1. Introduction
Disseminated intravascular coagulation (DIC) is a serious condition frequently linked to severe illnesses such as infectious diseases, hematological malignancies, and solid cancers. It is a major contributor to organ failure and bleeding complications, significantly worsening patient outcomes [1,2]. Effective management of DIC involves treating the underlying cause and providing supportive care, including blood product transfusions for significant bleeding [3]. In regions like Japan, early DIC treatment is prioritized [4], with therapies like antithrombin [5] and recombinant thrombomodulin [6] commonly used. Consequently, several diagnostic criteria for DIC have been developed by leading medical organizations, including the Japanese Ministry of Health, Labor and Welfare (JMHLW) [7], the International Society of Thrombosis and Haemostasis (ISTH) [8], the Japanese Association for Acute Medicine (JAAM) [9], and the Japanese Society of Thrombosis and Hemostasis (JSTH) [10].
Current diagnostic criteria for DIC often rely on complex scoring systems. These systems typically incorporate assessments of platelet count, prothrombin time (PT), fibrin-related markers, and fibrinogen levels [11]. However, these scoring systems can be cumbersome in clinical practice, and the standardization of fibrin-related markers remains a challenge [12]. Furthermore, there is inconsistency in the cut-off values used for various parameters across the different diagnostic criteria for DIC [11]. In fast-paced environments like the emergency room, simpler diagnostic tools are essential for facilitating rapid DIC diagnosis and initiating timely treatment. The sepsis-induced coagulopathy (SIC) score, a more recent development, offers a simplified approach using PT, platelet count, and the sequential organ failure assessment score, proving potentially useful for DIC diagnosis in sepsis patients [13]. However, variability in cut-off values for fibrin-related markers persists in DIC diagnosis using ISTH overt-DIC criteria [12]. Additionally, the JAAM criteria may not be as effective for DIC arising from non-infectious causes, and the JSTH criteria are considered complex. For these reasons, the JMHLW diagnostic criteria for DIC were utilized in this study as a reference standard.
This study aimed to establish practical cut-off values for prothrombin time–international normalized ratio (PT-INR), D-dimer, and platelet count to aid in DIC diagnosis. Based on these findings, we propose a streamlined “quick DIC score” system designed for rapid assessment and diagnosis of DIC.
2. Materials and Methods
This study included a cohort of patients admitted to Mie Prefectural General Medical Center between September 1, 2019, and December 28, 2020, with a range of conditions: sepsis (n = 58), critical illness without sepsis (n = 152), pneumonia (n = 107), obstetric disease (n = 16), solid cancer (n = 42), aneurysm (n = 78), other respiratory diseases (n = 28), peripheral arterial and venous thrombosis (PAVTE, n = 44), hematological disorders (n = 41), other infections (n = 77), other digestive disorders (n = 87), cerebrovascular disorders (n = 176), acute coronary syndrome (n= 67), other heart diseases (n = 94), urinary tract diseases (n = 125), unidentified clinical syndrome (n = 125), and other conditions (n = 50).
DIC diagnosis was determined using the Japanese Ministry of Health Labor and Welfare criteria for DIC (detailed in Supplementary Table S1) [7]. According to these criteria, patients were categorized as having DIC (score ≥ 7), pre-DIC (score 5–6), or non-DIC (score ≤ 4). Diagnostic methods for specific conditions included computed tomography or magnetic resonance imaging for cerebrovascular disorders, coronary angiography, electrocardiography, and troponin level assessment for acute coronary syndrome, and computed tomography or venous ultrasound for PAVTE. The study protocol (2019-K9) received ethical approval from the Human Ethics Review Committee of Mie Prefectural General Medical Center, and all participants provided informed consent. The study was conducted in accordance with the Declaration of Helsinki principles.
D-dimer measurements were performed using LPIA-Genesis (LSI Medience, Tokyo, Japan) on a STACIA system (LSI Medience). Prothrombin time (PT) international normalized ratio (INR) was measured with Thromborel S (Sysmex Co., Kobe, Japan) using a CS-5100 automatic coagulation analyzer (Sysmex Co.). Platelet counts were obtained using a XN-3000 full-automatic blood cell counter (Sysmex Co.). Reference ranges (median and 2.5–97.5 percentile) for healthy individuals at our institution were: D-dimer, 0.4 μg/mL (0.1–0.5 μg/mL); PT-INR, 0.96 (0.90–1.04); and platelet count, 21.9 × 1010/L (17.9–25.9 × 1010/L).
Statistical Analyses
Data are presented as median (25th–75th percentile). The Mann–Whitney U-test was used to assess the statistical significance of differences between groups. Multiple regression analysis was performed to evaluate PT-INR, D-dimer, and platelet counts as diagnostic markers for DIC or pre-DIC versus non-DIC. Cut-off values were determined at the intersection of sensitivity and specificity curves, and receiver operating characteristic (ROC) curve analysis was used to evaluate diagnostic performance. A p-value of < 0.05 was considered statistically significant.
3. Results
Based on the JMHLW diagnostic criteria, 70 patients were diagnosed with DIC and 109 with pre-DIC. DIC was more frequently observed in patients with sepsis, critical illness without sepsis, pneumonia, obstetric diseases, and solid cancer (Table 1).
Table 1. Patient Demographics and Underlying Diseases in DIC, Pre-DIC, and Non-DIC Groups.
| Underlying Diseases | Non-DIC | Pre-DIC | DIC | Number of DIC (%) |
|---|---|---|---|---|
| Sepsis | 26 | 21 | 11 | 11/58 (19.0%) |
| Critical illness without sepsis | 95 | 23 | 34 | 34/152 (22.4%) |
| Pneumonia | 78 | 17 | 12 | 12/107 (11.2%) |
| Obstetric diseases | 13 | 1 | 2 | 2/16 (12.5%) |
| Solid cancer | 33 | 6 | 3 | 3/42 (7.1%) |
| Aneurysm | 66 | 9 | 3 | 3/78 (3.8%) |
| Other respiratory diseases | 24 | 3 | 1 | 1/28 (3.6%) |
| Peripheral arterial and venous thrombosis | 39 | 4 | 1 | 1/44 (2.3%) |
| Hematological disorders | 40 | 0 | 1 | 1/41 (2.4%) |
| Other infections | 64 | 12 | 1 | 1/77 (1.3%) |
| Other digestive disorders | 84 | 2 | 1 | 1/87 (1.1%) |
| Cerebrovascular disorders | 174 | 2 | 0 | 0/176 (0%) |
| Acute coronary syndrome | 66 | 1 | 0 | 0/67 (0%) |
| Other heart diseases | 90 | 4 | 0 | 0/94 (0%) |
| Urinary tract diseases | 13 | 1 | 0 | 0/14 (0%) |
| Unidentified clinical syndrome | 125 | 0 | 0 | 0/125 (0%) |
| Others | 51 | 0 | 0 | 0/50 (0%) |
| Total | 1114 | 109 | 70 | 70/1293 (5.4%) |
DIC, disseminated intravascular coagulation; Non-DIC, DIC score ≤ 4; Pre-DIC, DIC score 5 or 6; DIC, DIC score ≥ 7 using the Japanese Ministry of Health, Labor and Welfare diagnostic criteria.
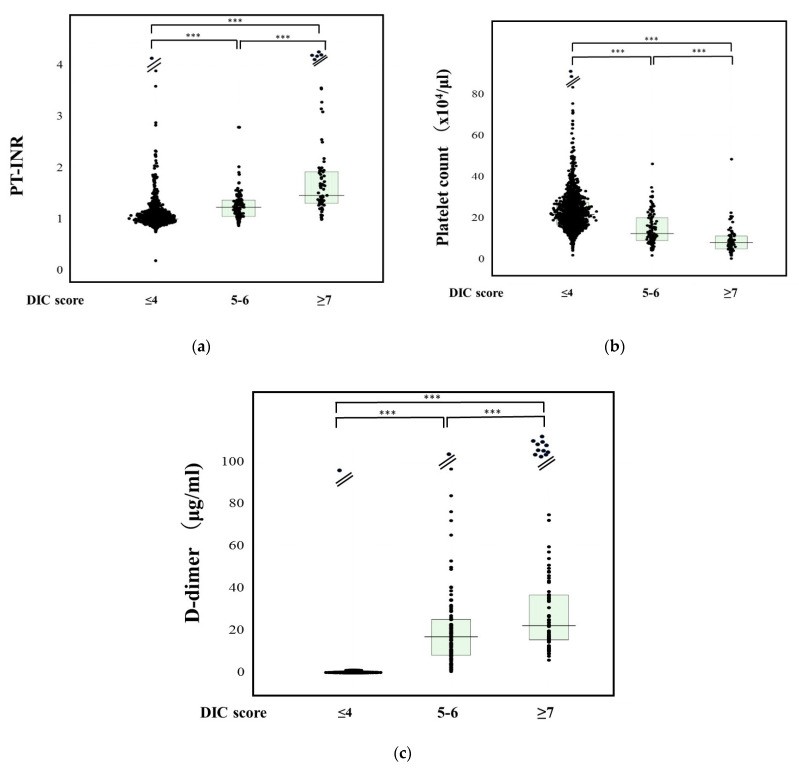
PT-INR levels were significantly higher in DIC patients (1.61: 1.34–1.96) compared to pre-DIC (1.24: 1.06–1.38) and non-DIC patients (1.01: 0.94–1.10), and also significantly higher in pre-DIC compared to non-DIC patients (Figure 1a). Multiple regression analysis showed D-dimer, platelet count, and PT-INR to be significant predictors for DIC or pre-DIC vs. non-DIC, with D-dimer exhibiting the highest standardized beta value (Table 2). ROC analysis for DIC vs. non-DIC identified a PT-INR cut-off value of 1.20, yielding 88.8% sensitivity and specificity, with an AUC of 0.934 and a negative predictive value (NPV) of 98.9% (Table 3).
Figure 1. Biomarker Levels in DIC, Pre-DIC, and Non-DIC Patients.
Comparative analysis of (a) PT-INR, (b) platelet counts, and (c) D-dimer levels across DIC, pre-DIC, and non-DIC patient groups. PT-INR, prothrombin time–international normalized ratio. ***, p < 0.001.
Table 2. Multiple Regression Analysis of PT-INR, D-dimer, and Platelet Counts for DIC and Pre-DIC Diagnosis.
| DIC/Pre-DIC | β | SE (β) | Stdβ | t | N | p |
|---|---|---|---|---|---|---|
| PT-INR | DIC | 0.09643 | 0.01059 | 0.2564 | 9.10465 | 1178 |
| DIC + Pre-DIC | 0.09596 | 0.01589 | 0.1660 | 6.04085 | 1287 | |
| PLT | DIC | −0.0034 | 0.00046 | −0.2098 | 7.36469 | 1178 |
| DIC + Pre-DIC | −0.0061 | 0.00068 | −0.2420 | 8.9452 | 1287 | |
| D-dimer | DIC | 0.00379 | 0.00027 | 0.3776 | 13.9969 | 1178 |
| DIC + Pre-DIC | 0.00501 | 0.00039 | 0.3365 | 12.8174 | 1287 | |
| Sex | DIC | 0.02700 | 0.01144 | 0.0686 | 2.35955 | 1178 |
| DIC + Pre-DIC | 0.03496 | 0.01686 | 0.0577 | 2.07386 | 1287 | 0.03829 |
| Age | DIC | −0.0007 | 0.00029 | −0.0740 | 2.54647 | 1178 |
| DIC + Pre-DIC | −0.0005 | 0.00043 | −0.0359 | 1.28869 | 1287 | 0.19774 |
The double correlation coefficient, DIC, R = 0.57684 (p < 0.001); DIC + Pre-DIC, R = 0.53189 (p < 0.001).
Table 3. ROC Analysis of PT-INR, Platelet Counts, and D-dimer for DIC and Pre-DIC Diagnosis.
| DIC/Pre-DIC | Cut-Off Value | Sensitivity | Specificity | AUC | NPV | Odds’ Ratio |
|---|---|---|---|---|---|---|
| PT-INR | DIC | 1.20 | 88.8% | 88.0% | 0.934 | 98.9% |
| DIC + Pre-DIC | 1.11 | 77.9% | 77.9% | 0.848 | 95.7% | 12.5 |
| PLT (×1010/L) | DIC | 12.0 | 93.6% | 80.0% | 0.925 | 44.1% |
| DIC + Pre-DIC | 16.4 | 76.2% | 76.2% | 0.828 | 34.0% | 10.2 |
| D-dimer (μg/mL) | DIC | 10.0 | 93.1% | 92.5% | 0.971 | 99.6% |
| DIC + Pre-DIC | 7.8 | 86.4% | 87.0% | 0.930 | 97.9% | 42.5 |
DIC, disseminated intravascular coagulation; Non-DIC, DIC score ≤ 4; Pre-DIC, DIC score 5 or 6; DIC, DIC score ≥ 7 using the Japanese Ministry of Health, Labor and Welfare diagnostic criteria. PT-INR, prothrombin time–international normalized ratio; AUC, area under the curve; NPV, negative predictive value.
Platelet counts were significantly lower in DIC patients (8.3 × 1010/L: 5.2–11.4 × 1010/L) than in pre-DIC (12.6 × 1010/L: 9.2–20.3 × 1010/L) or non-DIC patients (21.8 × 1010/L: 16.9–27.7 × 1010/L), and also lower in pre-DIC compared to non-DIC patients (Figure 1b). ROC analysis for DIC vs. non-DIC showed a platelet count cut-off value of 12.0 × 1010/L, with 93.6% sensitivity and 80.0% specificity, and an AUC of 0.925 and NPV of 44.1%.
D-dimer levels were significantly elevated in DIC patients (25.0 μg/mL: 16.1–47.8 μg/mL) compared to pre-DIC (17.0 μg/mL: 8.2–25.7 μg/mL) and non-DIC patients (1.7 μg/mL: 0.7–4.8 μg/mL), and also higher in pre-DIC compared to non-DIC patients (Figure 1c). For DIC vs. non-DIC, ROC analysis identified a D-dimer cut-off value of 10.0 μg/mL, achieving 93.1% sensitivity and 92.5% specificity, with a high AUC of 0.971 and NPV of 99.6%.
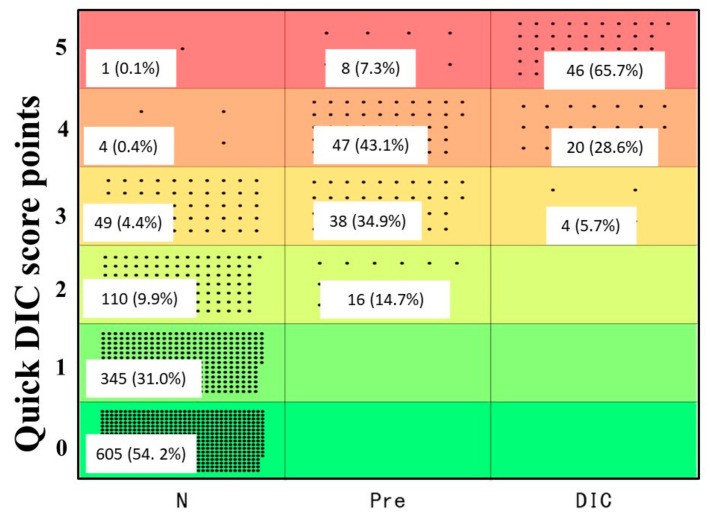
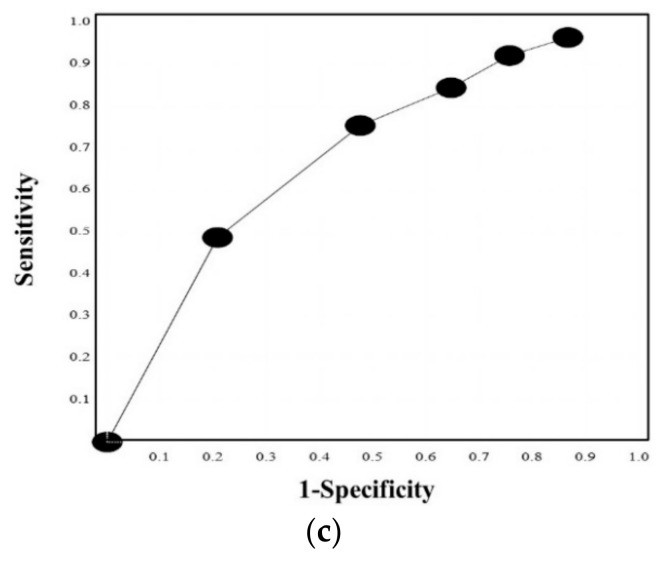
Based on these findings, we developed the quick DIC score system (Table 4). Using this system, all DIC patients scored ≥3 points, 85.3% of pre-DIC patients scored ≥3 points, and all pre-DIC patients scored ≥2 points (Figure 2). ROC analysis (Figure 3a,b) revealed an AUC of 0.997 for DIC vs. non-DIC and 0.984 for pre-DIC + DIC vs. non-DIC. The optimal cut-off score was 3 points for DIC and 2 points for DIC + pre-DIC (Table 4). Notably, quick DIC scores were significantly higher in non-survivors (2.0 points: 1.0–3.0 points) than in survivors (1 point: 0–1.0 points). ROC analysis for survival (Figure 3c) showed an AUC of 0.685, with a cut-off value of 2 points (Table 5).
Table 4. Quick DIC Diagnostic Criteria Based on Biomarkers and Underlying Conditions.
| Cut-Off Value | Points |
|---|---|
| PT-INR | ≥1.2 |
| Platelet count | ≤12.0 × 1010/L |
| D-dimer | ≥10.0 μg/mL |
| Underlying diseases * due to DIC | 1 |
| Total | ≥3 points |
DIC, disseminated intravascular coagulation; PT-INR, prothrombin time–international normalized ratio; * severe infections, hematological malignancy, solid cancer, aneurysm, obstetric diseases, critical illness such as trauma, shock and inflammation, multiple organ failure, etc.
Figure 2. Distribution of Quick DIC Scores Across Diagnostic Categories.
Quick DIC score distribution in patients categorized as non-DIC, pre-DIC, and DIC. DIC, disseminated intravascular coagulation.
Figure 3. ROC Curve Analysis for Quick DIC Score Performance.
ROC curves for the quick DIC score in diagnosing (a) DIC vs. non-DIC, (b) DIC and pre-DIC vs. non-DIC, and (c) survivor vs. non-survivor. ROC, receiver operating characteristic; DIC, disseminated intravascular coagulation; area under the curve, (a) 0.997, (b) 0.984, and (c) 0.685.
Table 5. Performance Metrics of Quick DIC Score for DIC and Pre-DIC Diagnosis.
| Score | Sensitivity | Specificity | PPV | NPV | Likelihood Ratio |
|---|---|---|---|---|---|
| DICAUC, 0.997Cut-off value, 3 | 5 | 65.7% | 99.9% | 97.9% | 94.1% |
| 4 | 94.3% | 99.6% | 93.0% | 99.6% | 210.1 |
| 3 | 100% | 95.1% | 56.0% | 100% | 20.3 |
| 2 | 100% | 85.5% | 30.3% | 100% | 6.9 |
| 1 | 100% | 54.6% | 12.2% | 100% | 2.2 |
| 0 | 100% | 0% | – | – | – |
| DIC + pre-DICAUC, 0.984Cut-off value, 2 | 5 | 30.2% | 99.9% | 98.2% | 89.9% |
| 4 | 67.6% | 99.5% | 96.0% | 95.0% | 462.7 |
| 3 | 91.1% | 95.1% | 74.8% | 98.5% | 18.4 |
| 2 | 100% | 85.5% | 52.6% | 100% | 6.9 |
| 1 | 100% | 54.6% | 26.1% | 100% | 2.2 |
| 0 | 100% | 0% | – | – | – |
DIC, disseminated intravascular coagulation; PPV, positive predictive value; NPV, negative predictive value.
4. Discussion
In this study, approximately 5% of patients, particularly those with critical illnesses such as sepsis, pneumonia, obstetric complications, solid cancers, and aneurysms, developed DIC. This observation supports the inclusion of severe infections, hematological malignancies, solid cancers, aneurysms, obstetric diseases, and critical illnesses (including trauma, shock, inflammation, and multiple organ failure) as underlying conditions contributing to DIC in our quick DIC scoring system. This aligns with the underlying conditions recognized in the JMHLW DIC diagnostic criteria [7] and generally accepted as DIC risk factors [8,9].
The ROC analysis demonstrated high AUC values for PT-INR, platelet counts, and D-dimer, indicating their utility in diagnosing DIC and pre-DIC. While D-dimer showed the highest AUC and standardized beta value, its measurement requires standardization [12], and the SIC scoring system omits D-dimer [13]. PT-INR, despite its high AUC and NPV, can be influenced by liver function and anticoagulation therapy [14]. Platelet count, a commonly used DIC marker with a high AUC, exhibited a low NPV in our study, suggesting that thrombocytopenia is prevalent even in the absence of DIC [15]. Recognizing the limitations of individual parameters, we propose the quick DIC scoring system, integrating PT-INR, platelet count, and D-dimer for improved DIC diagnosis. Furthermore, we adopted single cut-off values for each parameter, simplifying the scoring compared to the multiple cut-off values in JMHLW [7], ISTH [8], JAAM [9], and JSTH [10] criteria, making it a rapid and practical tool for DIC diagnosis in critical care settings.
Evaluation of the quick DIC score system revealed that it identified 100% of DIC patients and 85.3% of pre-DIC patients (diagnosed by JMHLW criteria) as “possible DIC,” demonstrating a remarkably high AUC for DIC diagnosis. These promising results warrant further large-scale studies to validate the clinical utility of the quick DIC score system for diagnosing both DIC and pre-DIC. The observation that approximately 50% of patients diagnosed with “possible DIC” by our quick score system died suggests its potential in predicting poor outcomes. This is consistent with the known association of DIC with adverse outcomes and its significant contribution to mortality in critically ill patients [3,16].
Key strengths of the quick DIC system include its focus on underlying conditions associated with DIC onset, the use of single cut-off values for PT-INR and D-dimer with high NPV for DIC diagnosis, and a single platelet count cut-off value with high sensitivity for DIC detection.
5. Conclusions
The quick DIC score, incorporating underlying diseases, PT-INR, platelet count, and D-dimer levels, effectively diagnosed DIC and showed promise in predicting poor patient outcomes. This simple and rapid diagnostic tool has the potential to improve the early identification and management of DIC in critical care settings.
Acknowledgments
We extend our gratitude to the physicians and laboratory technicians, Chief technicians Shinya Hiromori and Motoko Tanaka, at Mie Prefectural General Medical Center Hospital for their contributions in measuring laboratory biomarkers.
Supplementary Materials
The following supplementary information is available online at: https://www.mdpi.com/article/10.3390/jcm11041028/s1, Table S1: Modified diagnostic criteria for DIC established by Japanese Ministry of Health, Labor and Welfare.
Click here for additional data file. (141.9KB, zip)
Author Contributions
Methodology, Y.I. and M.E.; data curation, M.T. and S.F.; original draft preparation, H.W.; writing—review and editing, A.Y. and K.S. (Kei Suzuki); investigation, J.M., M.Y., I.M. and H.I. (Hidekazu Inoue); validation, H.I. (Hiroshi Imai); project administration, M.S.; funding acquisition, K.S. (Katsuya Shiraki); supervision, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant-in-aid (H30-015) from the Ministry of Health, Labor and Welfare of Japan.
Institutional Review Board Statement
The study protocol (O-0057) was approved by the Human Ethics Review committees of Mie Prefectural General Medical Center, and informed consent was obtained from each patient.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
D-dimer kits were provided by LSI Medience. The authors declare no other conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
[1] Levi, M.; Thachil, J. Disseminated intravascular coagulation in adults: recent advances in pathophysiology and diagnosis. Blood 2024, 143, 428–440.
[2] Connors, J.M. Mechanisms of thrombosis and disseminated intravascular coagulation in malignancy. J. Thromb. Haemost. 2020, 18, 1752–1764.
[3] Wada, H.; Thachil, J.; Di Nisio, M.; Mathew, P.; Reade, M.C.; Scully, M.; Ortel, T.L.; Levi, M.; Subcommittee on DIC of the ISTH. Guidance for diagnosis and treatment of disseminated intravascular coagulation (DIC): JSTH/ISTH shared recommendations. J. Thromb. Haemost. 2023, 21, 2113–2125.
[4] জাপানী স্বাস্থ্য, শ্রম ও কল্যাণ মন্ত্রণালয়। Disseminated intravascular coagulation诊疗指南。 জাপানী রক্ত栓止血学会。2015।
[5] জাপানী স্বাস্থ্য, শ্রম ও কল্যাণ মন্ত্রণালয়। Antithrombin III製剤の適正使用に関するガイドライン。厚生労働省。2015।
[6] জাপানী স্বাস্থ্য, শ্রম ও কল্যাণ মন্ত্রণালয়। Recombinant thrombomodulin製剤の適正使用に関するガイドライン。厚生労働省。2015।
[7] জাপানী স্বাস্থ্য, শ্রম ও কল্যাণ মন্ত্রণালয়। 播種性血管内凝固症候群(DIC)診断基準。厚生労働省。1987।
[8] Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M.; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330.
[9] জাপানী স্বাস্থ্য, শ্রম ও কল্যাণ মন্ত্রণালয়। 急性期DIC診断基準。日本救急医学会。2006।
[10] জাপানী স্বাস্থ্য, শ্রম ও কল্যাণ মন্ত্রণালয়। 血栓止血学会DIC診断基準。日本血栓止血学会。2017।
[11] Levi, M.; de Jonge, E.; van der Poll, T. Disseminated intravascular coagulation. Crit. Care Med. 2020, 48, 1558–1572.
[12] Levi, M.; Thachil, J.; Di Nisio, M.; Toh, C.H.; European Society of Haematology. Diagnosis and treatment of disseminated intravascular coagulation. Br. J. Haematol. 2019, 185, 6–17.
[13] Iba, T.; Nisio, M.D.; Levy, J.H.; Kitamura, T.; Thachil, J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis. BMJ Open 2017, 7, e017046.
[14] Tripodi, A.; Mannucci, P.M. The coagulopathy of chronic liver disease. N. Engl. J. Med. 2011, 365, 147–156.
[15] Levi, M.; Schultz, M.; van der Poll, T. Disseminated intravascular coagulation in cancer patients. Haematol. Oncol. Clin. North Am. 2000, 14, 577–601.
[16] Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Kramer, A.A.; Weaver, J.; Martin, D.P.; Neff, M.; Sternberg, A.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Click here for additional data file. (141.9KB, zip)
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy restrictions.