquely identified for ePsA. A key differentiating factor between PsA and rheumatoid arthritis (RA), the most common inflammatory arthritis, is the typical absence of rheumatoid factor in PsA [10]. However, numerous candidate biomarkers with potential utility in PsA have been investigated [10], including elevated levels of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and acute-phase serum amyloid A (A-SAA). These are non-specific inflammatory markers also elevated in RA. Certain cytokines, such as interleukin (IL)-1, IL-12p40, interferon alpha, IL-15, and chemokine ligand 3, are found to be elevated in the synovial fluid of PsA patients with polyarticular involvement compared to those with monoarticular disease, suggesting their potential to differentiate between PsA subtypes [10].
S100A8/S100A9 (calgranulin) levels correlate with disease activity in PsA, with higher levels observed in patients with active disease. Methotrexate treatment has been shown to reduce S100A8/S100A9 levels in conjunction with improvements in swollen joint count, Richie articular index, and disease activity scores [42]. Elevated S100A8/S100A9 levels are also associated with a higher number of involved joints (>10) compared to those with fewer joints affected [43]. Vascular endothelial growth factor and angiopoetin-2, angiogenic markers known to predict joint damage in RA, are found at higher levels in PsA than RA and also predict joint damage in PsA [43]. Radiographic progression in PsA patients has been linked to levels of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand [44]. Baseline levels of A-SAA have been shown to correlate with 1-year radiographic progression in PsA patients. A-SAA levels are also associated with matrix metalloproteinases (MMP) 1, MMP3, MMP13, and tissue inhibitors of matrix metalloproteinases [45]. A-SAA is known to induce MMP production in synovial fibroblast-like cells [46], and MMP1 and MMP3 have been implicated in radiographic progression in early RA [47], suggesting their potential as early disease progression markers in PsA.
Genetic markers have indicated potential distinctions between psoriasis and PsA as separate entities. While numerous molecules show differential prevalence in psoriasis and PsA, most may not be directly involved in pathogenesis and exhibit weak correlations with disease. HLA molecules remain the most consistently identified genetic risk factors for PsA [6,21].
Epigenetic markers, currently under investigation, may offer a means to differentiate psoriasis from PsA. IL-22 is one such candidate, with studies showing changes in its methylation levels between patients with only cutaneous psoriasis and those with both cutaneous and articular involvement [48].
5. The Role of Imaging Techniques in Early Diagnosis
Imaging techniques offer enhanced sensitivity over clinical examinations for detecting synovitis and enthesitis, as well as for assessing inflammatory activity in PsA. Integrating imaging modalities into the assessment and early intervention strategies for ePsA may be critical for preventing permanent disability. In early PsA, inflammatory changes occur in soft tissues and bone marrow that are often undetectable by standard X-rays [9]. Ultrasonography and MRI are valuable and sensitive tools for detecting inflammatory joint disease in early stages [49,50,51]. Ultrasonography is frequently utilized in clinical practice to evaluate arthritis. Studies by Zabotti et al. [52] have shown that psoriasis patients with arthralgia (PsOAr) are at a higher risk of developing PsA and exhibit more frequent sonographic findings of tenosynovitis, although tenosynovitis alone was not predictive of PsA progression in longitudinal studies. However, sonographically detected active enthesitis was significantly associated with disease progression to PsA.
The synovio-entheseal complex (SEC) is recognized as an initial site of inflammation in PsA, particularly in areas of biomechanical stress, and helps to distinguish PsA from RA. Research indicates that up to half of asymptomatic psoriasis patients show subclinical synovial or entheseal inflammation on imaging [27]. Ultrasonography findings suggestive of early PsA include enthesitis of metacarpophalangeal (MP) joints and proximal interphalangeal (PIP) joints of the hands [52]. While MRI may be necessary for diagnosing axial disease, both MRI and ultrasound (US) can visualize enthesitis [24]. Dynamic MRI may provide a clinically useful measure of synovial inflammation. High-resolution peripheral quantitative computed tomography (HRpQ-CT) is an emerging technique primarily used for diagnosis and monitoring disease progression, offering detailed bone imaging [53]. High-resolution fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT imaging of the wrist and hand has been shown to be feasible in RA and PsA patient populations, providing quantifiable measures of disease activity [54,55]. Furthermore, 18F-FDG PET/CT has been reported as a powerful tool for detecting subclinical arthritis in patients with psoriatic arthritis and/or psoriasis vulgaris [56].
6. Treatment Strategies for Early Psoriatic Arthritis
The clinical course of ePsA is often characterized by fluctuations in signs and symptoms, with a disease trajectory that is not always linear. Some patients with ePsA may rapidly progress to severe disease, while others experience transient symptoms that resolve over time. This variability highlights the challenge in determining which patients require early and aggressive intervention to prevent severe disease progression and which may have a milder or self-limiting course. Ideally, biomarkers or predictive tools would help distinguish between these patient groups, facilitating targeted treatment strategies. However, in the absence of such definitive markers, treatment decisions are largely guided by current disease severity.
6.1. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Methotrexate (MTX)
Initial pharmacological management for ePsA often involves non-steroidal anti-inflammatory drugs (NSAIDs). For some patients, NSAIDs alone may be sufficient to manage symptoms, with residual mild disease or symptoms resolving spontaneously over time [57].
For patients requiring more robust treatment, methotrexate (MTX) is frequently considered [57]. MTX is approved by regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for severe psoriasis (often associated with PsA) and rheumatoid arthritis (RA), and is commonly used in PsA management despite limited evidence from randomized controlled trials (RCTs). Pincus T et al. [58] have discussed the limitations of MTX clinical trials in PsA, suggesting that factors such as suboptimal MTX dosing, inadequate patient stratification, or insufficient statistical power in older trials may have contributed to the lack of conclusive evidence. They propose that the observed treatment advantage of MTX over placebo, while not statistically significant in some studies (p > 0.05), may still be clinically relevant.
MTX was a cornerstone of PsA treatment before the advent of biologics, although it was not approved in Japan until March 2019 [59]. Potential adverse effects, including liver toxicity and bone marrow suppression, are important considerations with MTX use.
Leflunomide, a selective pyrimidine synthesis inhibitor that inhibits T-cell activation and proliferation, has also demonstrated efficacy in improving joint and skin symptoms in PsA, although its effect on skin symptoms may be less pronounced [57]. Leflunomide has shown effectiveness in several randomized, double-blind, placebo-controlled trials in PsA, in contrast to MTX, which lacks such robust RCT evidence [60,61].
The European League Against Rheumatism (EULAR) guidelines for PsA management recommend conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) like MTX or leflunomide for peripheral arthritis with polyarticular involvement, monoarthritis, or oligoarthritis, particularly in the presence of poor prognostic factors such as structural damage, elevated ESR and CRP levels, dactylitis, or nail involvement [57]. Biologic DMARDs (bDMARDs) are recommended when csDMARDs prove to be ineffective.
6.2. Biologic Therapies for Psoriatic Arthritis
Tumor necrosis factor (TNF) antagonists are well-established and effective biologic agents for PsA treatment [57]. Currently, several anti-TNF agents, including infliximab, adalimumab, and certolizumab-pegol, are approved for PsA treatment in Japan [62]. These agents have demonstrated efficacy in improving articular symptoms in both peripheral and axial disease, radiological findings, and skin and nail lesions [63].
Anti-IL-17 antibodies, such as secukinumab, ixekizumab, and brodalumab, have also proven effective in treating PsA with both peripheral and axial involvement. EULAR guidelines recommend TNF inhibitors and IL-17 inhibitors as first-line biologic options for axial disease, noting that MTX and IL-23 inhibitors are less effective in this manifestation [57]. Conversely, IL-12/23 inhibitors are effective for peripheral arthritis and are recommended at a similar level to IL-17 inhibitors, and considered superior to TNF inhibitors for peripheral arthritis when csDMARDs are inadequate [57].
Anti-IL-23 antibodies, including guselkumab, risankizumab, and tildrakizumab, may have reduced efficacy in treating axial disease compared to anti-IL-17 and anti-TNF antibodies. This difference may be attributed to IL-17-producing cells in axial lesions, such as ɤδ T cells and mucosal-associated invariant cells, being independent of IL-23 stimulation [64]. Radiographic studies, including MRI and HRpQ-CT, have shown a lack of both erosive and bone anabolic damage progression with secukinumab and ixekizumab, suggesting these agents may arrest the progression of structural changes in PsA.
Treatment of psoriasis and psoriatic arthritis with biologics has shown benefits in protecting patients from systemic inflammatory comorbidities, such as cardiovascular diseases, diabetes, and abnormal lipid metabolism. However, caution is warranted as anti-IL-17 antibodies may trigger new onset or exacerbate existing inflammatory bowel diseases [63].
6.3. Janus Kinase (JAK) Inhibitors in PsA Therapy
Janus kinase (JAK) inhibitors are a more recent class of targeted synthetic DMARDs (tsDMARDs) approved for PsA treatment. Due to potential adverse effects associated with this class, JAK inhibitors are typically recommended for PsA treatment when bDMARDs are not effective. The efficacy and safety of JAK inhibitors in PsA have been extensively evaluated. Three JAK inhibitors—tofacitinib, baricitinib, and upadacitinib—are approved for autoimmune diseases, with tofacitinib specifically approved for PsA treatment. Tofacitinib, an oral JAK inhibitor, expands treatment options for PsA and other inflammatory conditions [65].
7. The Imperative of Early Intervention in ePsA
The optimal timing for initiating early intervention in PsA remains a subject of ongoing discussion. The Interception in Very Early PsA (IVEPSA) study, an open-label prospective exploratory trial, suggested that very early intervention with secukinumab, an IL-17A inhibitor, in PsA may lead to significant improvement in skin symptoms [66]. The Tight Control Of inflammation in early Psoriatic Arthritis (TICOPA) study demonstrated the benefits of tight control treatment strategies in early PsA [67]. Further trials assessing the efficacy of targeted biologic therapies and DMARDs in early PsA are crucial to validate early intervention as a strategy to modify the disease course [67].
Biologic treatment in psoriasis patients without clinically evident psoriatic arthritis has been reported to reduce the incidence of subsequent PsA development [68]. This patient group may include individuals at increased risk, those with subclinical arthritis detected through imaging, or those with undiagnosed musculoskeletal symptoms, as discussed in Section 2. Identifying patients who would benefit from bDMARDs to prevent PsA development is essential to avoid overtreatment in those who may not progress.
However, the complex and variable disease course of PsA, with its fluctuating nature, necessitates the identification of novel biomarkers to distinguish patients who require early intervention to prevent poor outcomes.
7.1. Treatment Guidelines for Psoriatic Arthritis
Several organizations, including the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), European League Against Rheumatism (EULAR), American College of Rheumatology/National Psoriasis Foundation, and national dermatological associations, have published guidelines for the management of psoriasis and psoriatic arthritis, with ongoing updates to incorporate the latest advancements in treatment [69,70,71,72]. GRAPPA takes a comprehensive approach, considering both dermatological and musculoskeletal manifestations, while EULAR focuses primarily on rheumatic diseases, referring to dermatologists for skin concerns and not providing specific recommendations for skin and nail manifestations. Variations in healthcare systems and insurance coverage across countries necessitate the development of country-specific guidelines, often adapted from EULAR and/or GRAPPA recommendations. EULAR utilizes the Oxford Centre for Evidence-Based Medicine: Levels of Evidence, while GRAPPA employs the newer Grading of Recommendations, Assessment, Development and Evaluation system [73], both relying on systematic literature reviews (SLRs). GRAPPA primarily emphasizes randomized controlled trials. However, RCT data may be lacking for older medications like methotrexate (MTX). EULAR recommends MTX as a first-line treatment for PsA based on expert opinion, whereas GRAPPA does not rank MTX but includes it as a potential DMARD option.
7.2. Economic Considerations in PsA Treatment
While biologics are highly effective in managing PsA manifestations and improving health-related quality of life, they can significantly increase the economic burden on healthcare systems [74]. Studies in five European countries have estimated the total annual cost per PsA patient ranging from US $10,924 to US $17,050, considering purchasing power parity [75]. The introduction of biologics has been reported to lead to a 3-fold to 5-fold increase in direct costs and a subsequent rise in total healthcare costs [76].
Both EULAR guidelines and Japanese guidelines for PsA treatment do not recommend biologics as first-line therapy, instead favoring csDMARDs or MTX [57,76]. While biologics are highly effective, their cost can pose a substantial burden on national economies. In contrast, csDMARDs, including MTX, are cost-effective and remain valuable treatment options for PsA, often recommended before bDMARDs in many healthcare systems. However, the American College of Rheumatology/National Psoriasis Foundation guidelines recommend biologics at a similar level to csDMARDs, reflecting differences in insurance systems, particularly the prevalence of private insurance in the US [77]. Each country adapts EULAR and GRAPPA recommendations to align with its specific insurance and healthcare system. Due to the high cost of newer biologics and targeted therapies, their use may be restricted to certain periods in some countries. Developing guidelines that effectively balance patient benefit with the sustainability of social insurance systems is crucial for ensuring continued access to quality medical treatments.
Even with insurance coverage, the economic realities of modern society create disparities, with some patients unable to afford biologic treatments for PsA due to economic constraints.
8. Conclusion: Advancing Early Diagnosis and Intervention in Psoriatic Arthritis
Despite significant advancements in PsA therapies and treatment strategies, there remains a critical unmet need to personalize therapeutic approaches for individual patients. Early diagnosis and intervention in ePsA are essential to prevent disease progression, structural joint damage, and permanent disability. Standardized imaging techniques, validated scoring systems, and treatment protocols are crucial to achieving this goal. While new imaging modalities like US, MRI, and PET/CT have emerged, a definitive gold standard technique for detecting ePsA is still lacking. Psoriatic arthritis is a heterogeneous condition, encompassing preclinical, subclinical, and varying degrees of clinical severity, with disease progression being highly individual. This heterogeneity complicates the development of universally applicable guidelines. Early intervention holds the promise of modulating inflammation and altering the disease course, emphasizing the importance of identifying patients who will benefit most from early aggressive treatment to avoid both undertreatment of progressive disease and overtreatment of milder forms and to optimize healthcare resource utilization. However, robust tools to accurately identify these patients and strong evidence to guide early intervention strategies are still needed. Further research into the pathophysiology, diagnosis, and intervention of ePsA is imperative to improve patient outcomes and refine management strategies.
Acknowledgments
We extend our sincere gratitude to all members of the Psoriasis Outpatient Clinic at the Department of Dermatology, Jichi Medical University, for their invaluable cooperation in the diagnosis and treatment of psoriasis patients.
Author Contributions
Conceptualization, T.H. and M.K.; writing—original draft preparation, T.H.; writing—review and editing, M.K.; supervision, M.O.; funding acquisition, M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.