In the fast-paced environment of the critical care pod, a sudden influx of patients with varied and complex presentations is the norm. Consider the case of a 60-year-old female with a history of hypertension, arriving in distress with facial swelling. This patient, exhibiting significant angioedema of the tongue and lips, labored breathing in a tripod position, rapid breathing, drooling, and noisy stridorous respirations, immediately commands urgent attention. Monitoring reveals an alarming oxygen saturation in the 80s. Communication is hampered by her severe swelling and respiratory distress, though a medication list reveals a recent prescription of Lisinopril for hypertension. This scenario underscores the critical need for emergency clinicians to efficiently navigate the broad differential diagnosis of facial swelling, swiftly distinguishing life-threatening conditions from less urgent presentations.
Facial swelling presents a diagnostic challenge due to its extensive differential, ranging from localized dental infections to life-threatening conditions like decompensated Ludwig’s angina compromising the airway. For emergency clinicians, rapidly differentiating critical etiologies from benign causes is paramount. Categorizing facial swelling by location can streamline the diagnostic process. While numerous causes exist, the following classification highlights key considerations, with bolded diagnoses indicating critical, “can’t miss” conditions demanding immediate attention due to their life-threatening nature.
- Orbital/Periorbital Space
- Cavernous Venous Thrombosis
- Orbital Cellulitis
- Myxedema Coma
- Subcutaneous
- Facial Cellulitis
- Submandibular Space
- Ludwig’s Angina
- Salivary Gland Pathology
- Intra-oral
- Angioedema
- Odontogenic Infection
- Miscellaneous Causes
- Trauma
- Superior Vena Cava Syndrome
- Malignancy
Immediate Life-Threatening Causes of Facial Swelling
Angioedema
Angioedema stands out as a critical “can’t miss” diagnosis in patients presenting with facial swelling. Characterized by subcutaneous swelling resulting from fluid leakage into interstitial tissues, angioedema typically manifests rapidly, often within minutes of onset. Emergency physicians must prioritize three primary etiologies:
- Anaphylaxis-related Angioedema
- Angiotensin Converting Enzyme (ACE) inhibitor-induced Angioedema
- C1 Inhibitor Deficiency (Hereditary Angioedema)
Anaphylactic angioedema is triggered by an IgE-mediated immune response. This reaction activates mast cells, releasing a cascade of inflammatory mediators that increase vascular permeability, leading to angioedema [1]. Conversely, both ACE inhibitor-induced angioedema and hereditary angioedema stem from disruptions in the bradykinin pathway. ACE inhibitors impede bradykinin degradation, while C1 inhibitor deficiency leads to increased bradykinin production [2].
Subtle clinical differences exist among these angioedema types. Generally, angioedema presents with rapid swelling affecting the face, lips, larynx, and potentially the bowel. Facial involvement commonly includes swelling of the lips, tongue, and uvula, particularly pronounced in ACE inhibitor-induced cases [3]. Determining the underlying cause is crucial as it dictates treatment strategies. A thorough medication review, specifically noting ACE inhibitor use, is essential.
Diagnosis is primarily clinical, based on observation of facial angioedema affecting the lips, tongue, uvula, or larynx. Anaphylaxis-related angioedema may present with accompanying urticaria, skin flushing, bronchospasm, or pruritus [4].
While most angioedema cases are benign, significant swelling of the tongue, upper airway, or larynx can lead to life-threatening upper airway obstruction, necessitating consideration of intubation. Severe tongue swelling might require fiberoptic nasal intubation. Given potential intubation challenges, preparing for surgical cricothyrotomy as a backup is crucial.
Beyond airway management, angioedema treatment is etiology-dependent. For anaphylaxis, immediate intramuscular epinephrine (0.3 mg for adults) is paramount, potentially requiring repeated doses based on symptom severity. Glucocorticosteroids and antihistamines may be considered, but epinephrine remains the cornerstone of treatment.
Managing ACE inhibitor-induced angioedema involves discontinuing the offending medication long-term. Various therapies, including Icatibant (a bradykinin receptor antagonist), C1 inhibitor concentrate (reducing kallikrein and subsequently bradykinin production), and Fresh Frozen Plasma (FFP), have been explored, but their efficacy is debated. Studies suggest early administration is key for therapeutic success [5-7]. In most cases, discontinuing the ACE inhibitor and airway management are the primary interventions. ACE inhibitor-induced angioedema typically resolves within 1-3 days [8].
Hereditary angioedema management, alongside airway support, includes C1 inhibitor concentrate, Icatibant, and Ecallantide (a kallikrein inhibitor). These therapies demonstrate greater effectiveness in hereditary angioedema compared to ACE inhibitor-induced cases.
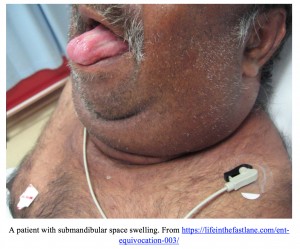
Submandibular Space Infection (Ludwig’s Angina)
Ludwig’s Angina, or submandibular space infection, is a rapidly progressing infection of the mouth floor with a high potential for morbidity and mortality. Early aggressive antibiotic therapy has dramatically reduced mortality rates from upwards of 50% to 0-4% [9]. Odontogenic origins, particularly involving the second and third mandibular molars, account for approximately two-thirds of Ludwig’s Angina cases [10]. The infection spreads swiftly, causing significant tongue and submandibular swelling, potentially extending to the epiglottis.
Diagnosis relies on clinical history and physical examination, often supported by imaging. Patients typically present with fever, chills, and oral pain. As the infection advances, neck stiffness, speech difficulty, drooling, and stridor may develop. Examination reveals symmetrical, tender submandibular swelling, sometimes with palpable crepitus, and potential tongue protrusion. CT imaging is the preferred modality for Ludwig’s angina evaluation, enabling assessment of submandibular space infection and localization for potential surgical drainage [11, 12]. However, imaging may be contraindicated if the airway is compromised.
Treatment goals for Ludwig’s Angina are airway management and prompt antibiotic administration. While emergent airway intervention isn’t always immediately necessary, a low threshold for early intervention is crucial. Securing airway control before airway compromise (stridor, hypoxia, asphyxia) is critical. Nasal fiberoptic intubation is often preferred, but video laryngoscopy can be considered on a case-by-case basis [13]. Anesthesia consultation for airway management assistance is advisable if needed.
Following airway assessment and management, initiating antibiotics is paramount. Antibiotic selection depends on the patient’s immune status. For immunocompetent individuals, recommended regimens [14] include:
- Ampicillin-Sulbactam (3 grams IV every 6 hours) or
- Penicillin G (2 to 4 MU IV every 4-6 hours) + Metronidazole (500 mg IV every 6-8 hours) or
- Clindamycin (600 mg IV every 6-8 hours)
Immunocompromised patients require broader coverage, such as:
- Cefepime (2 g IV every 12 hours) + Metronidazole (500 mg IV every 6-8 hours) or
- Imipenem (500 mg IV every 6 hours) or
- Meropenem (1 g IV every 8 hours) or
- Piperacillin-Tazobactam (4.5 g IV every hours)
Early consultation with otolaryngology (ENT) is important to determine need for operative incision and drainage (I&D) versus observation with IV antibiotics. ENT specialists can also provide backup for surgical cricothyrotomy if necessary. Depending on infection severity, ICU management, particularly for airway monitoring, may be required. While corticosteroids have been suggested to reduce swelling and inflammation, potentially decreasing surgical airway needs, randomized controlled trials supporting their effectiveness are lacking [15].
Decompensated Hypothyroidism/Myxedema Coma
Although facial swelling is not the primary presenting symptom of myxedema coma, it remains a crucial consideration, especially in patients with a history of hypothyroidism and other suggestive signs and symptoms. Myxedema coma carries a high mortality rate even with appropriate treatment.
Myxedema coma represents severe hypothyroidism leading to decreased mental status, hypothermia, and other hypothyroidism-related symptoms, including bradycardia, hypotension, and hypoglycemia [16]. It can arise from uncontrolled hypothyroidism or be triggered by events like myocardial infarction (MI), sepsis, or certain medications [17].
Facial swelling in myxedema coma manifests as diffuse facial puffiness, often accompanied by bilateral periorbital edema and lip/tongue swelling. This swelling results from abnormal protein and mucopolysaccharide deposits, causing non-pitting edema.
Diagnosis is primarily clinical, supported by laboratory findings. T4 levels are typically very low, while TSH levels can be variable depending on the etiology [19]. However, in cases of high suspicion, treatment should not be delayed for lab confirmation. Treatment involves intravenous thyroid hormone supplementation and consideration of adrenal insufficiency, often managed with hydrocortisone.
Myxedema coma carries a significant mortality rate (30%) [20]. Maintaining a high index of suspicion in patients presenting with facial swelling and other myxedema signs is critical. Mortality in myxedema coma is usually attributed to multi-organ failure.
Cavernous Sinus Thrombosis (CST)
Cavernous sinus thrombosis (CST), while uncommon, is a critical life-threatening diagnosis to exclude in patients with periorbital facial swelling. Untreated CST has a high mortality rate [21]. Pre-antibiotic era mortality approached 100%, but with current management, it is reduced to below 30%.
CST occurs when a thrombus forms within the cavernous sinus, a centrally located dural sinus lateral to the sphenoid sinus. Cavernous sinuses receive blood from facial veins, middle cerebral veins, and sphenoid veins, draining into the internal jugular veins. The absence of valves in these sinuses predisposes to infection spread from facial or sinus infections, with Staphylococcus aureus being the most common causative organism.
Initial CST signs and symptoms are variable. Headache, often localized to CN V distribution (Trigeminal Nerve), is common. Fever, eye pain, and vision changes may also occur. As severity increases, confusion, somnolence, and coma can develop. Examination may reveal chemosis, ptosis, proptosis, and ophthalmoplegia due to cranial nerve involvement (CN III, IV, VI proximity to cavernous sinuses) [22].
While non-contrast CT head scan is often the initial imaging, it can be normal in up to 30% of CST cases [23]. CT abnormalities may include cavernous sinus filling defects and superior ophthalmic vein dilation.
CT is often preferred initially due to speed and availability. However, MRI with venogram (MRV) is the most accurate imaging modality, demonstrating reduced or absent flow in the affected cavernous sinus [24], with sensitivity approaching 100% [25].
Immediate ED treatment includes early antibiotics. While S. aureus is common, CST can be polymicrobial, involving gram-positive, gram-negative, and anaerobic bacteria. Empiric antibiotic therapy should include a penicillinase-resistant penicillin and a 3rd or 4th generation cephalosporin. Vancomycin should be added if MRSA is suspected. Anticoagulation remains controversial in CST treatment. Limited data due to CST rarity hinders robust studies. Current evidence suggests heparin or low molecular weight heparin (LMWH) like enoxaparin to prevent thrombus progression is safe [26]. Corticosteroids can be considered as adjunctive therapy to reduce inflammation and edema, but antibiotics remain the mainstay. ICU admission is necessary for CST patients due to high mortality and morbidity.
Orbital Cellulitis
When facial swelling is primarily orbital or periorbital, orbital cellulitis becomes a crucial differential diagnosis. Untreated orbital cellulitis can lead to severe complications including permanent vision loss, abscesses, cavernous venous thrombophlebitis, and death [27]. Orbital cellulitis involves infection of orbital tissues, distinct from periorbital cellulitis, which is eyelid infection. Differentiating these is vital due to orbital cellulitis’s potential for serious complications.
Orbital cellulitis commonly affects young children but can occur in adults. Rhinosinusitis is the most frequent cause [28]. Other causes include recent eye surgery, trauma, and dental infections. Staphylococcus aureus and Streptococci are the most common causative organisms [29]. Less common causes include anaerobes, Pseudomonas Aeruginosa, Hemophilus Influenza, and fungal infections (mucormycosis in diabetic ketoacidosis) [29].
Clinical presentation includes eye pain, eyelid swelling, and double vision. Examination reveals pain with eye movements, proptosis, and ophthalmoplegia from extraocular muscle (EOM) inflammation and swelling. Periorbital cellulitis, in contrast, lacks pain with EOM and ophthalmoplegia [30].
Diagnosis combines clinical judgment and imaging. CT scan is a reasonable first-line imaging modality to evaluate orbits. Common CT findings include EOM inflammation, fat stranding, and anterior globe displacement [31].
Most orbital cellulitis cases are managed with antibiotics. Empiric antibiotic choices include:
- Vancomycin: 15 mg – 20 mg/kg IV daily every 8-12 hours AND
- Ceftriaxone: 2 g IV every 24 hours OR
- Cefotaxime: 2 g IV every 24 hours OR
- Ampicillin-Sulbactam: 3 g IV every 6 hours OR
- Piperacillin-Tazobactam: 4.5 g IV every 6 hours
While surgery is often not initially required, ophthalmology and otolaryngology consultation is important for further management and close monitoring for infection progression.
Other Differentials of Facial Swelling to Consider
Dental Infections
Dental infections are a frequent cause of facial swelling. Approximately 65 million Americans over 30 suffer from periodontal disease [32].
While isolated odontogenic infections are rarely life-threatening with antibiotic treatment, untreated infections can spread and lead to dangerous conditions like Ludwig’s Angina. Dental infections originate from bacterial colonization on tooth surfaces, forming plaque. Infection spread depends on plaque location. Supragingival plaque can cause dental caries, spreading to the pulp and alveolar bone, causing periapical abscesses [33]. Subgingival plaque causes gingivitis and periodontal infections, potentially spreading to deeper facial and neck fascial spaces [34]. Odontogenic infections can also extend to the jaw, resulting in osteomyelitis.
Dental caries, localized tooth structure destruction from plaque bacteria, are common in patients under 35 with odontogenic infections. Streptococcus mutans is a primary causative organism [35]. Caries progression can expose dental pulp, leading to pulpitis and periapical abscesses as infection reaches the tooth root.
Periodontal infections increase in prevalence in patients over 35 [36]. While infection progression is often slow, rapid periodontitis can occur, causing periodontal abscesses and deep fascial infections.
Thorough examination is crucial for odontogenic infection evaluation. Assess overall dentition, examining tooth roots for periapical abscesses and gingivae for periodontal abscesses. Due to the risk of spread to deep fascial spaces, evaluate the submandibular space for Ludwig’s angina signs.
Imaging is often unnecessary for uncomplicated odontogenic infections in the ED. However, CT can be useful to evaluate bony structures for osteomyelitis and deep fascial planes for infection spread [37].
Dental infection treatment depends on symptom severity. Dental caries can often be referred to a dentist for outpatient management. Uncomplicated gingivitis rarely requires systemic antibiotics; chlorhexidine 0.12% oral rinse, with antibacterial properties, can be used [38].
More severe cases or abscess presence may warrant systemic antibiotics:
- Amoxicillin 500 mg PO every 8 hours or
- Amoxicillin-Clavulanate 875 mg PO every 12 hours or
- Clindamycin 450 mg PO every 6 to 8 hours
Simple abscesses may be drained in the ED and referred to a dentist, while complex abscesses, especially with fascial plane spread, may require specialist surgical drainage.
Facial Cellulitis
Facial cellulitis, characterized by infection spreading to the dermis and subcutaneous tissue, can present with facial swelling. Streptococcus pyogenes and Staphylococcus species, including MRSA, are common causative organisms [39]. Overlying erythema and warmth are often present. Differentiating simple facial cellulitis from more severe infections like orbital cellulitis or deep space infections (Ludwig’s Angina) is crucial, as these can be life-threatening. Imaging is rarely needed unless deeper infection or orbital cellulitis is suspected, in which case CT is recommended. Treatment involves antibiotics targeting suspected organisms.
Superior Vena Cava Syndrome (SVC Syndrome)
Superior vena cava syndrome (SVC syndrome) is an important cause of facial swelling to recognize. SVC syndrome encompasses symptoms resulting from superior vena cava blood flow obstruction. Malignancy, particularly non-small cell lung cancer (NSCLC), is the most common underlying cause [40].
SVC obstruction from malignancy results from vessel compression or malignant invasion. Other causes include thromboses (especially with intravascular devices), infection, or radiation therapy. SVC syndrome presents clinically with dyspnea, facial swelling, or head fullness [41]. Arm swelling, cough, and chest pain may also occur. Symptom severity depends on SVC obstruction development rate. Examination often reveals facial edema and neck and chest venous distention. Severe cases may present with stridor due to acute compression.
Chest X-ray may show a mediastinal mass but is nonspecific. CT chest with IV contrast is the preferred imaging modality, defining vascular anatomy, blockage location, and etiology [42].
Treatment is primarily supportive, addressing the underlying SVC syndrome cause. Elevating the head of the bed is recommended. Upper extremity IM injections should be avoided due to delayed drug absorption from venous obstruction. Corticosteroids and diuretics may be considered, though their efficacy is debated [43, 44]. Radiation therapy is often indicated for malignancy-related SVC syndrome. Anticoagulation is recommended for thrombus-related SVC syndrome.
Malignancy
Head and neck malignancies are the 6th most common malignancies, with squamous cell carcinoma (SCC) being the most frequent [45]. Malignancies can affect various structures including the oral cavity, pharynx, soft tissue, bone, and larynx. Facial malignancies typically present as chronic, unilateral facial swelling [46]. Patients usually present with a slow-growing facial mass. Advanced imaging, including CT and/or MRI, is often necessary to assess anatomy and malignancy extent. Ruling out other contributing etiologies, such as cellulitis, and evaluating for airway involvement are important.
Trauma
Facial trauma should always be considered, particularly when history is limited. The face’s complex structures—bones, nerves, vasculature, glands, muscles—when injured, can lead to complications affecting breathing, vision, speech, and the central nervous system. Blunt trauma from violence or motor vehicle collisions (MVCs) is the most common cause of facial trauma [47]. Gunshot wounds (GSW) to the face often carry higher mortality.
As with all trauma, prioritize the primary survey, identifying life-threatening injuries. In severe facial trauma, identify injuries compromising breathing or airway.
After primary survey completion, perform a secondary survey with thorough examination to identify abnormalities. While detailed facial trauma discussion is beyond this scope, key pearls include:
- For ocular trauma, assess pupils for reactivity and size, extraocular movements, and visual acuity. Evaluate for proptosis and retrobulbar hematoma/open globe injuries in orbital trauma cases. These require urgent ophthalmologic surgical intervention.
- Lacerations near the medial eye/eyelid raise suspicion for nasolacrimal duct injury, typically requiring specialist repair.
- Bedside halo test for CSF leak is sensitive but cannot differentiate CSF from other clear fluids (saline, saliva, sweat) [48].
- Facial fractures requiring urgent specialist evaluation and admission include:
- LeFort midface fractures
- Facial fractures with multi-organ trauma
- Zygomatic arch fractures with trismus (airway monitoring)
- Nasoethmoid fractures (CSF leak evaluation)
Salivary Gland Pathology
Salivary gland pathology (stones or infection), while rarely life-threatening, can cause facial swelling. Salivary gland stones, or sialolithiasis, are common. Gland inflammation, sialoadenitis, can result from primary infection or secondary infection, such as obstructed sialolithiasis causing stasis [49]. Examples include viral parotitis (mumps), bacterial sialoadenitis, or secondary sialoadenitis.
Sialolithiasis is more common in men over 30. Risk factors include trauma, smoking, diuretics, anticholinergic medications, and dehydration [50].
The major salivary glands are parotid, submandibular, and sublingual. Most sialolithiasis cases (up to 90%) occur in the submandibular gland [51].
Patients typically present with gland-localized pain and swelling, exacerbated by eating or salivating. Palpable stones in the ductal system may be present in sialolithiasis. Inflamed glands may be tender and erythematous externally [52].
Imaging may not be necessary for diagnosis but can help locate stones and identify complications like tumors or abscesses. Ultrasound and CT can diagnose sialolithiasis. Ultrasound detects 90% of stones >2 mm. CT has high sensitivity (98%) and specificity (88%) for stone detection [53].
ED treatment is usually conservative, involving hydration, gland/duct massage, and salivary flow stimulants like lemon drops. Antibiotics may be indicated for secondary infection signs.
Conclusion
Returning to our initial case, the patient presented with ACE-inhibitor induced angioedema causing airway obstruction. Fiberoptic intubation was performed emergently without complications. She was admitted to the MICU for further management. Angioedema resolved after 3 days, and she was extubated and discharged home the following day. Lisinopril and ACE inhibitors were added to her allergy list.
Take Home Points
- While most facial swelling causes are benign, several etiologies are acutely life-threatening and should not be missed:
- Angioedema
- Submandibular Space Infection/Ludwig’s Angina
- Decompensated Hypothyroidism/Myxedema Coma
- Cavernous Sinus Thrombosis (CST)
- Orbital Cellulitis
- Swelling location aids in narrowing the differential diagnosis.
- Conduct a thorough examination to assess swelling location and extent, focusing on airway compromise potential.
- In cases of airway compromise concern, SECURE THE AIRWAY promptly. Early intervention is key.
- Develop an algorithm for airway management in significant facial swelling. Be proficient in intubation techniques, including Video Laryngoscopy, Fiberoptic Intubation, and Cricothyrotomy.
- For critically ill patients with significant facial swelling and suspected infection, initiate antibiotics immediately.
References/Further Reading
- Bernstein A, Cremonesi P, Hoffmann TK, et al. Angioedema in the emergency department: a practical guide to differential diagnosis and management. International Journal of Emergency Medicine. 2017;10:15.
- Bas M, Adams V, Suvorava T, et al. Nonallergic angioedema: role of bradykinin. Allergy. 2007 Aug. 62(8):842-56.
- Alcoceba E, Gonzalez M, Gaig P, et al. Edema of the uvula: etiology, risk factors, diagnosis, and treatment. J Investig Allergol Clin Immunol 2010; 20:80.
- Greaves MW, Sabroe RA. ABC of allergies. Allergy and the skin. I–Urticaria. BMJ 1998; 316:1147.
- Baş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med 2015; 372:418.
- Straka BT, Ramirez CE, Byrd JB, et al. Effect of bradykinin receptor antagonism on ACE inhibitor-associated angioedema. J Allergy Clin Immunol 2017; 140:242.
- Sinert R, Levy P, Bernstein JA, et al. Randomized Trial of Icatibant for Angiotensin-Converting Enzyme Inhibitor-Induced Upper Airway Angioedema. J Allergy Clin Immunol Pract 2017; 5:1402.
- Banerji A, Clark S, Blanda M, et al. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol 2008; 100:327.
- Furst IM, Ersil P, Caminiti M. A rare complication of tooth abscess–Ludwig’s angina and mediastinitis. J Can Dent Assoc 2001; 67:324.
- Boscolo-Rizzo P, Da Mosto MC. Submandibular space infection: a potentially lethal infection. Int J Infect Dis 2009; 13:327.
- Hurley MC, Heran MK. Imaging studies for head and neck infections. Infect Dis Clin North Am 2007; 21:305.
- Kulkarni AH, Pai SD, Bhattarai B, et al. Ludwig’s angina and airway considerations: a case report. Cases Journal. 2008;1:19.
- Ovassapian A, Tuncbilek M, Weitzel EK, et al. Airway management in adult patients with deep neck infections: a case series and review of the literature. Anesth Analg 2005; 100:585.
- Brook I. Microbiology and principles of antimicrobial therapy for head and neck infections. Infect Dis Clin North Am 2007; 21:355.
- Hasan W, Leonard D, Russell J, “Ludwig’s Angina—A Controversial Surgical Emergency: How We Do It,” International Journal of Otolaryngology, vol. 2011, Article ID 231816, 4 pages, 2011
- Kwaku MP, Burman KD. Myxedema coma. J Intensive Care Med 2007; 22:224.
- Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol 2017; 27:117.
- Berger T, James WD, Elston D. Andrews’ Diseases of the skin: clinical dermatology (11th ed.)
- Garber J, Cobin R, Gharib H, et al. Clinical Practice Guidelines for Hypothyroidism in Adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice: November 2012, Vol. 18, No. 6, pp. 988-1028.
- Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol 2017; 27:117.
- Laupland KB. Vascular and parameningeal infections of the head and neck. Infect Dis Clin North Am. 2007 Jun. 21(2):577-90, viii.
- Duong DK, Leo MM, Mitchell EL. Neuro-ophthalmology. Emerg Med Clin North Am. 2008 Feb. 26(1):137-80, vii.
- Bousser MG, Russell RR. Cerebral venous thrombosis. In: Major Problems in Neurology, Warlow CP, Van Gijn J (Eds), WB Saunders, London 1997. p.27, 104.
- Coutinho JM, Ferro JM, Canhão P, et al. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010 Nov. 41(11):2575-80
- Sadigh G, Mullins M, Saindane A, Diagnostic Performance Of MRI Sequences For Evaluation Of Dural Venous Sinus Thrombosis. American Journal of Roentgenology2016 206:6, 1298-1306
- Durand, ML. Periocular infections. In: Principles and Practice of Infectious Diseases, 7th ed, Mandell, GL, Bennett, JE, Dolin, R (Eds), Churchill Livingstone Elsevier, Philadelphia 2010. p.1569.
- Sobol SE, Marchand J, Tewfik TL, et al. Orbital complications of sinusitis in children. J Otolaryngol 2002; 31:131.
- McKinley SH, Yen MT, Miller AM, et al. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol 2007; 144:497.
- Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics 2011; 127:e566.
- Botting AM, McIntosh D, Mahadevan M. Paediatric pre- and post-septal peri-orbital infections are different diseases. A retrospective review of 262 cases. Int J Pediatr Otorhinolaryngol 2008; 72:377.
- Eustis HS, Mafee MF, Walton C, et al. MR imaging and CT of orbital infections and complications in acute rhinosinusitis. Radiol Clin North Am 1998; 36:1165.
- Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012; 91:914.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007; 369:51.
- Loesche W. Dental caries and periodontitis: contrasting two infections that have medical implications. Infect Dis Clin North Am 2007; 21:471.
- Chow AW. Infections of the oral cavity, neck and head. In: Principles and Practice of Infectious Diseases, 6th ed, Mandell GL, Bennett JE, Dolin R (Eds), Churchill Livingstone, Philadelphia 2005. p.787.
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol 1999; 70:13.
- Hurley MC, Heran MK. Imaging studies for head and neck infections. Infect Dis Clin North Am 2007; 21:305.
- Krayer JW, Leite RS, Kirkwood KL. Non-surgical chemotherapeutic treatment strategies for the management of periodontal diseases. Dent Clin North Am 2010; 54:13.
- Rath E, Skrede S, Mylvaganam H, et al. Aetiology and clinical features of facial cellulitis: a prospective study,Infectious Diseases, 2018; 50:1, 27-34.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59.
- Kim HJ, Kim HS, Chung SH. CT diagnosis of superior vena cava syndrome: importance of collateral vessels. AJR Am J Roentgenol 1993; 161:539.
- Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002; 14:338.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67:7.
- Farhood AI, Hajdu SI, Shiu MH, et al. Soft tissue sarcomas of the head and neck in adults. Am J Surg 1990; 160:365.
- Erdmann D, Follmar KE, Debruijn M, et al. A retrospective analysis of facial fracture etiologies. Ann Plast Surg 2008; 60:398.
- Dula DJ, Fales W. The ‘ring sign’: is it a reliable indicator for cerebral spinal fluid? Ann Emerg Med 1993; 22:718.
- Huoh KC, Eisele DW. Etiologic factors in sialolithiasis. Otolaryngol Head Neck Surg 2011; 145:935.
- Williams MF. Sialolithiasis. Otolaryngol Clin North Am 1999; 32:819.
- Mandel L. Salivary gland disorders. Med Clin North Am 2014; 98:1407.
- Paterson JR, Murphy MJ. Bones, groans, moans… and salivary stones? J Clin Pathol 2001; 54:412.
- Thomas WW, Douglas JE, Rassekh CH. Accuracy of Ultrasonography and Computed Tomography in the Evaluation of Patients Undergoing Sialendoscopy for Sialolithiasis. Otolaryngol Head Neck Surg 2017; 156:834.