Background
Rhabdomyolysis is a serious medical condition characterized by the rapid breakdown of damaged skeletal muscle tissue.
Methods
This article provides an in-depth review of rhabdomyolysis, covering its epidemiology, pathophysiology, various causes, clinical presentation, diagnostic approaches, potential complications, management strategies, and considerations for anesthesia.
Results
Rhabdomyolysis can be triggered by any form of muscle injury or any factor that leads to muscle damage. A critical aspect of managing suspected rhabdomyolysis is the prevention of acute kidney injury.
Conclusion
It is essential for all healthcare professionals to have a thorough understanding of the common causes, effective diagnostic methods, and appropriate treatment options for rhabdomyolysis.
Keywords: Rhabdomyolysis, Diagnosis Rhabdomyolysis
INTRODUCTION
Rhabdomyolysis is a complex and potentially life-threatening condition that involves the rapid disintegration of injured skeletal muscle. This breakdown of muscle fibers results in the leakage of intracellular components, such as myoglobin, creatine kinase (CK), aldolase, lactate dehydrogenase, and electrolytes, into the bloodstream and extracellular space. The severity of rhabdomyolysis can vary significantly, ranging from an asymptomatic state with only elevated CK levels to a critical condition marked by extremely high CK levels, electrolyte imbalances, acute renal failure (ARF), and disseminated intravascular coagulation.1 While traumatic injury is a common cause, rhabdomyolysis can also be induced by various factors including drugs, toxins, infections, muscle ischemia, electrolyte and metabolic disturbances, genetic predispositions, excessive exertion, prolonged immobilization, and temperature-related conditions like neuroleptic malignant syndrome (NMS) and malignant hyperthermia (MH).2 The hallmark of both traumatic and non-traumatic rhabdomyolysis is massive muscle necrosis, clinically manifesting as muscle weakness, myalgia, swelling, and often the presence of dark urine without blood (gross pigmenturia).3
Historical accounts of rhabdomyolysis can be traced back to the Old Testament, specifically the Book of Numbers, which describes a plague affecting the Israelites after consuming large quantities of quail during their exodus from Egypt.4 This plague is widely interpreted as an early description of myolysis, a recognized consequence in the Mediterranean region following quail consumption.5,6 The myolysis is believed to result from the poisonous hemlock ingested by quail during their spring migration.7,8 In modern medicine, the earliest documented description of rhabdomyolysis appeared in German medical literature in the early 1900s, termed Meyer-Betz disease.9 Bywaters and Beall are often credited with providing the first comprehensive understanding of the pathophysiological mechanisms of the syndrome and establishing the crucial link between rhabdomyolysis and ARF.10,11
The classic clinical presentation of rhabdomyolysis is characterized by a triad of symptoms: muscle pain (myalgia), muscle weakness, and myoglobinuria, which manifests as the characteristic tea-colored urine. However, it’s important to note that this triad is not universally present, observed in only about 50% of patients. Many individuals may not experience muscle pain or weakness, with the initial symptom being discolored urine.2 For diagnosis of rhabdomyolysis, measuring creatine kinase (CK) levels is the most sensitive laboratory test to detect muscle injury that could lead to rhabdomyolysis, provided there is no concurrent cardiac or brain injury.1 While attempts to correlate CK elevation with the severity of muscle damage and renal failure have yielded inconsistent results, significantly elevated CK levels, generally above 5,000 IU/L, are indicative of substantial muscle injury.1,9 Initial treatment for rhabdomyolysis is primarily supportive, focusing on managing the ABCs (airway, breathing, circulation) and implementing measures to protect kidney function, most importantly through aggressive fluid resuscitation.
EPIDEMIOLOGY
Historically, determining the precise incidence of myopathic events and rhabdomyolysis in clinical research has been challenging due to the absence of standardized clinical definitions. In 2002, the American College of Cardiology (ACC), American Heart Association (AHA), and National Heart, Lung, and Blood Institute (NHLBI) jointly issued a Clinical Advisory on the Use and Safety of Statins to address this issue and provide clarity.12 Their recommended definitions for muscle toxicity and rhabdomyolysis are outlined in Table 1.
Table 1. Definitions of Muscle Toxicity and Rhabdomyolysis by Clinical Advisory
Given that acute renal failure (ARF) represents the most critical and immediately life-threatening complication of rhabdomyolysis, understanding the relationship between these two conditions is vital. It is estimated that ARF develops in 10%-40% of patients with rhabdomyolysis, and rhabdomyolysis is implicated in up to 15% of all ARF cases.13 Earlier studies suggest that the incidence of ARF in children with rhabdomyolysis might be even higher, ranging from 42%-50%.14,15 However, obtaining more precise estimates remains difficult due to variations in clinical settings, patient populations, and confounding factors introduced by co-existing medical conditions.
PATHOPHYSIOLOGY
While the specific cause of rhabdomyolysis can often be identified, the exact mechanisms by which various insults lead to muscle injury and necrosis are less well-defined. However, the downstream events common to diverse causes of rhabdomyolysis are better understood. Regardless of the initial trigger, the final pathways leading to rhabdomyolysis involve either direct damage to muscle cells (myocytes) or a failure of energy supply within these cells.16
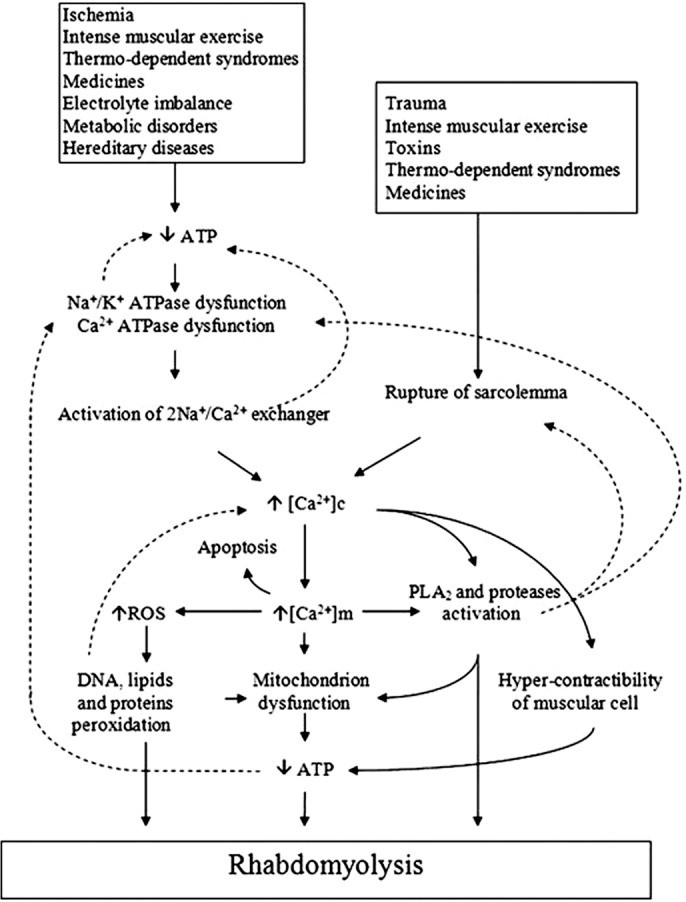
Under normal physiological conditions, at rest, ion channels (including Na+/K+ pumps and Na+/Ca2+ exchangers) located on the muscle cell membrane (sarcolemma) maintain low intracellular concentrations of Na+ and Ca2+ and high intracellular K+ concentrations. Muscle cell depolarization leads to an influx of Ca2+ from the sarcoplasmic reticulum, an intracellular storage site, into the cytoplasm (sarcoplasm). This calcium influx initiates muscle contraction through actin-myosin cross-linking. These processes are energy-dependent, requiring sufficient adenosine triphosphate (ATP). Consequently, any factor directly damaging ion channels or reducing ATP availability will disrupt the delicate balance of intracellular electrolyte concentrations.
When muscle injury or ATP depletion occurs, there is an uncontrolled influx of Na+ and Ca2+ into the muscle cell. Increased intracellular Na+ draws water into the cell, disrupting cellular integrity. Persistently high intracellular Ca2+ levels lead to sustained muscle fiber contraction, further depleting ATP reserves.16 Elevated intracellular Ca2+ also activates calcium-dependent proteases and phospholipases, promoting the breakdown of cellular membranes and further damaging ion channels.1 These intracellular alterations initiate an inflammatory, self-perpetuating cascade of myolysis, resulting in muscle fiber necrosis and the release of muscle contents into the extracellular space and bloodstream.10 Figure 1 visually summarizes this process, illustrating how diverse insults converge on a common pathway to initiate the rhabdomyolysis cascade.
Figure 1. Mechanisms of Rhabdomyolysis
Figure 1. Mechanisms of rhabdomyolysis. Reproduced with permission from Elsevier.17 ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; DNA, deoxyribonucleic acid; PLA, polylactic acid; ROS, reactive oxygen species.
CAUSES
In theory, any form of muscle damage, and consequently, any factor that induces muscle damage, can trigger rhabdomyolysis. In adults, data indicates that the most prevalent causes of rhabdomyolysis include drug or alcohol abuse, medication use, trauma, neuroleptic malignant syndrome (NMS), and prolonged immobility.18 In pediatric populations, the leading causes differ, with viral myositis, trauma, connective tissue disorders, exercise, and drug overdose being more commonly implicated. Viral myositis alone may account for up to one-third of pediatric rhabdomyolysis cases.14,15,19 Table 2 and Table 3 provide lists of many physical and non-physical causes, and specific drugs and agents that can induce rhabdomyolysis, respectively, though these are not exhaustive. Some of the more frequently encountered causes are discussed in greater detail below.
Table 2. Physical and Nonphysical Causes of Rhabdomyolysis
Table 3. Drugs and Other Agents That Can Cause Rhabdomyolysis
Statins
The association between statins, inhibitors of 3-hydroxymethyl-3-methylglutaryl coenzyme A reductase, and drug-induced muscle pain (myalgia) and rhabdomyolysis has been extensively studied since statins were introduced in the 1980s. Statins have become one of the most commonly prescribed drug classes worldwide due to their effectiveness in reducing mortality in patients with pre-existing cardiovascular disease, a leading cause of death in industrialized nations.20 However, the risk of statin-induced myopathy is a real concern and should always be considered when initiating statin therapy.
In 2012, the US Food and Drug Administration (FDA) issued a notification regarding potential side effects of statins, including liver injury, cognitive impairment, type 2 diabetes mellitus, and myopathy/rhabdomyolysis. Warning labels for all statin medications were updated to include these potential adverse effects. Specifically, the label for Mevacor (lovastatin) was revised to include contraindications for co-administration with various agents, such as human immunodeficiency virus (HIV) protease inhibitors and certain antibacterial and antifungal medications.21 Simultaneously, the FDA removed the recommendation for routine periodic monitoring of liver enzymes in patients taking statins, as monitoring had not proven beneficial in detecting or preventing serious liver injury.21 The FDA now recommends obtaining baseline liver enzyme levels before starting statin therapy and checking enzyme levels only if clinically indicated thereafter.
Randomized controlled trials estimate the incidence of myopathic events in statin users to be between 1.5%-5.0%; however, rates observed in clinical practice have varied more widely, from 0.3%-33%.22–24 This discrepancy likely arises from two primary factors: first, the lack of a universally accepted definition for myopathic events may lead to underreporting and underdiagnosis. Second, many randomized controlled trials exclude patient populations at higher risk of muscle toxicity, such as those with renal insufficiency, hepatic insufficiency, pre-existing muscle complaints, hypertriglyceridemia, and poorly controlled diabetes.25,26
Risk factors for developing statin-induced rhabdomyolysis include high statin dosages, advanced age, female gender, renal or hepatic impairment, and diabetes mellitus.1 Table 4 provides a more comprehensive list of potential risk factors.
Table 4. Proposed Risk Factors for Statin-Induced Rhabdomyolysis
Despite the relatively higher incidence of general muscle toxicity associated with statin use, rhabdomyolysis specifically attributed to statin therapy remains a rare occurrence.23 Guyton suggests that the mortality risk associated with rhabdomyolysis is considerably less than the reduction in overall mortality achieved with statin use.27 An analysis of 30 randomized controlled trials (n=83,858) reported 7 cases of rhabdomyolysis in patients receiving statin therapy compared to 5 cases in the placebo group.28 The FDA Adverse Event Reporting System (FAERS) documented rhabdomyolysis rates of 1.07 cases per 1 million statin prescriptions between 1998-2000, increasing to 3.56 cases per 1 million prescriptions from 2002-2004.29 FAERS data also indicated that rhabdomyolysis rates were lowest for pravastatin (1.63 cases) and highest for rosuvastatin (13.54 cases).29 It is important to note that FAERS data are limited by reliance on self-reporting and the requirement for significantly higher CK elevations for rhabdomyolysis classification compared to ACC/AHA/NHLBI definitions.29
Davidson et al. analyzed 2001 FAERS data and found that fatal rhabdomyolysis rates varied depending on the specific statin.29 The 2001 data showed one reported case of fatal rhabdomyolysis per 5.2 million lovastatin prescriptions, 8.3 million simvastatin prescriptions, 23.4 million atorvastatin prescriptions, and 27.1 million pravastatin prescriptions.29 Staffa et al. reported no fatal rhabdomyolysis events in a separate study of patients taking fluvastatin.30 According to Cervellin et al., 2001 FAERS data showed a rate of one fatal rhabdomyolysis case per 316,000 prescriptions for cerivastatin, a statin withdrawn from the market in 2001 due to high fatal rhabdomyolysis rates, especially when combined with fibrates, particularly gemfibrozil.2 Cerivastatin was shown to increase rhabdomyolysis risk up to 12-fold.31 An FDA study over 29 months found that rhabdomyolysis was most common with simvastatin (36%) and cerivastatin (32%), with less frequent occurrences for atorvastatin (12%), pravastatin (12%), lovastatin (6%), and fluvastatin (2%).32
Cytochrome P450 Inhibitors
The potential for increased myopathy and rhabdomyolysis risk when statins are used concurrently with other common medications further complicates statin therapy. Drug interactions contribute to approximately 60% of statin-induced rhabdomyolysis cases.33 These interactions often occur because both statins and frequently co-administered drugs are metabolized by the cytochrome P450 system.
Statins vary in their physiochemical properties. Some statins (atorvastatin, simvastatin, and lovastatin) undergo phase I metabolism by cytochrome P450 3A4 and are classified as 3A4 substrates, while others (pravastatin, fluvastatin, and rosuvastatin) are not metabolized by the 3A4 isoenzyme.34 The 3A4 isoenzyme is also responsible for metabolizing over 50% of marketed pharmaceuticals.34 Administering 3A4 inhibitors with a statin that is a 3A4 substrate can lead to significantly elevated plasma statin levels, resulting in increased statin toxicity.34 Commonly prescribed drugs that are 3A4 inhibitors include fibrates (especially gemfibrozil), calcium channel blockers, histamine H2 antagonists, antibiotics (e.g., clarithromycin), antifungals (e.g., itraconazole), antidepressants, antiretroviral drugs (e.g., protease inhibitors), and immunosuppressants (e.g., cyclosporine).
A 2004 pharmacokinetic study by Jacobson assessing multiple-dose drug interaction profiles showed that simvastatin and atorvastatin (3A4 substrates) exhibited significant pharmacokinetic changes when co-administered with various 3A4 inhibitors, with up to a 5-fold increase in myopathy incidence.35 Fluvastatin (primarily metabolized by cytochrome P450 2C9) and pravastatin (not a major cytochrome P450 substrate) showed no significant pharmacokinetic changes when administered with 3A4 inhibitors.35 Similarly, Law and Rudnicka demonstrated that rhabdomyolysis incidence was higher for patients receiving lovastatin, simvastatin, and atorvastatin, and lower for those receiving fluvastatin and pravastatin, aligning with FAERS data.36 They proposed this difference is due to the metabolism of lovastatin, simvastatin, and atorvastatin by cytochrome P450 3A4, while fluvastatin and pravastatin are not.
One clinically significant drug interaction, particularly relevant given patient comorbidities, is between statins and fibrates. A review of 36 published clinical trials on statin-fibrate combination therapy found a 0.12% prevalence of myopathic events.37 The Davidson et al. FAERS study also examined rhabdomyolysis rates when fenofibrate or gemfibrozil were combined with various statins.29 Data showed that while both fenofibrate and gemfibrozil use with any statin increased rhabdomyolysis risk compared to statin monotherapy, fenofibrate use with a statin resulted in fewer rhabdomyolysis reports (4.5 cases per million prescriptions) than gemfibrozil with a statin (87 cases per million prescriptions).29 Notably, only 2.3% (14 of 606) of rhabdomyolysis reports with fibrate/statin therapy involved cerivastatin and fenofibrate, whereas 88% (533 of 606) were associated with cerivastatin and gemfibrozil.38 Recent studies suggest that the increased myotoxicity observed with gemfibrozil/statin therapy may be partly due to gemfibrozil’s inhibition of statin glucuronidation, leading to reduced statin elimination and increased plasma statin concentrations.38 Fenofibrate has not shown any effect on statin glucuronidation or oxidation.39,40
Trauma
Blunt and crush injuries are common causes of trauma-induced rhabdomyolysis. Interestingly, in crush injuries associated with major disasters like earthquakes or bombings, rhabdomyolysis onset often occurs after the acute muscle compression is relieved, allowing muscle breakdown products to enter circulation.17 High-voltage electrical injuries, such as electrocution or lightning strikes, are also causes of trauma-induced rhabdomyolysis. It is estimated that up to 10% of electrical accident survivors develop rhabdomyolysis.10
Exercise/Exertion
Diagnosing rhabdomyolysis due to exertion can be challenging because serum CK levels naturally increase after strenuous exercise in almost all individuals, potentially reaching up to 10 times the upper limit of normal.41 The extent of CK elevation varies significantly among individuals, and exertional rhabdomyolysis may develop in one person exercising under the same conditions as another who does not experience it.41 Elevated temperature and humidity during exercise may also contribute to higher rhabdomyolysis rates.42 A retrospective study of military personnel in basic training reported 22.2 cases of exertional rhabdomyolysis per 100,000 recruits per year.43 This study also indicated that the incidence and recurrence risk of exertional rhabdomyolysis were low in young, physically active individuals.
Temperature, NMS, and MH
Heat stroke, neuroleptic malignant syndrome (NMS), and malignant hyperthermia (MH) can all lead to rhabdomyolysis.
Heat stroke occurs when core body temperature exceeds 40.5°C. Prolonged exposure to extreme heat can result in rhabdomyolysis, as well as associated hypotension, lactic acidosis, hypoglycemia, disseminated intravascular coagulation, and multi-organ failure.43 Interestingly, exertional heat stroke is less common in women, possibly due to the protective effect of estrogen on muscle.44 Therefore, women presenting with rhabdomyolysis seemingly due to heat stroke should be evaluated for underlying muscle disease or other contributing factors.44
NMS, often associated with antipsychotic medications (particularly first-generation/atypical antipsychotics like haloperidol), can cause rhabdomyolysis, likely due to the significant heat generation from muscle rigidity and tremor in NMS patients.43 The proposed mechanism involves either central nervous system dopamine receptor blockade or withdrawal of a dopaminergic agonist.1
MH is a genetic disorder, autosomal dominant in 50% and autosomal recessive in 20% of cases.1 Symptoms are similar to NMS, including skeletal muscle rigidity, hyperventilation, tachycardia, fever, hemodynamic instability, and lactic acidosis.43 MH typically occurs during general anesthesia in susceptible individuals, with an estimated incidence of 1 in 15,000 anesthetics in children and 1 in 50,000-100,000 in adults.45
Muscle Ischemia
Prolonged oxygen deprivation to muscle tissue can lead to muscle cell necrosis, ultimately precipitating rhabdomyolysis and acute kidney injury. Causes of localized muscle ischemia include blood vessel compression during surgery or otherwise, thromboses, emboli, compartment syndrome, carboxyhemoglobinemia, or sickle cell disease.17 Hypothermia, though rare, can also cause rhabdomyolysis by reducing muscle perfusion.1,17
Infection
Rhabdomyolysis has been reported in association with various types of infections, ranging from localized muscle infections with erythema (bacterial pyomyositis) to sepsis without direct muscle infection.1,46 Proposed mechanisms include tissue hypoxia due to sepsis or dehydration, toxin release, fever, direct bacterial invasion of muscle, or rigors/tremors.1 Legionella bacteria are classically associated with bacterial rhabdomyolysis.47 Viral infections, particularly influenza A and B viruses, are also implicated.48,49 Rhabdomyolysis has also been described with other viruses, including HIV,50 coxsackie virus,51 Epstein-Barr virus,52 Cytomegalovirus,53 herpes simplex virus,54 varicella zoster virus,55 and West Nile virus.56
SYMPTOMS/PRESENTATION
Presenting symptoms of rhabdomyolysis often reflect the underlying cause as well as complications such as renal failure or muscle injury. The classic symptom triad includes myalgia, weakness, and tea-colored urine. Urine color can be affected by muscle mass, urine concentration, and kidney function. A study of 87 cases (CK >500 IU/L) by Gabow et al. found that 26% of patients tested negative for myoglobin in urine using the orthotolidine-toluidine dipstick test.57 Mannix et al. reported that common presenting symptoms in pediatric rhabdomyolysis, regardless of kidney injury, were muscle pain, fever, and viral prodromes.19 Dark or tea-colored urine was reported in only 3.6% of these cases.19 Patients may also present with tense and swollen muscles upon physical examination. It’s important to reiterate that the classic triad is only present in a minority of patients, and many do not experience muscle pain or weakness. Systemic symptoms like tachycardia, malaise, fever, nausea, and vomiting can occur but are non-specific. Clinical manifestations of ARF, disseminated intravascular coagulation, and multi-organ failure may develop subsequently.2
DIAGNOSIS OF RHABDOMYOLYSIS
Accurate and timely diagnosis of rhabdomyolysis relies on a high degree of clinical suspicion, a thorough patient history, and a comprehensive physical examination. Given that the classic triad of symptoms is often absent, clinicians must be vigilant in considering rhabdomyolysis in at-risk patients.
The cornerstone of laboratory diagnosis of rhabdomyolysis is the measurement of plasma creatine kinase (CK). While a definitive CK cutoff has not been established, a level at least five times the upper limit of the normal reference range (i.e., >1,000 IU/L) is commonly used to indicate rhabdomyolysis.2 CK levels are generally considered predictive of the likelihood of developing acute renal failure (ARF), with concentrations exceeding 5,000 IU/L strongly associated with kidney damage. CK has a half-life of approximately 1.5 days, meaning elevated blood levels persist longer than myoglobin, which has a shorter half-life of 2-4 hours. Myoglobin concentrations typically normalize within 6-8 hours after muscle injury.2 Due to its short half-life and rapid clearance, plasma myoglobin is less sensitive than CK for rhabdomyolysis diagnosis, leading to potential false-negative results.16
Urine myoglobin can be detected using urine dipstick tests, which will show a positive result for erythrocytes (blood) due to the orthotoluidine component of the dipstick reacting with myoglobin, causing a color change to blue. However, it’s crucial to differentiate myoglobinuria from hematuria (blood in urine). Microscopic examination of the urine can help distinguish between these conditions. In myoglobinuria, the urine dipstick will be positive for blood but microscopic examination will not reveal red blood cells.
Differential diagnosis of rhabdomyolysis should also consider other conditions that can present with similar symptoms, such as:
- Myositis: Inflammation of muscles, which can also cause muscle pain and weakness, but typically has a different etiology and may be associated with autoimmune conditions.
- Polymyalgia rheumatica: Causes muscle pain and stiffness, particularly in older adults, but CK levels are usually normal.
- Fibromyalgia: A chronic pain condition characterized by widespread musculoskeletal pain, fatigue, and tenderness in localized areas. CK levels are normal.
- Hypothyroidism: Can cause muscle weakness and pain, but is associated with other systemic symptoms and specific thyroid function tests are diagnostic.
- Electrolyte imbalances: Conditions like hypokalemia and hypophosphatemia can cause muscle weakness and mimic some symptoms of rhabdomyolysis, but CK levels are usually not as dramatically elevated as in rhabdomyolysis.
In summary, the diagnostic approach to rhabdomyolysis involves:
- Clinical suspicion: Considering rhabdomyolysis in patients with risk factors or presenting symptoms.
- Creatine Kinase (CK) measurement: The primary laboratory test for diagnosis. A CK level >1000 IU/L is suggestive, and >5000 IU/L is highly indicative and associated with increased risk of complications.
- Urine dipstick for myoglobin: Helpful if positive, but a negative result does not rule out rhabdomyolysis.
- Microscopic urine examination: To differentiate myoglobinuria from hematuria.
- Assessment of renal function: Blood urea nitrogen (BUN) and creatinine levels to monitor for acute kidney injury.
- Electrolyte panel: To assess for electrolyte imbalances (hyperkalemia, hypocalcemia, hyperphosphatemia, hyperuricemia).
- Consideration of underlying causes: Thorough history and physical exam to identify potential triggers (trauma, medications, exertion, etc.).
Early and accurate diagnosis of rhabdomyolysis is crucial for prompt management and prevention of serious complications, particularly acute kidney injury.
SELECTED COMPLICATIONS
Potential complications of rhabdomyolysis are serious and require immediate attention. These include compartment syndrome and acute kidney injury, among others. Figure 2 lists these and other common complications associated with rhabdomyolysis, along with initial treatment strategies for each.
Figure 2. Complications of Rhabdomyolysis
Figure 2. Complications of rhabdomyolysis. Reproduced with permission from Springer.59 IV, intravenous; Rx, treatment.
Compartment Syndrome
Post-traumatic and ischemic muscle damage, especially in muscle groups enclosed by inelastic fascia, can lead to increased pressure within muscle compartments (intracompartmental hypertension).58 When crushed muscle becomes engorged with blood and edematous after the initial injury, it can exacerbate this pressure, a phenomenon known as rebound hyperperfusion. This increased blood flow and compromised lymphatic drainage can impair arteriolar perfusion. Once intracompartmental pressure exceeds a critical threshold, typically >30 mmHg, it can collapse arterioles and halt effective muscle and nerve perfusion, resulting in compartment syndrome.2 Further muscle damage can manifest as a “second wave phenomenon,” characterized by persistent or rebound elevation in CK levels 48-72 hours after the initial injury.17
Acute Kidney Injury
Acute kidney injury (AKI) is the most severe complication of rhabdomyolysis in the days following initial presentation, occurring in approximately 33% of patients.17 AKI in rhabdomyolysis is primarily attributed to the nephrotoxicity of myoglobin accumulation in the kidneys. Hypovolemia, contributing to renal hypoperfusion, is another significant factor.
Various clinical parameters, such as serum CK, creatinine, potassium, and calcium levels, and urine myoglobin levels, have been used to predict AKI risk, but no single parameter has been definitively established as a reliable predictor.17
MANAGEMENT
When rhabdomyolysis is suspected, regardless of the underlying cause, the primary treatment objective is to prevent acute kidney injury. Given the potential for fluid accumulation in muscle compartments and associated hypovolemia, fluid management is crucial to prevent prerenal azotemia. Aggressive hydration, typically at a rate of 1.5 L/h, is the cornerstone of management.59 Another approach involves alternating hourly infusions of 500 mL of saline solution with 500 mL of 5% glucose solution containing 50 mmol of sodium bicarbonate for every 2-3 L of total solution. The target urinary output is 200 mL/h, with a urine pH >6.5 and plasma pH 2 Table 5 summarizes the goals of early vigorous fluid resuscitation in rhabdomyolysis. It’s important to note that the efficacy of urinary alkalization with sodium bicarbonate or sodium acetate, and the use of mannitol to promote diuresis, remain unproven.60 Any medications known to increase rhabdomyolysis risk, such as statins, should be immediately discontinued. In cases of compartment syndrome, fasciotomy may be necessary to relieve pressure and limit muscle and kidney damage.
Table 5. Aims of Early Vigorous Fluid Resuscitation in Rhabdomyolysis
ANESTHETIC CONSIDERATIONS
Specific data regarding anesthetic choices in patients with rhabdomyolysis is limited, possibly due to the relative rarity of the condition. However, retrospective studies on patients with muscular dystrophies, who are at increased risk of anesthesia-related complications including rhabdomyolysis,61 offer some insights. Muenster et al., in a review of 232 patients with Duchenne muscular dystrophy, used total intravenous anesthesia (TIVA) without volatile anesthetic agents, employing opioids and non-depolarizing muscle relaxants as needed based on the procedure type and duration, guided by the anesthetist’s discretion.61 Succinylcholine was avoided, and neuromuscular blockade was monitored using acceleromyography.61 Nitrous oxide and propofol were used for induction. Muenster et al. reported no serious anesthetic complications and no cases of rhabdomyolysis in their study.61 Segura et al., reviewing 117 patients with dystrophinopathies, found that succinylcholine could trigger rhabdomyolysis, hyperkalemia, and cardiac arrest; however, evidence regarding inhalational anesthetic use was lacking.62 Segura et al. also reported no rhabdomyolysis cases with TIVA in their retrospective study and no conclusive evidence for or against volatile anesthetic usage in this patient population.62 It is important to recognize that no anesthetic agent is entirely risk-free; rhabdomyolysis has been reported with non-triggering anesthetics, barbiturates, benzodiazepines, propofol, ketamine, and even fasting.62
Ketamine
Ketamine hydrochloride is commonly used as a dissociative anesthetic for procedural sedation in the operating room.63 Ketamine, a phencyclidine analog, is hypothesized to induce agitation and prolonged muscle activity, potentially leading to rhabdomyolysis.63 Weiner et al.’s case study of 20 patients aged 15-40 presenting to the emergency room after ketamine abuse showed that 2 of the 20 patients developed clinical rhabdomyolysis.64
Succinylcholine
Succinylcholine is a widely used neuromuscular depolarizing agent in operating rooms to induce muscle relaxation and short-term paralysis, often prior to tracheal intubation. Succinylcholine is known to cause succinylcholine-induced malignant hyperthermia (MH), which frequently co-occurs with clinically significant rhabdomyolysis.65 Despite these potentially life-threatening complications, succinylcholine remains popular in operating rooms, particularly in trauma settings, due to its rapid onset and short duration of action compared to other muscle relaxants.
While the exact mechanism of succinylcholine-induced rhabdomyolysis is not fully understood, numerous case reports exist.66 Rhabdomyolysis following succinylcholine administration can lead to severe hyperkalemia and potentially cardiac arrest.65 Most patients experiencing rhabdomyolysis after succinylcholine administration are subsequently found to have underlying MH or undiagnosed muscular dystrophy.65 In fact, succinylcholine-induced rhabdomyolysis after puberty is rare in the absence of an underlying predisposing condition.67 The mortality rate for patients with undiagnosed muscular dystrophies who develop cardiac arrest due to succinylcholine-induced rhabdomyolysis is approximately 30%.65 However, the association between succinylcholine and rhabdomyolysis in patients with muscular dystrophies requires further investigation, as rhabdomyolysis can still occur in this population under general anesthesia even when succinylcholine is not used.65
Propofol
Propofol-infusion syndrome (PRIS), a term coined by Bray in 1998, initially described a clinical syndrome observed in critically ill children undergoing prolonged propofol sedation.68 Since its initial description, PRIS has been reported in adults as well, with the first adult PRIS-related death in 2000.69 PRIS is not limited to critically ill patients; it has been reported in healthy individuals and patients receiving short-term, high-dose propofol infusions.70,71 Rhabdomyolysis is a commonly observed symptom within the diverse range of signs and symptoms associated with PRIS, typically occurring later in the syndrome’s progression.68 Risk factors for PRIS include severe head injury, airway infection, young age, high cumulative propofol dose, elevated catecholamine and serum glucose levels, low carbohydrate/high fat intake, critical illness, and inborn metabolic errors.68 Rhabdomyolysis has been identified as an independent risk factor for mortality in PRIS.68
The first large, prospective study of PRIS investigated its development in intensive care unit patients across 11 medical centers who received propofol infusions for >24 hours.72 PRIS was strictly defined as metabolic acidosis and cardiac dysfunction, plus one of the following: rhabdomyolysis, hypertriglyceridemia, or renal failure.72 The study reported a 1.1% PRIS incidence in this patient population and found that 91% of patients who developed PRIS were receiving vasopressors. Interestingly, only 18% of patients had received propofol at infusion rates >5 mg/kg/h, suggesting that the total cumulative dose, rather than infusion rate, may be a better predictor of PRIS development.72
CONCLUSION
Rhabdomyolysis is a complex medical condition associated with significant morbidity and mortality. While often caused by direct trauma, a wide range of etiologies exist, including drugs, toxins, infections, muscle ischemia, electrolyte and metabolic disorders, genetic conditions, exertion, prolonged immobilization, and temperature-related states like NMS and MH. The classic triad of symptoms includes myalgia, weakness, and myoglobinuria, and elevated CK levels are the most sensitive indicator of muscle injury-induced rhabdomyolysis. Prompt and accurate diagnosis of rhabdomyolysis is crucial for initiating timely treatment and preventing severe complications such as acute kidney injury. All clinicians should maintain a strong awareness of the common causes, effective diagnostic strategies, and current treatment options for rhabdomyolysis to ensure optimal patient outcomes.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.