Introduction
Clostridium difficile, now reclassified as Clostridioides difficile, was initially recognized as a significant cause of antibiotic-associated diarrhea in 1978.1 However, since the emergence of the epidemic BI/NAP1/027 strain around the year 2000,2 C. difficile infections (CDI) have become more prevalent and increasingly challenging to treat.2–4 The rise in CDI incidence and severity underscores the critical need for accurate and timely Diagnosis Of Clostridium Difficile to guide effective management and improve patient outcomes.
In the United States, hospital discharge diagnoses for CDI more than doubled between 2001 (approximately 148,900 discharges) and 2005 (around 301,200 discharges).5 The incidence of CDI has climbed from 4.5 per 1000 adult discharges in 2001 to 8.2 per 1000 discharges in 2010.6 This surge not only impacts patient health but also significantly increases healthcare costs, with annual attributable expenses exceeding $1.5 billion in the U.S.7
CDI development is a two-step process involving the acquisition of C. difficile and a disruption of the normal gut microbiota. While the precise mechanism of symptomatic CDI is still being elucidated, it’s understood that C. difficile itself is non-invasive, and toxin production is the primary driver of pathogenesis. Non-toxigenic strains do not cause diarrhea. The toxins disrupt the epithelial barrier by targeting microtubules and tight junctions between cells, leading to the release of cytokines like IL-8.8 This cascade of events promotes inflammation in the colonic mucosa, fluid shifts resulting in diarrhea, and epithelial necrosis. Antibiotic use is a major risk factor as it disturbs the normal gut flora, increasing susceptibility to CDI.9 Other factors associated with increased CDI risk include advanced age, recent and prolonged hospitalization, multiple antibiotic courses, extended antibiotic use, proton pump inhibitors, chemotherapy, chronic kidney disease, and feeding tubes.10–14 This article focuses on the current best practices for the diagnosis of clostridium difficile and its treatment in adults, encompassing both established and novel diagnostic and therapeutic approaches.
Methods
To gather evidence for this review, a comprehensive literature search was performed using Ovid Medline and Cochrane databases. Search terms included “Clostridium difficile” and related synonyms, focusing on diagnostic testing and treatment strategies (Appendix A). We included studies published between January 1978 and October 31, 2014. Studies not in English, as well as those involving animals or children, were excluded. The initial search yielded 4,682 articles. After reviewing abstracts and bibliographies of retrieved studies and previous reviews, we selected 196 articles for closer examination, ultimately including the 116 most clinically pertinent articles (Appendix B). We also reviewed meta-analyses, systematic reviews, and clinical practice guidelines published within the last decade.
Diagnosing C. difficile Infection: Patient Selection and Criteria
A critical aspect of effective diagnosis of clostridium difficile infection is recognizing when testing is appropriate. Laboratory tests alone cannot differentiate between asymptomatic colonization and active infection. Therefore, CDI diagnosis requires two key criteria: 1) the presence of diarrhea, defined as three or more unformed stools within a 24-hour period, and 2) a positive stool test for toxigenic C. difficile or its toxins, or colonoscopic/histopathologic findings indicative of pseudomembranous colitis.15–17 The definitive diagnosis, considered the gold standard, involves detecting toxigenic C. difficile in stool alongside colonic histopathology demonstrating pseudomembranes in a patient exhibiting clinical symptoms.18 Many clinical laboratories rightly restrict C. difficile testing to diarrheal stool samples only.15,16,19–21
It’s important to note that asymptomatic shedding of C. difficile spores can persist for up to six weeks in a significant proportion of patients (56%) even after successful treatment.22,23 Consequently, “test of cure” is generally not recommended.15 Studies have documented chronic shedding and a higher prevalence of asymptomatic colonization, particularly in healthcare settings, supporting the idea of long-term asymptomatic carriage post-CDI.24,25 Recurrent symptoms in the weeks following CDI resolution can occur due to transient functional bowel disorders in up to 35% of patients. However, persistent symptoms beyond three months due to post-infectious irritable bowel syndrome are less common, affecting only about 4.3% of patients.26 Clinical practice guidelines advise against treating asymptomatic C. difficile carriage,15 emphasizing the need to distinguish between recurrent CDI symptoms and those stemming from functional bowel disorders or irritable bowel syndrome. Currently, validated methods to definitively differentiate these conditions are lacking.
C. difficile Testing Methods: A Comprehensive Overview
Organism Detection Methods
Toxigenic culture (TC) is considered the gold standard for detecting toxigenic C. difficile in stool (Table 1).19 This method involves anaerobically culturing stool specimens on specialized media27 for 24–48 hours. After colony selection and taxonomic confirmation, isolates are incubated for another 48 hours followed by a cell cytotoxicity assay (CCA) (Table 1). However, the independent performance of TC can be challenging to ascertain as most studies compare other diagnostic methods against TC or CCA,19 and variations exist in media choices and sample pretreatment protocols.
Table 1. Diagnostic Tests for Toxigenic C. difficilea
| Testing Method | Target(s) | Notes |
|---|---|---|
| Gold Standard Tests | ||
| Toxigenic Culture | Toxigenic C. difficile | • Reference standard • Difficult to perform • Time consuming (24–48 hours) |
| Cell Cytotoxicity Assay | Toxins A or Bb | • Reference standard • Highly sensitive for toxin compared to EIA • Difficult to perform • Time consuming (24–48 hours) |
| Rapid Diagnostic Tests | ||
| EIA | GDH | • GDH alone insufficient for diagnosis (must be paired with a test for toxin) • Rapid • Variable sensitivity and specificity |
| EIA | Toxins A or Bb | • Rapid • Variable sensitivity and specificity |
| NAAT | tcdB or tcdC genes | • Rapid but more expensive than EIA • Highly sensitive and specific for presence of toxigenic C. difficile • May increase detection of colonization and not true CDI |
| RT-PCR | tcdB or tcdC genes | • tcdA– / tcdB+ strains can cause disease |
| LAMP | tcdA or tcdB genes | • *tcdA+ / tcdB– not well-described in human disease • Caution required in interpreting negative results based on tcdA* testing alone by LAMP |



Abbreviations: CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; LAMP, loop-mediated isothermal amplification; NAAT, nucleic acid amplification testing; RT-PCR, real-time polymerase chain reaction.
aRefer to the text or Table 2 / Appendix C for sensitivity / specificity of the diagnostic tests
bC. difficile can produce toxin A and/or toxin B. Although both play a role in clinical disease, it is not known if strains producing only toxin A are associated with symptomatic infection in humans.
Despite being the reference standard, TC is labor-intensive, time-consuming, and requires specialized equipment and skilled personnel. Diagnostic delays associated with TC can impact treatment initiation and infection control measures.29,30 Rapid diagnostic tests address these limitations. One such approach involves detecting glutamate dehydrogenase (GDH), a C. difficile enzyme, typically using enzyme immunoassay (EIA). However, GDH is produced by both toxigenic and non-toxigenic C. difficile strains. Given that asymptomatic colonization studies suggest a significant proportion (up to 46%) of C. difficile isolates are non-toxigenic,31 GDH testing alone is insufficient for CDI diagnosis and must be combined with a toxin detection assay. Performance characteristics of GDH EIA tests show considerable variability (Table 2).
Table 2. Systematic Reviews and Meta-Analyses Examining Performance Characteristics of Rapid Diagnostic Tests for Clostridium difficile Infection
| Test | Source | Number of Included Studies | Sensitivity | Specificity |
|---|---|---|---|---|
| Organism Detection | ||||
| GDH EIA | Crobach et al., 200919 | 11 | 0.88 (0.6–0.97)ae | 0.89 (0.75–0.97)ae |
| Shetty et al., 2011111 | 13 | 0.92 (0.8–1)ae | 0.93 (0.83–1)ae | |
| NAAT | Crobach et al., 200919 | 4 | 0.91 (0.86–1)ae | 0.96 (0.94–1)ae |
| Deshpande et al., 2011112 | 19 | 0.9 (0.88–0.91)be | 0.96 (0.96–0.97)be | |
| O’Horo et al., 2012113 | 25 | 0.92 (0.91–0.94)bc | 0.94 (0.94–0.95)bc | |
| 0.87 (0.84–0.9)bd | 0.97 (0.97–0.98)bd | |||
| Toxin Detection | ||||
| Toxin A/B EIA | Crobach et al., 200919 | 60 | 0.73 (0.32–0.99)ae | 0.98 (0.65–1)ae |
| Planche et al., 2008114 | 18 | 0.87 (0.69–0.99)ae | 0.97 (0.92–1)ae |
Abbreviations: EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; NAAT, nucleic acid amplification testing.
aMean (range)
bPooled (95% confidence interval)
cCompared to TC
dCompared to CCA
eCompared to TC+CCA or another mixed reference standard
Nucleic acid amplification tests (NAAT), including RT-PCR and loop-mediated isothermal amplification (LAMP), can detect the tcdA/tcdB genes (which regulate toxin A/B production) or the tcdC gene (a negative regulator of toxin A and B production) in a single step, thus identifying the presence of toxigenic C. difficile (Table 1).19,21,32,33 NAATs generally exhibit high sensitivity and specificity (typically >0.90 range, as shown in Table 2). However, this enhanced sensitivity can also detect toxigenic C. difficile in asymptomatic carriers. This highlights the importance of testing only symptomatic patients and has led some experts to caution against using NAAT-based testing alone for routine CDI diagnosis.16,19,34
Toxin Detection Assays
The cell cytotoxicity assay (CCA) is considered the gold standard for directly detecting C. difficile toxins A and/or B.27 CCA is performed either directly on stool samples or as part of a toxigenic culture. The process involves inoculating filtrates of stool suspensions or culture supernatants into cell cultures and then assessing for cytopathic effects after 24 to 48 hours.27 This highly sensitive test can detect minute amounts of toxin (as low as 3 picograms) and boasts high sensitivity (0.94–1) and specificity (0.99), particularly when combined with antiserum.27,35 The primary drawbacks of CCA are its longer turnaround time and technical complexity.
Enzyme immunoassays (EIAs) for toxin A and/or B show variable sensitivity and specificity (Table 2). Repeating EIA testing does not significantly improve sensitivity. A systematic review indicated that the vast majority (91%) of positive EIA results are obtained with the initial test, and the probability of subsequent tests becoming positive after two negative results is very low 36.
Multistep Diagnostic Algorithms for CDI
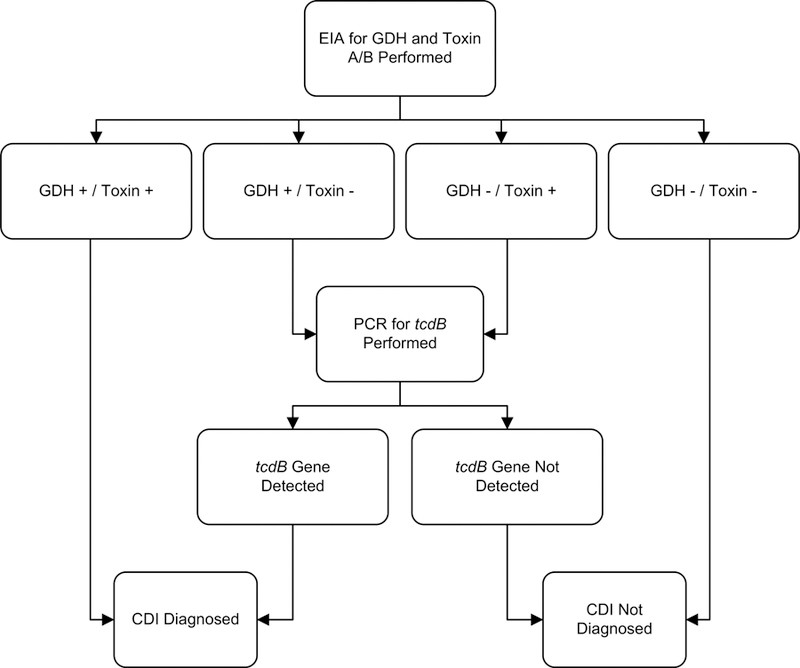
Given the limitations of some toxin EIA kits in terms of sensitivity, and the potential for over-diagnosis of asymptomatic colonization with single-step NAAT algorithms, multistep algorithms have gained favor among experts and are recommended by some guidelines for rapid diagnosis of clostridium difficile infection.15,16,19,34 A representative example is shown in Figure 1, demonstrating a sensitivity of 0.91, specificity of 0.98, and a negative predictive value of 0.99.37
Figure 1. Sample Multistep Algorithm for Rapid Diagnosis of C. difficile Infection.
Abbreviations: CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction.
Footnotes: Adapted under Creative Commons License from Rao K, Erb-Downward JR, Walk ST, et al. The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE. 2014;9(3):e92578.
We reviewed studies evaluating rapid testing algorithms in diagnosis of clostridium difficile, comparing them to at least one gold standard method (Appendix C). Generally, multistep algorithms incorporating NAAT demonstrated excellent sensitivity (0.68–1) and specificity (0.92–1). In contrast, algorithms relying solely on GDH or toxin EIA testing showed poorer and more variable performance. A large, multi-center study by Planche et al.38 reported that a GDH/NAAT-based algorithm achieved the highest sensitivity (0.91–0.98) and specificity (0.96–0.98) (Appendix C).
Treatment of C. difficile Infection (CDI): Strategies and Considerations
Since 2000, treatment failures and recurrences of CDI have become increasingly common.2–4 These challenges are likely due to a complex interplay of patient-specific factors, bacterial virulence, and the ability to achieve effective drug concentrations in the colon. Some C. difficile strains exhibit higher minimum inhibitory concentrations to metronidazole, potentially contributing to treatment failures.39 Current guidelines recommend tailoring CDI treatment based on disease severity, recurrence risk, and potential for complications.15,16
Markers of Disease Severity in CDI
Clinical presentations of CDI span a spectrum from mild diarrhea to life-threatening illness. Various prediction rules have been developed to assess the risk of recurrence, complications, and mortality.40 However, many of these studies have been limited by small sample sizes and significant heterogeneity.40 One prospective study of 746 CDI patients proposed a risk scoring system for predicting fulminant CDI based on factors such as age >70 years, WBC counts (≥20,000 or ≤2,000 cells/mL), cardiorespiratory failure, and diffuse abdominal tenderness. Patients with a score ≥6 were categorized as high risk.41 Another scoring system used age, systemic antibiotic treatment, leukocyte count, albumin, and serum creatinine to predict response to vancomycin or fidaxomicin.42
Clinical practice guidelines categorize CDI severity based on clinical and laboratory findings. Mild CDI is typically defined as diarrhea without systemic signs of infection and a WBC count <15,000 cells/mL and serum creatinine <1.5 times premorbid level. Severe, complicated CDI is characterized by hypotension or shock, ileus, or megacolon.15 European guidelines define severe CDI as an episode with a complicated course or signs/symptoms of severe colitis, systemic toxin effects, and shock requiring ICU admission, colectomy, or resulting in death. Key findings include WBC >15 X 109/L and serum albumin <30 g/L.16 The term “fulminant” is often used to describe severe, complicated CDI.42–44 (Table 3)
Table 3. C. difficile Infection (CDI) Classification Based on Disease Severity
| Disease Category | Clinical and Laboratory Signs | Associated Risk Factors |
|---|---|---|
| Mild to moderate CDI | Diarrhea without systemic signs of infection, WBC < 15,000 cells/mL, serum creatinine < 1.5 times premorbid level 15 | Antibiotic use, previous hospitalization, longer duration of hospitalization, use of proton pump inhibitors, receipt of chemotherapy, chronic kidney disease, and presence of a feeding-tube 10–14. |
| Severe CDI | Systemic signs of infection, and/or WBC ≥ 15,000 cells/mL, or serum creatinine ≥ 1.5 times the premorbid level 15 | Advanced age, infection with BI/NAP1/027 strain 115,116. |
| Severe, complicated CDI | Systemic signs of infection including hypotension, ileus, or megacolon 15 | See above, plus recent surgery, history of inflammatory bowel disease and intravenous immunoglobulin treatment 43 |
| Recurrent CDI | Recurrence within 8 weeks of successfully completing treatment for CDI 16,20 | Patient age ≥65 years, concomitant antibiotic use, presence of significant comorbidities, concomitant use of proton pump inhibitors, and increased initial disease severity 16 |
Asymptomatic C. difficile Carriers
Asymptomatic carriage of C. difficile is prevalent, ranging from 10% to 52% in various populations.45–49,25 Asymptomatic fecal shedding may be transient. Interestingly, asymptomatic colonization does not appear to increase the risk of developing symptomatic CDI and may even offer protection against future symptomatic disease.31,47,51 A study by Shim et al. involving 618 non-colonized patients and 192 asymptomatic carriers found that symptomatic CDI developed in 3.6% of non-colonized patients but only in 1% of asymptomatic carriers 31.
Antibiotic Withdrawal as a Strategy
The gut microbiota plays a crucial role in preventing pathogen overgrowth, including C. difficile. Antibiotics disrupt this protective microbiota, with penicillins, cephalosporins, and clindamycin being particularly strongly associated with CDI risk.52–54 Fluoroquinolones are linked to an increased risk of infection with the virulent BI/NAP1/027 strain.12
Historically, antibiotic withdrawal alone was sometimes used as CDI treatment.55 A study by Olson et al. (1982-1991) found that 15% of CDI patients experienced symptom resolution with antibiotic withdrawal alone.56 While the effectiveness of antibiotic withdrawal as a standalone treatment for mild CDI remains uncertain, evidence suggests it may be beneficial when combined with standard CDI therapies.57 Conversely, continuing the offending antibiotics is associated with a higher risk of CDI recurrence.58
Metronidazole versus Vancomycin: First-Line Therapies
Metronidazole and vancomycin have been the primary treatments for CDI since the 1980s. Early studies suggested comparable efficacy between oral metronidazole and oral vancomycin, with similar tolerability and relapse rates.56,59,60 However, more recent data indicate higher treatment failure rates with metronidazole in severe or complicated CDI.3,61–64
A large retrospective study observed an increase in oral metronidazole treatment failures (from 10% to 26%) and a rise in the 60-day recurrence probability (from 21% to 47%) after the emergence of the BI/NAP1/027 strain.4 However, other studies have not consistently demonstrated increased metronidazole failures post-BI/NAP1/027 emergence.65,66
A randomized trial by Zar et al. comparing metronidazole and vancomycin in 150 patients stratified by CDI severity revealed no significant difference in cure rates for mild CDI (90% vs. 98%, respectively). However, in severe CDI, vancomycin showed significantly better cure rates (76% vs. 97%).63 A systematic review (2001-2010) reported higher treatment failure rates with metronidazole compared to vancomycin (22.4% vs. 14.2%; P = 0.002), while recurrence rates were similar. Metronidazole failures were more frequent in North America than in Europe.3 A large clinical trial comparing tolevamer (a toxin-binding polymer) with vancomycin and metronidazole found tolevamer inferior to both. Importantly, metronidazole was also found to be inferior to vancomycin in terms of success rates, particularly in severe CDI (66.3% for metronidazole vs. 78.5% for vancomycin).64
Factors associated with metronidazole treatment failures include age >60 years, fever, hypoalbuminemia, peripheral leukocytosis, ICU admission, and abnormal abdominal CT imaging.61–63 Patients with hematologic malignancies and CDI tend to respond poorly to both metronidazole and vancomycin (53.7% and 50% response rates, respectively).67
Patients treated with metronidazole may experience a longer time to symptomatic improvement compared to those receiving vancomycin.60,68 A retrospective study of 102 patients after the emergence of the BI/NAP1/027 strain found that only 71% of patients responded to metronidazole within 6 days, although the overall response rate was 91%. Failures were associated with greater disease severity.62
Oral vancomycin is generally well-tolerated. However, both oral and rectal vancomycin administration can rarely lead to systemic absorption.69 Metronidazole is associated with gastrointestinal side effects, a disulfiram-like reaction with alcohol, and peripheral neuropathy with prolonged use.70
Treatment Strategies Based on Disease Severity
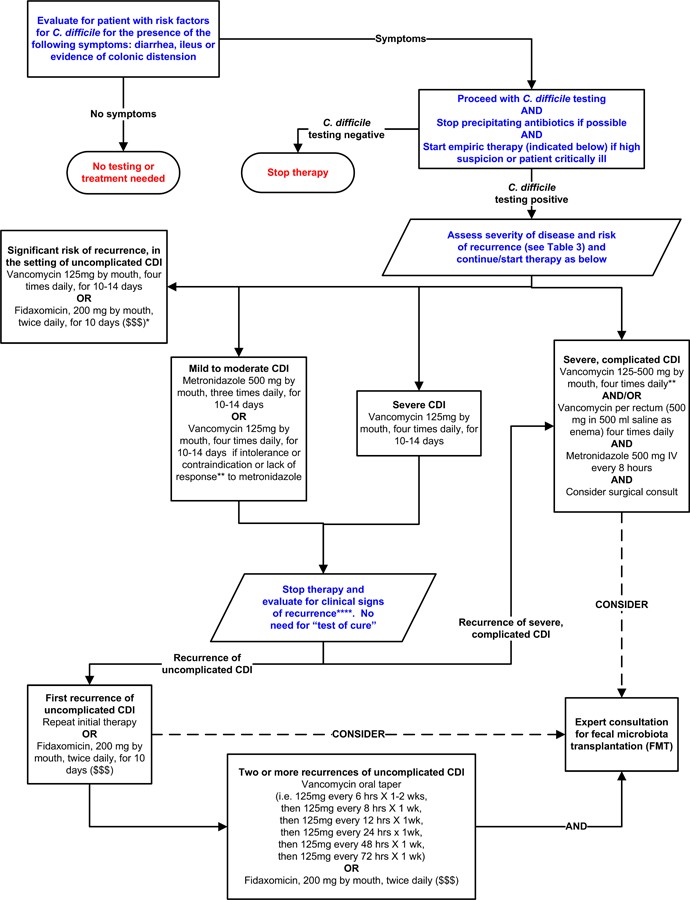
Table 3 outlines CDI severity classifications, definitions of recurrent disease, and recurrence risk factors.15,16,20 Figure 2 presents a potential algorithm for CDI treatment based on disease severity, although it’s important to note this approach is not formally validated.71–73,74,75
Figure 2. Possible Approach for the Treatment of C. difficile Infection (CDI).
Footnotes: *Suggested approach for CDI treatment according to disease severity based on current guidelines, recent reviews/meta-analyses of fecal microbiota transplantation and randomized controlled trials of fidaxomicin. This approach is not validated. There are no data supporting the use of fidaxomicin for complicated CDI. **Treatment response is defined by clinical improvement in diarrhea or other signs of infection; response may require 3–5 days after starting therapy, but therapy escalation can be considered sooner based on disease severity. ***Duration of therapy depends on treatment response. ****Consider post-infectious irritable bowel syndrome rather than recurrent CDI for mild symptoms. “$ $ $” indicates that costs are substantially higher. References: 15,16,71–73,75
Treatment of Mild to Moderate CDI
For mild to moderate CDI, oral metronidazole remains a frequently recommended first-line therapy, partly due to its lower cost.15,16,63 The standard regimen is 500mg orally three times daily for 10–14 days. For patients unable to take oral medication, intravenous metronidazole can be administered at the same dose, although it is not recommended as monotherapy intravenously.15,16 Given recent evidence suggesting lower clinical success rates for metronidazole compared to vancomycin,64 vancomycin may be a reasonable alternative even for mild to moderate CDI.
Treatment of Severe or Complicated CDI
Vancomycin is the preferred treatment for severe or complicated CDI.15,16,63 A dose of 125 mg oral vancomycin four times daily for 10–14 days is considered non-inferior to higher doses in the absence of complicated infection.22 However, expert opinion often favors higher doses in severe or complicated cases.15,16
In cases of ileus, vancomycin can be administered rectally as an adjunctive therapy, although evidence is primarily limited to case reports.15,76,77 Rectal vancomycin is not typically used alone as it may not reach the entire affected colonic area.78 Intravenous metronidazole can achieve detectable levels throughout the colon79 and may be used as an adjunctive therapy in ileus or severe/complicated CDI, often in conjunction with oral and/or rectal vancomycin. However, randomized trials supporting this practice are lacking.15,16 Treatment failures have been reported with IV metronidazole monotherapy in patients with ileus.56,77
Prompt surgical consultation is crucial for patients with complicated CDI. Early surgical intervention can improve mortality rates.80,81 Subtotal or total colectomy with end ileostomy is frequently performed when surgery is necessary, although newer colon-preserving surgical techniques are emerging.80,81
Treatment of Recurrent C. difficile Infection
Recurrent CDI is more common in older patients and those with concomitant antibiotic use, comorbidities, proton pump inhibitor use, and more severe initial disease.11,16 Inadequate antibody response following a CDI episode is also associated with increased recurrence risk.82,83
For the first recurrence of mild-moderate CDI, guidelines recommend oral metronidazole or vancomycin.15,16 Vancomycin is generally recommended for subsequent recurrences, often using pulsed or tapered regimens.84 Randomized trials are limited, but case series and reports support this practice.23,84,85 A study by McFarland et al. involving 163 patients with recurrent CDI reported an overall subsequent recurrence rate of 44.8%. Tapered and pulsed vancomycin courses resulted in fewer recurrences (31%, p=0.01 and 14.3%, p=0.02, respectively), although patient numbers in these subgroups were small.23
Fidaxomicin, approved for CDI treatment in 2011, has demonstrated similar cure rates to oral vancomycin in randomized studies.74,86 A double-blind randomized trial by Cornely et al. showed comparable clinical cure rates between fidaxomicin (87.7%) and vancomycin (86.8%).74 Louie et al. also reported non-inferior clinical cure rates with fidaxomicin (88.2% versus 85.8%) in 629 patients, but significantly lower recurrence rates with fidaxomicin (15.4% vs. 25.3%, P = 0.005).86
When antibiotics cannot be discontinued due to ongoing infections, fidaxomicin has shown higher clinical cure rates for concomitant CDI compared to vancomycin.58 Fidaxomicin may also better preserve the gut microbiota compared to other treatments.75 While not considered first-line for mild or uncomplicated CDI due to its higher cost,87 fidaxomicin is a valuable option for recurrent CDI, initial episodes with high recurrence risk, or following vancomycin courses in patients with multiple recurrences.16,84,88 Data supporting fidaxomicin use in complicated or fulminant disease are currently lacking.16
Anecdotal evidence suggests rifaximin as an adjunctive therapy for recurrent CDI, typically following a standard CDI treatment course.89,90 Monotherapy is discouraged due to the potential for resistance.89 Nitazoxinide is not a first-line agent for initial CDI episodes but may be considered as adjunctive therapy for recurrent CDI, although data are limited.15
Probiotics and Fecal Microbiota Transplantation (FMT)
Recurrent CDI can arise from relapse of the original infection or reinfection with a different strain. Preserving the diversity of the normal gut microbiota is crucial for preventing and treating recurrences.91
Probiotics, live microorganisms that can restore gut microbiota, have a poorly defined role in CDI treatment. However, evidence suggests they may help prevent initial episodes and recurrence.92–94 While rare cases of probiotic-associated bacteremia and fungemia have been reported, primarily in immunocompromised or critically ill patients,95 probiotics are generally well-tolerated with minimal side effects.96 A recent case series suggested that daily kefir administration (a fermented milk probiotic) alongside tapered vancomycin or metronidazole doses was beneficial for recurrent CDI.97
Fecal microbiota transplantation (FMT) aims to restore gut microbiota diversity by transferring donor stool into the gastrointestinal tract of a CDI patient. FMT has shown high clinical response rates and a favorable safety profile in refractory or recurrent CDI.71–73 A systematic review in 2011 (317 patients) reported clinical resolution in 92% of recurrent CDI cases treated with FMT (89% after a single treatment) without serious adverse effects.73 A more recent review of 536 patients reported an 87% clinical response rate.72
A randomized trial demonstrated symptom resolution in 94% of patients receiving vancomycin for 5 days followed by one or two FMT treatments, compared to 31% in those receiving vancomycin alone for 14 days and 23% in those receiving vancomycin plus bowel lavage. This study was stopped early due to the clear superiority of FMT. Among patients in the other treatment groups who subsequently received FMT, 83% achieved symptom resolution.98
Stool substitute preparations made from purified fecal cultures from a single healthy donor have also shown promise in treating recurrent CDI.99 Frozen fecal capsules prepared from pre-screened donors have demonstrated a 90% response rate in recurrent CDI in a feasibility study.101 Pre-screened, filtered, and frozen donor stool for FMT is also commercially available.102 However, in some regions, regulatory agencies consider FMT investigational, requiring specific applications for its use. Anecdotal reports also support FMT for refractory or complicated CDI in the context of ileus or megacolon.103
Other Emerging Therapies for CDI
Alternative Antibiotics
Teicoplanin has been shown to be non-inferior to vancomycin for CDI treatment but is not available in the U.S.59 Case reports suggest tigecycline efficacy for severe or recurrent CDI,[104](#R104] but its role remains unclear. Phase III trials are ongoing for novel antibiotics like surotomycin and cadazolid.
Toxin Binders
Randomized trial data indicate that nonabsorbable anionic polymers like colestipol and cholestyramine are ineffective for CDI. Tolevamer, an anionic polymer binding C. difficile toxins A and B, has also been found inferior to vancomycin and metronidazole.64 Polymers can bind other medications, such as vancomycin, and should not be co-administered with standard CDI therapies.15
Immunotherapy
Serum antibody response to toxin A may protect against recurrent symptomatic CDI.45,82 C. difficile vaccines are under development for both primary and recurrent CDI prevention.105 )106,107
Pooled immunoglobulin can neutralize C. difficile toxins in vitro, but limited data support intravenous immunoglobulin for recurrent CDI,[108](#R108] and its role in severe CDI remains unclear. Human monoclonal antibodies targeting C. difficile toxins A (CDA1) and B (CDB1), combined with standard therapy, have shown promise in reducing recurrent infection rates in a randomized, double-blind, placebo-controlled study (7% vs. 25%).109 Phase III trials are evaluating monoclonal antibodies like MK-3415, MK-6072, and MK-3415A to prevent recurrent CDI in patients receiving standard therapies.110
Discussion
C. difficile infections present a wide range of clinical manifestations, from asymptomatic colonization to fulminant disease. Laboratory testing cannot distinguish between colonization and infection, emphasizing the importance of testing only symptomatic individuals.15 Numerous diagnostic strategies exist for CDI, with multistep algorithms often recommended for optimal sensitivity and specificity.15,16,19,34
Treatment decisions for C. difficile should be guided by disease severity and recurrence risk. Oral vancomycin is recommended for severe, complicated, or recurrent CDI, while oral metronidazole is often used for mild to moderate disease. However, evolving evidence may shift recommendations towards vancomycin even for milder cases if further studies confirm metronidazole’s inferiority.15,16,64 Fidaxomicin is a valuable option when recurrence risk is high, although cost may be a limiting factor. The evidence base supporting FMT for recurrent CDI is growing rapidly,71–73,[98](#R98] but standardization and regulatory frameworks for FMT are still developing. Research continues to explore synthetic stool substitutes99 and convenient FMT administration methods like capsules.101
Conclusion
C. difficile continues to be a significant cause of morbidity and mortality. Effective treatment strategies must consider disease severity and recurrence risk. Fecal microbiota transplantation is demonstrating significant promise in resolving recurrent CDI and may play an expanding role in CDI management in the future.
Key Messages: Diagnosis and Treatment of Clostridium difficile Infection in Adults
DIAGNOSIS
- Diagnosis of clostridium difficile infection (CDI) requires the presence of diarrhea (three or more unformed stools in 24 hours) AND a positive stool test for toxigenic C. difficile or its toxins, or colonoscopic/histopathologic evidence of pseudomembranous colitis. Laboratory testing alone cannot differentiate colonization from infection. CDI testing should be limited to symptomatic patients.
- Diagnostic strategies for diagnosis of clostridium difficile vary. Multistep algorithms utilizing polymerase chain reaction (PCR) for toxin gene detection or single-step PCR on liquid stool samples exhibit the highest sensitivity and specificity.
- “Test of cure” is not recommended following CDI treatment.
TREATMENT
- CDI treatment should be tailored to disease severity and the risk of recurrence or complications.
- Vancomycin and metronidazole are first-line therapies for CDI.
- Vancomycin is the preferred treatment for severe or complicated CDI.
- Recurrent CDI is more common in older patients and those with concomitant antibiotic use, comorbidities, proton pump inhibitor use, and more severe initial disease.
- Oral metronidazole or vancomycin are recommended for the first recurrence of mild-moderate CDI.
- Vancomycin is recommended for patients experiencing two or more recurrences.
- Fidaxomicin can be considered for recurrent CDI.
- Fecal microbiota transplantation (FMT) is associated with symptom resolution in recurrent CDI.
Acknowledgements:
The authors extend their gratitude to Mrs. Whitney Townsend, MLIS (University of Michigan), for her invaluable assistance with the literature search.
Funding/Support:
This work was supported by grants from the National Institutes of Health (1U19AI090871-01, Drs. Rao and Malani), the Claude D. Pepper Older Americans Independence Center (AG-024824, Dr. Rao), and the Michigan Institute for Clinical and Health Research (2UL1TR000433, Dr. Rao).
Footnotes
Conflicts of Interest Disclosures: All authors have completed and submitted the ICJME Form for Disclosure of Potential Conflicts of Interest: None reported. JAMA Associate Editor, Dr. Malani, had no role in the review or acceptance decision for this paper.
Role of the Sponsor: The funding bodies had no role in the study design, conduct, data collection, management, analysis, interpretation, manuscript preparation, review, approval, or the decision to submit the manuscript for publication.
REFERENCES
1. Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531–4. [PubMed]
2. McDonald LC, Killgore GE, Thompson A, Owens RC Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–41. [PubMed]
3. Nelson WW, Katz DA, Rosenblatt RE. Comparison of outcomes in metronidazole versus vancomycin treatment of Clostridium difficile-associated diarrhea. J Hosp Med. 2011;6(8):429–34. [PubMed]
4. Zilberberg MD, Nardi EP, McDonald M, Dumbadze S, Tessier KM, Slater J, et al. Comparative effectiveness of antibiotics for Clostridium difficile infection in hospitalized patients. Am J Gastroenterol. 2008;103(7):1761–7. [PubMed]
5. Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009. HCUP Statistical Brief #124. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
6. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55 Suppl 2:S88–92. [PubMed]
7. Dubberke ER, Gerding DN, Classen D, Arias KM, Podgorny K, Anderson DJ, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29 Suppl 1:S81–92. [PubMed]
8. Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and contribution to disease. Clin Microbiol Rev. 2005;18(2):247–63. [PubMed]
9. Bartlett JG. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15(4):573–81. [PubMed]
10. Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med. 2008;359(18):1932–40. [PubMed]
11. Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107(1):89–95. [PubMed]
12. Pepin J, Valiquette L, Cossette P. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171(5):466–72. [PubMed]
13. Loo VG, Bourgault AM, Miller MA, Shetty AP, Boyd DA, Salgado CD, et al. Association of proton pump inhibitor use with Clostridium difficile infection in a case-control study. Gastroenterology. 2007;133(4):969–75. [PubMed]
14. Dial S, Delaney JA, Schneider V, Suissa S. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294(23):2989–95. [PubMed]
15. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–55. [PubMed]
16. Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases. European guidelines for the diagnosis and treatment of Clostridium difficile infection. Clin Microbiol Infect. 2014;20 Suppl 2:1–26. [PubMed]
17. Kelly CP. Clostridium difficile–induced colitis. N Engl J Med. 1994;330(4):257–62. [PubMed]
18. Kelly CP, Pothoulakis C, Lamont JT. Clostridium difficile colitis. N Engl J Med. 1994;330(4):257–62.
19. Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI). Clin Microbiol Infect. 2009;15(12):1053–66. [PubMed]
20. Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SJ, Gilligan PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–98. [PubMed]
21. Miller MA, Valiquette L. Clostridium difficile infection: a problem for all ages. CMAJ. 2004;171(5):453–4. [PubMed]
22. Johnson S, Samore MH, Gerding DN. Recurrence rates and outcomes of Clostridium difficile infection in patients aged ≥65 years. J Am Geriatr Soc. 2008;56(10):1824–9. [PubMed]
23. McFarland LV, Elmer GW, Surawicz CM, Greenberg RN, Sobel JD,etal. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–75. [PubMed]
24. Alonso CD, Cohen SH, Jiang ZD, Garey KW. Asymptomatic Clostridium difficile colonization in hospitalized patients. Infect Control Hosp Epidemiol. 2012;33(1):78–80. [PubMed]
25. Curry SR, Muto CA, Schlackman JL, Pasculle AW, Shutt KA, Marsh JW, et al. Asymptomatic Clostridium difficile colonization in newly admitted patients: prevalence and attributable costs. Infect Control Hosp Epidemiol. 2009;30(10):977–84. [PubMed]
26. Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(10):1432–42. [PubMed]
27. Reller LB, Weinstein MP, Stratton CW, Cockerill FR 3rd, Edson DC, Gilligan PH, et al. Cumitech 17A, Laboratory diagnosis of Clostridium difficile infection. Washington, D.C.: American Society for Microbiology; 2001.
28. Gilligan PH. Re: Cumitech 17A, laboratory diagnosis of Clostridium difficile infections. Clin Microbiol Newsl. 2002;24(13):103–4.
29. Peterson LR, Manson RU, Pauker SG, Kassirer JP. Decision analysis of diagnostic strategies for Clostridium difficile-associated diarrhea. J Gen Intern Med. 1988;3(4):334–42. [PubMed]
30. Gerding DN, Brazier JS. Optimal methods for Clostridium difficile testing. Clin Infect Dis. 1993;16 Suppl 4:S193–201. [PubMed]
31. Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Asymptomatic colonization with Clostridium difficile and risk of subsequent Clostridium difficile infection in hospitalized patients. Am J Med. 2011;124(5):477–83. [PubMed]
32. Wilcox MH. Diagnostic dilemmas in Clostridium difficile infection. J Antimicrob Chemother. 2009;63 Suppl 1:i1–14. [PubMed]
33. Barbut F, Jones G, Eckert C, Charriot J, Thai HT, Ducournau A, et al. Evaluation of a rapid and automated real-time PCR assay for toxigenic Clostridium difficile detection in stools. J Clin Microbiol. 2011;49(4):1474–7. [PubMed]
34. Kelly CP, LaMont JT. Clostridium difficile infection. Annu Rev Med. 1998;49:375–90. [PubMed]
35. Lyerly DM, Krivan HC, Wilkins TD. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1(1):1–18. [PubMed]
36. Sethi AK, Johnson S, Gerding DN, Peterson LR. Probability of positive Clostridium difficile toxin assay results after initial negative result. Infect Control Hosp Epidemiol. 2011;32(1):89–91. [PubMed]
37. Rao K, Erb-Downward JR, Walk ST, Young VB, Burnham CA, Foxman B, et al. The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE. 2014;9(3):e92578. [PubMed]
38. Planche T, Davies J, Coen P, Enoch DA, Harding I, Wilks M, et al. Comparison of diagnostic assays for Clostridium difficile infection by use of a Bayesian latent class model. J Clin Microbiol. 2013;51(4):1295–301. [PubMed]
39. Ackermann G, Patel S, Rose R, Hussain S, Russo R, Nguyen M, et al. Minimum inhibitory concentrations of metronidazole and vancomycin for Clostridium difficile isolates in relation to patient outcome. Anaerobe. 2012;18(1):109–11. [PubMed]
40. Longtin Y, Miller MA, Khanna S, Mullane KM, Gorbach SL, Valiquette L, et al. Update on markers of Clostridium difficile infection severity: review of the literature. Clin Infect Dis. 2013;56(4):603–15. [PubMed]
41. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. T