Corticobasal degeneration (CBD) is a complex neurodegenerative disorder that poses significant diagnostic challenges. Initially identified by its clinicopathological features as “corticodentatonigral degeneration with neuronal achromasia,” CBD is now understood to encompass a broader spectrum of clinical presentations than initially recognized. The classic presentation, known as corticobasal syndrome (CBS), while still relevant, often leads to misdiagnosis as other pathologies can mimic its symptoms. The pathological hallmark of CBD is the accumulation of hyperphosphorylated 4-repeat tau protein within neurons and glial cells, notably forming astrocytic plaques in specific brain regions. Despite the existence of various clinical diagnostic criteria, accurately diagnosing CBD antemortem remains a hurdle, with pathological confirmation occurring in only a fraction of clinically diagnosed cases. Furthermore, existing criteria predominantly focus on CBS, overlooking the increasingly recognized behavioral and cognitive manifestations of CBD.
The imperative for refined diagnostic criteria is underscored by the emergence of potential neuroprotective therapies targeting tauopathies. To address the limitations of current diagnostic approaches, an international consortium of experts in behavioral neurology, neuropsychology, and movement disorders collaborated to develop updated criteria. This initiative combined expert consensus, analysis of brain bank cases, and a comprehensive review of existing literature. However, the inherent clinicopathologic heterogeneity of CBD presents a significant challenge in creating highly specific diagnostic criteria, unlike some other neurodegenerative conditions. Consequently, this effort resulted in the proposition of two sets of criteria: more specific clinical research criteria for probable CBD and broader criteria for possible CBD. The latter, while more inclusive, acknowledges a higher likelihood of encompassing other tau-based pathologies.
Methods for Refining CBD Diagnosis Criteria
The development of these new diagnostic criteria involved a rigorous methodology. Existing clinical diagnostic criteria for CBD were thoroughly reviewed. An international panel of specialists convened to leverage their collective expertise and literature reviews to identify core clinical phenotypes and formulate preliminary CBD diagnostic criteria.
To further refine these criteria, a systematic literature search was conducted using MEDLINE (1950 to April 2012) and EMBASE (1980 to April 2012). This search targeted English-language publications detailing pathologically confirmed CBD cases, employing search terms such as “corticobasal,” “corticobasal degeneration,” and “CBD” in conjunction with “pathology.” Stringent inclusion criteria were applied, focusing on studies with at least five pathologically proven CBD cases to mitigate bias from atypical case reports and ensure sufficient data for analysis. Data extraction encompassed clinical phenotypes and symptoms/features. Only cases with definitive pathological confirmation of CBD were included to maintain diagnostic accuracy. The broad inclusion criteria aimed to maximize sample size and minimize biases related to case selection and variations in feature reporting. A comprehensive database was constructed to house reported features, including those deemed pertinent by the expert panel. Measures were taken to prevent case duplication across publications and brain bank data. Data from five brain bank centers specializing in CBD cases were incorporated, prioritizing original brain bank data over potentially less detailed published summaries. Two overlapping datasets were created: one for feature extraction and another for clinical diagnosis/phenotype information.
Clinical features were documented at initial presentation and throughout the disease course (“ever”), focusing on time points with the most consistent data availability. Presentation data was typically captured approximately 3.0 years post symptom onset. Feature presence was recorded only when explicitly described in the source material. While this approach might slightly overestimate feature frequency, it was deemed appropriate given the complexity of CBD and the variability in retrospective data detail. Consequently, the denominator for feature frequency calculations reflects the number of cases where that feature was reported, not the total sample size. Finally, a targeted literature review of clinicopathologic correlation studies was performed to identify clinical features that could enhance the differentiation of CBD from other pathologies. The insights gleaned from expert panel discussions, case reviews, and clinicopathologic studies were synthesized to formulate the proposed diagnostic criteria. A detailed glossary of terms is provided for clarity.
Advancements in Understanding CBD Diagnosis: Key Findings
Limitations of Previous Clinical Diagnostic Criteria
Prior diagnostic criteria for CBD, while termed as such, largely described the clinical presentation now recognized as CBS. These criteria emphasized an asymmetric movement disorder profile coupled with lateralized higher cortical dysfunction. The evolving understanding of dementia’s role in CBD is a prime example of diagnostic refinement. Earlier criteria often excluded “early dementia” to enhance diagnostic specificity. However, current knowledge recognizes dementia as a significant and often presenting feature in numerous CBD cases. This shift highlights the need for updated criteria that reflect the full clinical spectrum of CBD.
Insights from Systematic Literature Review
The systematic literature review yielded 37 articles meeting the inclusion criteria from an initial pool of 808 non-overlapping publications. Clinical feature data was extracted from 103 published and 106 brain bank cases, representing unique CBD patients. Brain bank data contributions came from several prominent institutions, enhancing the robustness of the dataset.
Comprehensive Clinical Features of CBD
Motor Impairments in CBD
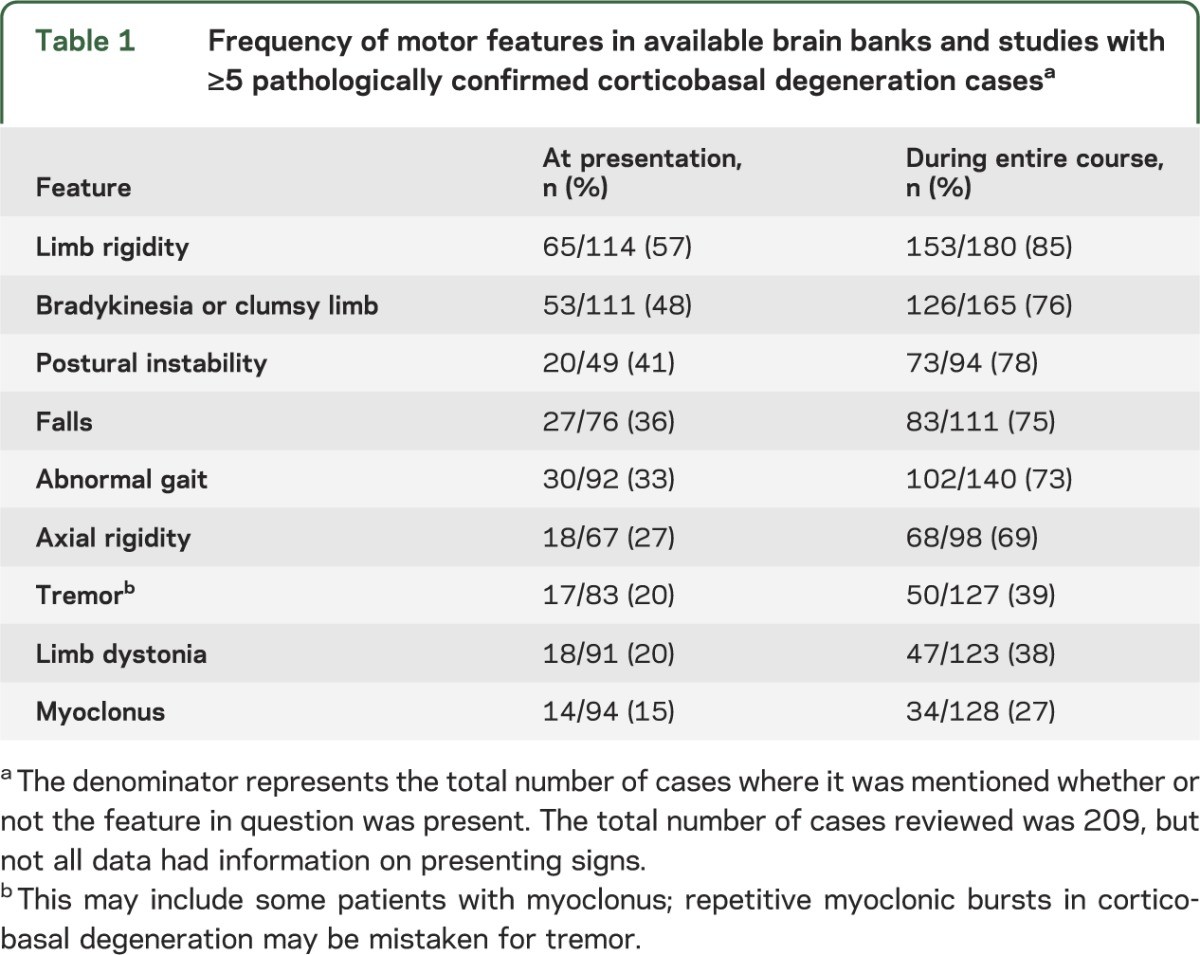
Early descriptions of CBD, often based on cases lacking complete pathological confirmation, emphasized the CBS presentation. This typically involved asymmetric, levodopa-resistant parkinsonism, dystonia, and myoclonus. Asymmetry in parkinsonism was documented in a significant majority (73%) of CBD patients exhibiting parkinsonian features. Limb rigidity (85%) and bradykinesia (76%) were the most prevalent motor findings. At presentation, limb rigidity was observed in 57% and bradykinesia in 48% of cases where these details were available. While often described as severe, the specific characteristics of limb rigidity were infrequently detailed, potentially encompassing parkinsonism, dystonia, or gegenhalten/paratonia. Axial rigidity was reported in 27% at presentation and 69% during the disease course. Notably, CBD series focusing on cognitive or behavioral presentations reported lower rates of early parkinsonism, indicating phenotypic variability.
Table 1. Frequency of Motor Features in Pathologically Confirmed Corticobasal Degeneration Cases
Tremor was documented in 39% of CBD cases throughout the disease, encompassing a mix of resting, postural, action, and undefined tremor types. The tremor phenotype in CBD is not well-defined, but CBS series suggest it differs from the typical rest tremor seen in Parkinson’s disease (PD). Low-amplitude action myoclonus may be misidentified as tremor.
Gait abnormalities, often poorly characterized, were reported in 73% overall, but only 33% at onset. Postural instability and falls occurred at similar frequencies. The timing of falls was often unspecified.
Sustained levodopa responsiveness has been a criterion for excluding CBD in prior diagnostic frameworks. While some CBD patients may exhibit transient, mild to moderate improvement with levodopa, and rarely develop levodopa-induced dyskinesias, a sustained beneficial response is uncommon. Levodopa response presence or absence was infrequently documented in the analyzed cases.
Dystonia is reported in a significant proportion (59%–71%) of patients in series combining CBS and CBD. However, limb dystonia was observed in only 38% of compiled CBD cases “ever” and in just 20% at presentation.
Clinical series report myoclonus in a substantial proportion (55%–93%) of CBS patients. In contrast, myoclonus occurred in only 27% of compiled CBD cases. Myoclonus in CBD can be superimposed on dystonia and is most commonly described in the upper extremities, but can also affect the face. Descriptions include “focal myoclonus,” “stimulus-sensitive myoclonus,” and “action myoclonus.” Studies of myoclonus in CBS suggest a very short latency may be characteristic, but this is less clear in CBD pathology-confirmed cases.
Higher Cortical Dysfunction in CBD
Higher cortical features in CBD include apraxia, alien limb phenomena, cortical sensory loss, cognitive impairment, behavioral changes, and aphasia.
Apraxia is a core feature in all previous diagnostic criteria. Limb apraxia was found in 57% of compiled CBD cases. Ideomotor apraxia is the most frequently described type in CBD, with some series also reporting limb-kinetic apraxia. The presence of limb dystonia, bradykinesia, and rigidity can complicate the assessment of ideomotor apraxia and make limb-kinetic apraxia diagnosis very challenging. Orobuccal apraxia and “apraxia of eyelid opening” are also described in CBD. However, the latter often represents pretarsal blepharospasm rather than true apraxia.
Table 2. Frequency of Higher Cortical Features in Pathologically Confirmed Corticobasal Degeneration Cases
Alien limb phenomena are included in prior criteria, but the specific behaviors constituting this phenomenon remain debated. Alien limb phenomena, encompassing complex unintentional limb movements interfering with tasks and the sensation of a limb being foreign or having its own will, were described in 30% of compiled CBD cases.
Cortical sensory loss, when reported (in less than half of compiled CBD cases), was present in about a quarter of cases. Visual neglect occurs in CBD, but also in Alzheimer’s disease (AD), highlighting the need for differential diagnosis.
Language impairments are now recognized as a common and often presenting feature of CBD. Aphasia occurred in 40% of compiled CBD cases at presentation and in 52% over the disease course. Reports commonly categorized the aphasia as primary progressive aphasia (PPA), progressive aphasia, or progressive nonfluent aphasia (PNFA), terms that have been revised in contemporary classifications. Aphasic CBD patients may progress to mutism. Apraxia of speech (AOS) has been described in CBD, both independently and with aphasia. Diagnostic challenges in AOS limit accurate frequency estimation. Speech abnormalities in general were described in 53% of cases (23% at presentation). Some patients exhibited dysarthria, while details were limited for others.
Table 3. Frequency of Other Features in Pathologically Confirmed Corticobasal Degeneration Cases
Cognitive impairment was present in over half of compiled CBD cases at onset and in 70% during the disease course. This encompassed subjective memory concerns and objective cognitive disturbances in areas like executive function and language. Neuropsychological testing in CBD patients has revealed difficulties in learning tasks, word fluency, verbal comprehension, perceptual organization, and cognitive flexibility. Impairments in executive, language, and visuospatial domains with relatively preserved episodic memory have also been noted. Conversely, prominent memory loss was a presenting symptom in some series. The presence of memory impairment in CBD is further emphasized by numerous series where clinical diagnoses of AD or atypical AD were ultimately found to be CBD at pathology. Acalculia and visuospatial difficulties are infrequently described in CBD.
Behavioral changes and executive dysfunction are common in CBD, with many patients presenting with a behavioral variant frontotemporal dementia (FTD) syndrome. Symptoms include apathy, bizarre or antisocial behavior, personality changes, irritability, disinhibition, and hypersexuality. Behavioral changes were observed in 55% of reviewed CBD cases, often at presentation. Clinical depression (rather than formal diagnosis) was described in 51% of patients where mood was recorded, but this information was available for less than half of cases. Hallucinations were rare, with only a single published patient reporting them coincident with levodopa treatment, and rare reports in brain bank subjects not associated with levodopa.
Other Clinical Features
Eye movement abnormalities may occur in CBD, but details are often lacking and terminology is ambiguous. Eye movement abnormalities were present in 60% of compiled cases, and 33% at onset. Studies of CBS patients describe increased saccadic latency, but this finding was not consistently replicated in a publication including oculographic measurements in a small CBD cohort, where visually guided saccades were often normal, but antisaccade performance was consistently abnormal. Upper motor neuron signs are another described feature in CBD cases, but their presence is not highly specific as they also occur in other atypical parkinsonisms.
CBD Phenotypes: Expanding the Diagnostic Landscape
While clinical features were extracted from 209 CBD cases, a broader analysis of 267 CBD cases from literature and brain banks provided information on clinical diagnoses or phenotypes. Among 210 cases with final clinical diagnoses, five phenotypes accounted for 87.1% of cases: CBS (37.1%), progressive supranuclear palsy syndrome (PSPS, also known as Richardson syndrome) (23.3%), FTD (13.8%), AD-like dementia (8.1%), and aphasia (typically PPA or PNFA) (4.8%). Mixed diagnoses involving these phenotypes accounted for an additional 5.7%. PD was diagnosed in 3.8%, and dementia with Lewy bodies (DLB) in a smaller number.
In 129 cases with initial clinical diagnoses, CBS was the most frequent (27.1%), followed by FTD and PD/atypical PD (each 15.5%), aphasia (14.7%), AD/dementia (9.3%), and PSPS (6.2%). The presence of PD as an early diagnosis and the shift in phenotype frequency from initial to final diagnosis highlights the challenges in early and accurate diagnosis of CBD and the evolving clinical presentation over time.
Having identified these phenotypes, further literature review sought clinical features from clinicopathologic series that could aid in predicting underlying pathology, given that these phenotypes are also associated with non-CBD pathologies.
Age, Disease Duration, and Family History
The mean age at CBD symptom onset was 63.7 years, with a range from 45 to 77.2 years. Mean disease duration was 6.6 years, ranging from 2.0 to 12.5 years. CBD typically does not present as a rapidly progressive dementia compared to conditions like Creutzfeldt-Jakob disease (CJD).
Most CBD patients lack a family history of the condition. However, a family history of FTD spectrum disorder has been reported in a small subset of CBD cases. Rare familial CBD-like disorders linked to tau mutations exist, but sporadic CBD is the norm. Familial CBS may be associated with granulin (GRN) mutations and frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions (FTLD-TDP) pathology, rather than CBD pathology.
Phenotype Comparisons and Biomarkers
Studies directly comparing different CBD phenotypes are limited in definitively identifying clinical or imaging features that reliably distinguish CBD from other pathologies. Potential differentiating features require validation in larger studies.
Neuroimaging and laboratory markers in CBD have been sparsely investigated. Existing imaging studies in clinical cohorts often show abnormalities consistent with clinical findings but not specific to CBD pathology. Recent research on atrophy patterns in CBS associated with CBD, progressive supranuclear palsy (PSP), AD, and FTLD-TDP pathology shows promise but requires prospective validation before integration into diagnostic criteria. Imaging can be helpful in excluding conditions mimicking CBS, such as CJD. CSF biomarkers hold potential but are not yet adequately studied in CBD. Therefore, a laboratory-supported diagnostic category is not currently proposed.
Proposed CBD Diagnostic Criteria: Probable and Possible CBD
The complex clinicopathologic correlations and evolving phenotypes of CBD make diagnosis of CBD challenging. While AD is a frequent clinical misdiagnosis, detailed information on how these diagnoses were reached and the specific features prompting them is limited. Given the higher prevalence of AD compared to CBD, including an AD phenotype in CBD criteria would likely result in a high false-positive rate. Therefore, the AD phenotype was excluded. The four clinical phenotypes deemed most representative of CBD are CBS, frontal behavioral-spatial syndrome (FBS), nonfluent/agrammatic variant of primary progressive aphasia (naPPA), and PSPS.
Table 4. Proposed Clinical Phenotypes Associated with Corticobasal Degeneration Pathology
While these four phenotypes are common clinical presentations of CBD, identifying features that consistently predict CBD pathology versus other pathologies like FTLD or PSP remains difficult. This led to the development of two diagnostic classifications: clinical research criteria for probable CBD (cr-CBD) and possible CBD criteria (p-CBD). Cr-CBD aims to maximize specificity for classic CBD pathology, minimizing contamination from other pathologies. P-CBD criteria are broader, less restrictive, but still emphasize presentations consistent with underlying tau pathology. P-CBD criteria are expected to capture more CBD cases but also have a higher false-positive rate, potentially including patients with PSP or other neurodegenerative conditions. This broader approach may be relevant for research studies, including therapeutic trials targeting tauopathies in general.
Table 5. Diagnostic Criteria for Corticobasal Degeneration
Both cr-CBD and p-CBD criteria require insidious onset and gradual progression over at least one year to exclude rapidly progressive conditions suggestive of other pathologies (e.g., CJD). Age at onset ≥50 years is required for cr-CBD to capture the majority of CBD cases and exclude pathologies with younger onset (e.g., FTLD). No age minimum is set for p-CBD to allow for young-onset familial CBD cases related to tau mutations. Similarly, a family history (>1 relative) of a comparable neurodegenerative disease is an exclusion criterion for cr-CBD but not p-CBD.
Accepted phenotypes differ slightly between the two criteria sets. While PSPS is a CBD phenotype, it is more commonly associated with PSP than CBD. Thus, PSPS is only an acceptable phenotype in p-CBD criteria. Features suggestive of idiopathic PD (classic 4- to 6-Hz resting tremor, excellent and sustained levodopa response) are exclusion criteria for both, as are hallucinations, which are more indicative of PD or DLB. Prominent dysautonomia and cerebellar signs, suggestive of multiple system atrophy, are also exclusion criteria based on expert consensus. Coincident upper and lower motor neuron signs or semantic- or logopenic-variant PPA are excluded due to their association with non-tau pathology.
While tau mutations are permitted for p-CBD, GRN, TDP-43, or FUS mutations are exclusion criteria for both cr-CBD and p-CBD. Given the emerging role of amyloid imaging and CSF Aβ42/tau ratio in AD diagnosis, and their incorporation into AD diagnostic criteria, results suggestive of AD on these studies are exclusion criteria for CBD. This acknowledges the current lack of definitive validation for distinguishing AD from CBD using these tests. Mutations known to cause AD are also exclusion criteria. Similarly, imaging suggestive of other pathologies (e.g., CJD) are exclusion criteria for CBD, while CBD-supportive imaging is not yet included as a diagnostic criterion due to the need for further validation.
Discussion and Future Directions in CBD Diagnosis
The proposed diagnostic criteria were developed through expert consensus and rigorous review of brain bank and published clinical-pathologic series. Limitations inherent in retrospective data, incomplete records, and subspecialty biases were acknowledged and addressed through strategic case selection, brain bank data utilization, and maximizing sample size. The decision to record features as present only when explicitly described may lead to an overestimation of feature frequency, which should be considered in interpretation.
The ongoing challenge in diagnosis of CBD lies in identifying highly specific clinical phenotypes and features. The proposed two-tiered criteria (probable and possible CBD) address this by providing both a more specific research-oriented approach and a broader, more inclusive clinical approach. Ultimately, definitive diagnosis of CBD may rely on the identification of specific biomarkers and associated genetic mutations. In the interim, broader criteria may be valuable, particularly as therapeutic strategies may target tauopathies as a class, rather than focusing on individual tau-based diagnoses.
Our understanding of CBD has significantly advanced since its initial description, yet diagnostic complexities persist. These new CBD diagnostic criteria, based on recent progress and a large cohort of pathologically proven cases, represent a significant step forward. Continued revisions and refinements are anticipated as our understanding of CBD deepens and as imaging and biomarker technologies evolve and become validated for differentiating phenotypes and diagnoses.
Acknowledgment
The authors gratefully acknowledge the contributions of the CBD patients and their families, and the brain bank contributions from Keith A. Josephs, Paul McMonagle, Andrew Kertesz, Peachie Moore, Murray Grossman, Suzee E. Lee, and Dennis W. Dickson.
Glossary
- AD: Alzheimer disease
- AOS: apraxia of speech
- CBD: corticobasal degeneration
- CBS: corticobasal syndrome
- CJD: Creutzfeldt-Jakob disease
- cr-CBD: clinical research criteria for probable corticobasal degeneration
- DLB: dementia with Lewy bodies
- FTD: frontotemporal dementia
- FTLD-TDP: frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions
- GRN: granulin
- p-CBD: possible corticobasal degeneration criteria
- PD: Parkinson disease
- PNFA: progressive nonfluent aphasia
- PPA: primary progressive aphasia
- PSP: progressive supranuclear palsy
- PSPS: progressive supranuclear palsy syndrome