1. Introduction to Kaposi Sarcoma and Diagnostic Challenges
Kaposi Sarcoma (KS) stands as the most frequently diagnosed malignancy affecting individuals with HIV globally. In Sub-Saharan Africa, it ranks among the most prevalent cancers in men overall. While strongly associated with HIV, KS also affects other immunocompromised populations, including the elderly, children in regions where Kaposi Sarcoma-associated herpesvirus (KSHV) is endemic, and organ transplant recipients. The key etiological agent for all forms of KS is KSHV, also known as human herpesvirus 8.
Diagnosing KS hinges on identifying the viral protein LANA within biopsy samples. However, the clinical presentation of KS is remarkably diverse, and treatment responses vary significantly across different KS subtypes. Despite two decades of research advancements in understanding KS, the standard therapeutic approaches have remained largely unchanged. This article aims to provide an updated overview of KS diagnosis, emphasizing current best practices and recent advances in the field to better inform clinicians and improve patient outcomes. Accurate and timely Diagnosis Of Kaposi Sarcoma is paramount for effective management and treatment planning.
2. Understanding Kaposi Sarcoma-associated herpesvirus (KSHV) in Diagnosis
All forms of Kaposi Sarcoma are unequivocally linked to Kaposi sarcoma-associated herpesvirus (KSHV). KSHV is a complex double-stranded DNA virus encoding over 80 proteins and numerous microRNAs. Its replication mechanism involves a virus-encoded DNA-dependent DNA polymerase (orf9), which is sensitive to antiviral drugs like ganciclovir and foscarnet, but not acyclovir. Like other herpesviruses, KSHV possesses genes that enable it to evade immune surveillance during initial infection, including mechanisms to inhibit interferon responses, anti-apoptotic factors, autophagy inhibitors, and countermeasures against Natural Killer (NK) cell-mediated immunity.
A crucial aspect of KSHV biology is its ability to establish latency, a state where the virus persists within host cells, including KS tumor cells, without active replication in most cells. During latency, only a limited set of viral genes, primarily latent genes and viral miRNAs, are expressed. This restricted gene expression is sufficient to maintain KS lesions. The latency-associated nuclear antigen (LANA) plays a critical role in maintaining the viral episome within dividing infected cells, as illustrated in Figure 1. LANA’s consistent presence in KS lesions makes it a vital diagnostic marker. Although latency is dominant, evidence suggests that lytic replication is also necessary for systemic persistence, oncogenesis, and disease progression, and is essential for viral transmission.
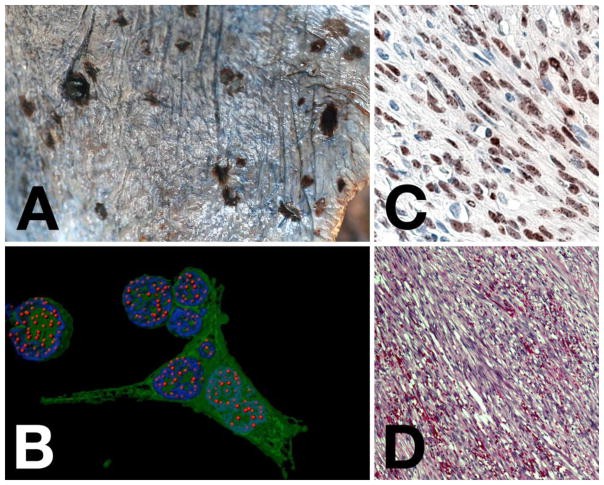
Figure 1. Microscopic Pathology Essential for Kaposi Sarcoma Diagnosis.
Panel A illustrates the macroscopic appearance of disseminated Kaposi Sarcoma on the lung surface, showing both nodular and flat lesions. Panel B is a computer-enhanced immunofluorescence image of KSHV-infected PEL cells, with LANA staining in red, nuclear DNA in blue, and GFP indicating infected cells in green, highlighting “LANA dots” where viral genomes attach to host chromosomes. Panel C shows LANA staining in a KS lesion via immunohistochemistry (brown) with hematoxylin counterstain (blue), demonstrating nuclear LANA staining with darker spots. Panel D is an H&E stain of a KS lesion, revealing spindle-shaped endothelial cells and slit-like spaces with extravasated red blood cells, key histological features for diagnosis of Kaposi Sarcoma.
KSHV transmission primarily occurs horizontally through saliva, requiring close and repeated contact, such as mother-to-child or sexual interactions. This contrasts with Epstein-Barr Virus, which is more readily transmitted. Among adults, sexual transmission, particularly among men who have sex with men (MSM), is a significant factor in KSHV prevalence. While transmission through blood transfusion is rare, organ transplantation has been linked to isolated cases. Vertical transmission routes appear less significant. The development of KS in both HIV-negative transplant patients and AIDS patients indicates that HIV is not a prerequisite for KS, but HIV-related immunosuppression significantly elevates the risk compared to other forms of immune compromise. This suggests that HIV infection may induce a more profound immune deficiency that is particularly conducive to KSHV reactivation and KS development.
3. Clinical and Pathological Spectrum in Kaposi Sarcoma Diagnosis
The diagnosis of Kaposi Sarcoma is complicated by its broad clinicopathological variability. This variation is influenced by: (i) the anatomical location of lesions (cutaneous, lymph node, or visceral), (ii) the clinical stage of lesions (patch, plaque, or nodular), and (iii) the epidemiological classification of KS. Epidemiological classifications include classic KS in elderly men, endemic African KS (affecting younger men and children in central Africa), iatrogenic KS (primarily in transplant recipients, but also associated with chemotherapy and immunosuppressive therapies), and epidemic HIV/AIDS-associated KS. Endemic KS further subdivides into a clinically indolent nodular form, an aggressive variant with invasive cutaneous tumors affecting soft tissue and bone, and a pediatric variant manifesting as lymphadenopathy in young children. Epidemic HIV-associated KS is predominantly seen in MSM, but also affects women, children, intravenous drug users, and transplant recipients. Subgroups within epidemic KS include HIV-associated KS in patients on combined antiretroviral therapy (cART), those not on or failing cART (end-stage AIDS), pediatric HIV-associated KS, and KS associated with immune reconstitution inflammatory syndrome (IRIS). Pediatric KS, regardless of epidemiological variant, tends to be more aggressive and disseminated compared to adult KS.
Given this wide spectrum, it’s crucial to recognize that KS is not a singular disease entity. However, current treatment protocols are largely uniform: localized superficial lesions may be managed with surgery or cryotherapy, while systemic or extensive KS typically requires first-line therapy with liposomal doxorubicin (Doxil™). The diagnostic challenge lies in accurately identifying KS across its various presentations and distinguishing it from other conditions that may mimic its clinical and histological features.
4. Diagnostic Pathology: The Cornerstone of Kaposi Sarcoma Identification
While clinical suspicion is vital, histopathological confirmation remains the gold standard for diagnosis of Kaposi Sarcoma. However, the diagnosis can be challenging, especially for pathologists less familiar with the diverse histopathological spectrum of KS. Early-stage KS, in particular, may present subtle histological clues that are easily overlooked. Established KS lesions typically exhibit characteristic histopathological features that can be reliably diagnosed by experienced histopathologists.
The morphological diversity of KS can mimic numerous benign and malignant conditions, posing a diagnostic pitfall. Pathologists must be aware of recognized KS variants, including anaplastic, telangiectatic, lymphangioma-like, cavernous hemangioma–like, pyogenic granuloma–like, intravascular, bullous, ecchymotic, hyperkeratotic, keloidal, micronodular, glomeruloid, solid, desmoplastic, KS with myoid nodules, KS with sarcoid-like granulomas, and pigmented KS. A unifying histological feature across all KS variants is the presence of spindle-shaped cells. These cells are not only diagnostic but also constitute the majority of the proliferating cell population within KS lesions.
Immunohistochemical staining for LANA has become the definitive diagnostic marker for KS. Commercially available, robust monoclonal antibodies targeting the highly antigenic EQEQE repeat motif in LANA provide a reliable tool for automated immunohistochemical systems. A positive LANA stain, in the appropriate clinicopathological context, unequivocally confirms the diagnosis of Kaposi Sarcoma. However, LANA expression is not exclusive to KS and can be found in other KSHV-associated conditions such as multicentric Castleman’s disease, primary effusion lymphoma, and lymphomas arising in KSHV-associated multicentric Castleman’s disease. Thus, while highly specific, LANA positivity must be interpreted in conjunction with clinical and histological findings.
5. Challenges in LANA Detection and Alternative Diagnostic Approaches
LANA staining intensity can vary, depending on the clinical stage of KS, particularly in cutaneous lesions, and in lesions from patients on stable cART. Superficial or regressing lesions may exhibit fewer LANA-positive cells. Biopsies from patients with multiple lesions may inadvertently target milder lesions with fewer spindle cells and consequently fewer LANA-positive cells. This variability in LANA staining is not correlated with patient demographics, KS subtype, lesion distribution, or CD4 count, but is influenced by disease stage and immunohistochemical technique sensitivity. Factors affecting LANA expression levels remain largely unknown.
In cases where LANA expression is undetectable by immunohistochemistry, technical issues or very low viral copy numbers within KS cells might be responsible. However, the absence of detectable LANA does not definitively exclude KS in a clinically suggestive scenario. In such instances, LANA-stained sections should be meticulously re-examined for subtle granular nuclear staining in KS cells. PCR-based assays can reliably detect HHV-8 DNA in KS lesions even when LANA expression is undetectable, offering a valuable adjunct diagnostic tool. However, potential contamination of KS biopsies leading to false-positive PCR results must be considered. Therefore, negative LANA expression should prompt a thorough re-evaluation of all clinicopathological features and consideration of alternative diagnoses in the differential. In complex cases, integrating PCR results with careful histological review enhances diagnostic accuracy in diagnosis of Kaposi Sarcoma.
6. Advances in Kaposi Sarcoma Treatment and Implications for Diagnosis
While FDA-approved treatments for KS have remained largely unchanged for two decades, a deeper understanding of KS pathogenesis and the role of KSHV has led to advancements in therapeutic strategies. AIDS-KS often responds to immune reconstitution and HIV suppression through cART. In resource-limited settings and depending on disease severity, cART alone can induce remission in up to 50% of AIDS-KS cases. Effective cART and diligent monitoring are essential in managing AIDS-KS, whether KS is the initial manifestation of HIV infection or indicates cART failure. However, a subset of AIDS-KS patients may experience disease progression upon cART initiation, termed KS-IRIS, particularly in African populations. Managing KS-IRIS requires a careful balance, as withdrawing cART is not advisable, but concurrent chemotherapy and immune modulation may be beneficial in the short term.
Transplant-associated KS management involves immune reconstitution, but reducing immunosuppression risks graft rejection. Switching from cyclosporine A/FK506 to mTOR inhibitors like rapamycin/sirolimus/everolimus often results in KS regression. Cytotoxic chemotherapy, particularly liposomal doxorubicin and paclitaxel, remains a standard treatment for KS, achieving response rates of 60–80%. However, treatment outcomes are less favorable in resource-limited settings and in populations with limited access to HIV and cancer care.
Emerging therapies targeting VEGF signaling, such as bevacizumab and other VEGF-receptor inhibitors, have shown variable clinical efficacy. mTOR inhibitors like sirolimus have demonstrated effectiveness in transplant KS and are being explored in AIDS-KS. Immunomodulatory agents like IFN-alpha and imiquimod, as well as thalidomide derivatives, are also under investigation. Immune checkpoint inhibitors, which have revolutionized treatment in other cancers, are being evaluated for KS, with ongoing trials exploring nivolumab and ipilimumab. These therapeutic advances underscore the importance of precise diagnosis of Kaposi Sarcoma to tailor treatment strategies effectively and improve patient outcomes. Accurate diagnosis not only guides initial therapy selection but also helps in monitoring treatment response and identifying patients who may benefit from newer targeted therapies.
7. Conclusion: Enhancing Diagnostic Precision for Improved Kaposi Sarcoma Management
Kaposi Sarcoma remains a significant health concern, particularly for people living with HIV/AIDS. Even with effective HIV management through cART, the risk of KS persists and increases with age in co-infected individuals. The histopathological diagnosis of Kaposi Sarcoma, while crucial, can be challenging due to the disease’s morphological diversity. Immunohistochemical detection of LANA has significantly improved diagnostic accuracy, although limitations and variability in LANA expression necessitate a comprehensive diagnostic approach. While treatments like Doxil and Paclitaxel are effective, they carry significant toxicity, driving the need for newer, targeted therapies. Emerging treatments, including sirolimus, VEGF/VEGF receptor inhibitors, and immunomodulatory agents, hold promise, but their optimal application requires precise patient stratification based on accurate diagnosis and KS subtype characterization. Future advancements in diagnostic techniques and a deeper understanding of KS pathogenesis will be essential to refine treatment strategies and improve outcomes for patients with Kaposi Sarcoma.
Key points for Kaposi Sarcoma Diagnosis.
- Kaposi Sarcoma continues to be a major cause of morbidity and mortality in people living with HIV globally, highlighting the ongoing need for improved diagnostic and therapeutic strategies.
- The diverse clinical presentations and histological variants of Kaposi Sarcoma necessitate a multi-faceted diagnostic approach, integrating clinical context, histopathology, and immunohistochemistry.
- Accurate diagnosis of Kaposi Sarcoma, particularly through reliable LANA detection, is critical for guiding appropriate treatment decisions and monitoring disease progression.
Acknowledgments
We extend our gratitude to B. Damania, Marcia Sanders, and Anthony Eason for their critical review and insightful discussions that contributed to the quality of this article.
Funding. This work received support from PHS grants DE018304, CA190152 to DPD and CA192744 to JWS and DPD. The authors are affiliated with the AIDS Malignancy Consortium (AMC), supported by PHS grant CA121947, and JWS is associated with the AIDS Cancer Specimen Resource (ACSR), supported by PHS grant CA181255.
Footnotes
Compliance with Ethical Standards
Conflict of interest. DPD has disclosed receiving reagents from and a consulting agreement related to KS with Delenex AG and Navidea Inc. These affiliations did not influence the opinions or conclusions presented in this review.