Disseminated intravascular coagulation (DIC) is a serious condition characterized by the abnormal activation of the coagulation system, leading to widespread thrombin and fibrin generation within blood vessels. This process results in both thrombosis in small and medium-sized vessels and, paradoxically, an increased risk of bleeding due to the consumption of platelets and coagulation factors. Understanding the nuances of DIC diagnosis is crucial for effective management and improved patient outcomes. This review provides an in-depth exploration of DIC diagnosis, drawing upon established guidelines and recent advancements in the field.
Pathophysiology of DIC: Implications for Diagnosis
The hemostatic imbalances in DIC arise from a complex interplay between hypercoagulation and hyperfibrinolysis. As illustrated in Figure 1, the clinical presentation of DIC varies depending on the dominant vector in this dynamic process.
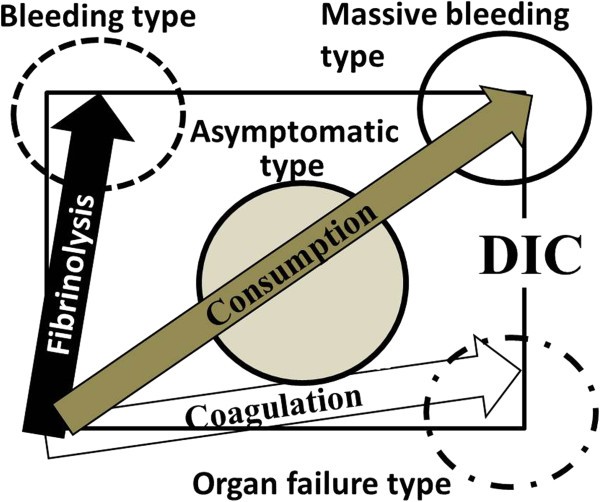 Bleeding, organ failubre, massive bleeding, and non-symptomatic types of DIC
Bleeding, organ failubre, massive bleeding, and non-symptomatic types of DIC
Figure 1: Spectrum of DIC Types Based on Hemostatic Imbalance. Alt text: Diagram illustrating the four types of Disseminated Intravascular Coagulation (DIC) – Bleeding, Organ Failure, Massive Bleeding, and Non-symptomatic – based on the balance between hypercoagulation and hyperfibrinolysis vectors, crucial for accurate Diagnosis Of Dic.
When hyperfibrinolysis predominates, the primary clinical manifestation is bleeding. This “bleeding type” or “hyperfibrinolysis predominance type” of DIC is frequently observed in patients with acute promyelocytic leukemia (APL), obstetric complications, or aortic aneurysms. Conversely, when hypercoagulation is the dominant force, organ failure becomes the prominent feature. This “organ failure type,” also known as the “hypercoagulation predominance type” or “hypofibrinolysis type,” is commonly associated with infections, particularly sepsis. Hypofibrinolysis in these cases is often linked to elevated levels of plasminogen activator inhibitor I (PAI-I), triggered by cytokines and lipopolysaccharide (LPS). Furthermore, neutrophil extracellular traps (NETs), released during sepsis, contribute to the procoagulant state by promoting vascular endothelial cell apoptosis and platelet aggregation, while also degrading tissue factor pathway inhibitor (TFPI), further enhancing thrombus formation. High mobility group box 1 (HMGB-1), released from damaged cells, amplifies the inflammatory response, exacerbating the DIC process.
In cases where both hypercoagulation and hyperfibrinolysis are markedly elevated, patients may develop “massive bleeding” or “consumptive type” DIC. This severe form is characterized by major hemorrhage, often seen post-surgery or in obstetric emergencies, and carries a high risk of mortality if not promptly managed with blood transfusions. Finally, the “non-symptomatic type” of DIC, or “pre-DIC,” occurs when both vectors are weakly activated. Patients may exhibit minimal or no clinical symptoms, with abnormalities primarily detected through laboratory testing. Early diagnosis of non-symptomatic DIC is important, as intervention at this stage may prevent progression to more severe forms. The varying pathophysiology of these DIC types underscores the need for tailored diagnostic approaches and treatment strategies.
Diagnostic Approaches for DIC
The diagnosis of DIC poses a significant clinical challenge due to the wide range of underlying conditions that can trigger this syndrome and the variability in its clinical presentation. Several international guidelines have been developed to standardize the diagnostic process, including those from the British Committee for Standards in Haematology (BCSH), the Japanese Society of Thrombosis and Hemostasis (JSTH), and the Italian Society for Thrombosis and Haemostasis (SISET). The International Society of Thrombosis and Haemostasis (ISTH) has also published harmonized guidance to reconcile differences among these guidelines, aiming to improve consistency in DIC diagnosis and management.
Scoring Systems for DIC Diagnosis
Recognizing the complexity of DIC diagnosis, all major guidelines recommend the use of scoring systems. These systems integrate various clinical and laboratory parameters to estimate the probability of DIC. Three prominent scoring systems are widely used: the ISTH/SSC overt DIC score, the Japanese Ministry of Health, Labour and Welfare (JMHLW) score, and the Japanese Association of Acute Medicine (JAAM) score.
The JMHLW score demonstrates a strong correlation with DIC severity and can predict patient outcomes. The ISTH overt DIC score is valuable for diagnosing DIC across diverse etiologies, both infectious and non-infectious. The JAAM score is particularly sensitive for detecting DIC in sepsis and correlates well with both the ISTH and JMHLW scores, as well as disease prognosis. A comparative study in Japan found no significant differences in the predictive power of these three scoring systems for DIC outcomes, highlighting the need to incorporate molecular hemostatic markers and dynamic changes in coagulation tests alongside scoring systems for a comprehensive diagnostic approach. Repeated testing and trend analysis are crucial for accurate DIC diagnosis, especially in patients with evolving clinical conditions.
While these scoring systems are invaluable for diagnosing bleeding type and massive bleeding type DIC (ISTH and JMHLW criteria) and organ failure type DIC (JAAM criteria), they may be less effective in identifying non-symptomatic DIC. The diagnosis of non-symptomatic DIC often necessitates the inclusion of hemostatic molecular markers. Furthermore, a non-overt DIC scoring system, incorporating global coagulation tests, their dynamic changes, and molecular markers, has been proposed to improve the early detection of DIC.
Laboratory Tests in DIC Diagnosis
Laboratory investigations are indispensable for confirming the clinical suspicion of DIC and for differentiating between its various types. Global coagulation tests provide essential insights into the extent of coagulation factor activation and consumption.
Common Coagulation Tests
- Prothrombin Time (PT): Prolongation of PT is observed in approximately 50% of DIC patients. However, PT prolongation is not specific to DIC and can also occur in liver disease and vitamin K deficiency. PT is particularly relevant in diagnosing organ failure, bleeding, and massive bleeding types of DIC.
- Platelet Count: Thrombocytopenia, or a declining platelet count, is a sensitive indicator of DIC, reflecting platelet consumption. However, it can also be seen in bone marrow disorders. Platelet counts are important across all DIC types, including non-symptomatic, organ failure, bleeding, and massive bleeding.
- Fibrinogen Level: Reduced fibrinogen levels are suggestive of DIC, especially in bleeding type DIC associated with leukemia or obstetric complications. However, fibrinogen levels may be normal or even elevated in septic DIC. Fibrinogen measurement is particularly useful in diagnosing bleeding and massive bleeding DIC.
- Fibrin-Related Markers (FRMs): Elevated levels of FRMs, such as fibrin degradation products (FDP), D-dimer, and soluble fibrin (SF), indicate fibrin formation and breakdown. D-dimer is a widely used marker for DIC, with elevated levels reflecting ongoing coagulation and fibrinolysis. SF assays may offer advantages in DIC detection by directly reflecting thrombin activity on fibrinogen, although SF has a shorter half-life. It’s crucial to note that elevated FRMs are not specific to DIC and can be found in conditions like trauma, surgery, bleeding, and venous thromboembolism (VTE). FRMs are valuable in diagnosing bleeding, non-symptomatic, and organ failure DIC.
Advanced Hemostatic Markers
- Natural Anticoagulants (Antithrombin (AT) and Protein C): Reduced levels of natural anticoagulants, such as AT and protein C, are common in DIC, reflecting their consumption during the coagulation process. AT activity measurement is also important for guiding heparin therapy. However, levels can be influenced by liver function and albumin concentration. Reduced AT and protein C levels are particularly relevant in organ failure type DIC.
- ADAMTS13 Activity: Reduced ADAMTS13 (a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13) activity and elevated soluble thrombomodulin (TM), PAI-I, and von Willebrand factor propeptide levels are often observed in DIC and have prognostic implications. Elevated TM levels can also be seen in renal dysfunction and organ failure in general. These markers are particularly relevant in organ failure type DIC.
- Activated Partial Thromboplastin Time (APTT) Waveform: A biphasic waveform in the APTT assay has been associated with DIC and may have positive predictive value, particularly in the context of infection. This abnormality is primarily associated with organ failure type DIC.
- Plasmin-Plasmin Inhibitor Complex (PPIC): Elevated PPIC levels indicate plasmin generation and fibrinolysis, which are prominent features of DIC. PPIC levels are particularly relevant in bleeding and massive bleeding types of DIC.
Table 2: Laboratory Tests for Diagnosis of DIC. Alt text: Table summarizing key laboratory tests for diagnosing Disseminated Intravascular Coagulation (DIC), outlining abnormalities in DIC, other potential causes for these abnormalities, and the DIC types for which each test is most relevant in diagnosis.
Table 2 summarizes the laboratory tests relevant to DIC diagnosis, highlighting their abnormalities in DIC, other potential causes for these abnormalities, and the DIC types for which they are most diagnostically useful. It is crucial to recognize that no single laboratory marker is sufficient for DIC diagnosis. The guidelines emphasize that DIC diagnosis should be based on a combination of laboratory markers, interpreted in the clinical context. The selection of appropriate laboratory tests should be guided by the suspected type of DIC. For instance, PT, fibrinogen, and platelet count are particularly important in massive bleeding DIC, while fibrinogen, FDP, and PPIC are crucial for bleeding type DIC. Platelet count, PT, and AT are key markers for organ failure DIC, and hemostatic molecular markers like SF and thrombin-AT complex are valuable in diagnosing non-symptomatic DIC.
Treatment Strategies for DIC: Tailoring Therapy to Diagnosis
The management of DIC is complex and multifaceted, requiring a tailored approach based on the underlying cause, the type of DIC, and the patient’s clinical status. While this review primarily focuses on diagnosis, understanding the treatment principles is essential for appreciating the clinical significance of accurate DIC typing.
Addressing the Underlying Cause
The cornerstone of DIC treatment is to identify and treat the underlying condition that triggered the syndrome. This may involve antibiotics or surgical drainage for infections, anticancer therapy or surgery for malignancies, or obstetric interventions for pregnancy-related complications. While direct evidence supporting this approach is limited, clinical experience and expert consensus strongly advocate for addressing the root cause. In many cases, DIC resolves spontaneously once the underlying condition is effectively managed. However, supportive therapies targeting the coagulation abnormalities are often necessary, particularly in severe DIC. Notably, treatment strategies differ depending on the DIC type. For bleeding, organ failure, and non-symptomatic DIC, addressing the underlying condition is the priority, while massive bleeding DIC necessitates immediate blood transfusions alongside treatment of the primary cause.
Supportive Therapies for Coagulation Abnormalities
- Blood Transfusion: Transfusion of platelet concentrates (PC) and fresh frozen plasma (FFP) is recommended in DIC patients with active bleeding or at high bleeding risk, especially before invasive procedures. The platelet transfusion threshold typically is ≤50 × 10^9/l in bleeding patients, but a lower threshold (10-20 × 10^9/l) may be acceptable in non-bleeding patients, such as those with chemotherapy-induced DIC. Higher platelet counts may be targeted in patients at high bleeding risk based on clinical and laboratory assessments. FFP transfusion is indicated to correct coagulation factor deficiencies, aiming for PT and APTT ratios less than 1.5 times normal and fibrinogen levels above 1.5 g/dl. An initial FFP dose of 15 ml/kg is commonly used. Prothrombin complex concentrates or purified fibrinogen concentrates/cryoprecipitate may be considered in specific situations, particularly in massive bleeding DIC. Blood component therapy should be guided by clinical and laboratory monitoring. Recombinant factor VIIa should be used cautiously in life-threatening bleeding, ideally within clinical trials. Blood transfusions are primarily used in massive bleeding and bleeding types of DIC.
- Heparin: The role of heparin in DIC is complex and varies across guidelines. Therapeutic heparin doses may be considered in DIC cases where thrombosis predominates, particularly in non-symptomatic DIC to prevent deep vein thrombosis (DVT). Low molecular weight heparin (LMWH) may be preferred over unfractionated heparin (UFH). However, heparin is generally contraindicated in bleeding and massive bleeding DIC due to the bleeding risk. VTE prophylaxis with UFH, LMWH, or mechanical methods is recommended in DIC patients at risk of thromboembolism. AT levels should be considered when using heparin. Heparin is recommended for non-symptomatic DIC but not for bleeding or massive bleeding DIC.
- Anti-Xa Agents: Fondaparinux and danaparoid sodium, which selectively inhibit factor Xa, are used for DVT prophylaxis, but their role in DIC treatment is limited. Danaparoid sodium is used for DIC treatment in Japan, but robust evidence of benefit is lacking. These agents are generally not recommended for bleeding or massive bleeding DIC, nor in patients with renal failure. Anti-Xa agents are not typically recommended for DIC treatment, especially in bleeding types.
- Synthetic Protease Inhibitors: Gabexate mesilate and nafamostat, synthetic protease inhibitors with multi-faceted effects, are used in Japan for DIC treatment. They have mild anticoagulant and antifibrinolytic properties and may be considered in bleeding, massive bleeding, and non-symptomatic DIC. However, strong evidence from randomized controlled trials (RCTs) is lacking. Synthetic protease inhibitors may be used in non-symptomatic, organ failure, bleeding, and massive bleeding DIC.
- Natural Protease Inhibitors: Antithrombin (AT), recombinant human activated protein C (rhAPC), and recombinant human thrombomodulin (rhTM) are natural protease inhibitors that have been investigated in DIC, primarily in the context of sepsis-associated DIC. AT concentrates may be considered, particularly in organ failure DIC, especially in patients not receiving heparin. rhAPC is no longer widely used in sepsis, and its role in DIC is limited. rhTM has shown promise in improving DIC resolution and organ failure severity in some studies, particularly in organ failure DIC. Further RCT evidence is needed to definitively establish the role of these agents. AT and rhTM may be considered in organ failure DIC.
- Antifibrinolytic Agents: Antifibrinolytic agents like tranexamic acid can be effective in managing bleeding in hyperfibrinolytic DIC, particularly in bleeding and massive bleeding types. However, they are generally contraindicated in organ failure and non-symptomatic DIC due to the risk of thrombosis. Early administration may be more beneficial before PAI-1 levels rise significantly. Antifibrinolytic agents are recommended for bleeding and massive bleeding DIC but not for organ failure or non-symptomatic DIC.
Table 3: Treatment of DIC in Four DIC Types. Alt text: Table outlining recommended treatments for the four types of Disseminated Intravascular Coagulation (DIC) – Non-symptomatic, Organ Failure, Bleeding, and Massive Bleeding – including underlying condition treatment, blood transfusion, heparin, protease inhibitors, and antifibrinolytic therapy, guiding tailored treatment approaches based on DIC diagnosis.
Table 3 summarizes the recommended treatments for the four DIC types, highlighting the tailored approach based on the specific diagnostic category.
Conclusion: Precision Diagnosis for Effective DIC Management
Accurate diagnosis of DIC, including the identification of its specific type, is paramount for guiding effective treatment strategies and improving patient outcomes. By integrating clinical assessment, scoring systems, and comprehensive laboratory testing, clinicians can achieve a more precise diagnosis of DIC. This nuanced diagnostic approach allows for tailored therapeutic interventions, addressing both the underlying cause and the specific hemostatic derangements characteristic of each DIC type. Continued research and refinement of diagnostic and treatment algorithms are essential to further optimize the management of this complex and life-threatening syndrome.
Acknowledgements
This study was supported in part by research grants from the Japanese Ministry of Health, Labour and Welfare and the Japanese Ministry of Education, Science, Sports and Culture.
Abbreviations
ADAMTS13 a disintegrin-like and metalloproteinase with thrombospondin type 1 motifs 13
APL acute promyelocytic leukemia
APTT activated partial thromboplastin time
AT antithrombin
ATRA all-trans retinoic acid
BCSH British Committee for Standards in Haematology
DIC disseminated intravascular coagulation
FDP fibrinogen and fibrin degradation products
FFP fresh frozen plasma
FRMs fibrin-related markers
HMGB-1 high mobility group box 1
ISTH International Society of Thrombosis and Haemostasis
JAAM Japanese Association of Acute Medicine
JMHLW Japanese Ministry Health, Labour and Welfare
JSTH Japanese Society of Thrombosis and Hemostasis
LMWH low molecular weight heparin
LPS lipopolysaccharide
NETs neutrophil extracellular traps
PAI-I plasminogen activator inhibitor I
PC platelet concentrate
PT prothrombin time
RCT randomized controlled trial
rh recombinant human activated protein C
SF soluble fibrin
SISET Italian Society for Thrombosis and Haemostasis
SSC Scientific and Standardization Committee
TFPI tissue factor pathway inhibitor
TM thrombomodulin
UFH unfractionated heparin
VTE venous thromboembolism.
Footnotes
Competing interests
None of the authors disclose any financial or personal relationships with other people or organizations that could inappropriately influence (bias) their work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Authors’ contributions
HW mainly contributed to write this paper. TM and YY mainly contributed to review references. All of authors discussed for this review. All authors read and approved the final manuscript.
Contributor Information
Hideo Wada, Email: [email protected].
Takeshi Matsumoto, Email: [email protected].
Yoshiki Yamashita, Email: [email protected].