Familial adenomatous polyposis (FAP) is a hereditary condition notably increasing the risk of colorectal cancer. However, individuals with FAP also face a significant risk of developing neoplasia in the small intestine, particularly in the duodenum. Diagnosis and surveillance are critical in managing these risks and improving patient outcomes.
Duodenal adenomas are a common finding in FAP patients, observed in a significant majority, ranging from 50% to 90% of individuals. These polyps are predominantly located in the second and third portions of the duodenum, with fewer occurrences in the distal small bowel. Within the duodenum, adenomas are not evenly distributed; they tend to cluster around and distal to the ampulla of Vater. The periampullary region, encompassing the duodenal papilla and ampulla of Vater, is particularly susceptible, with adenomatous polyps found in at least half of classic FAP cases.
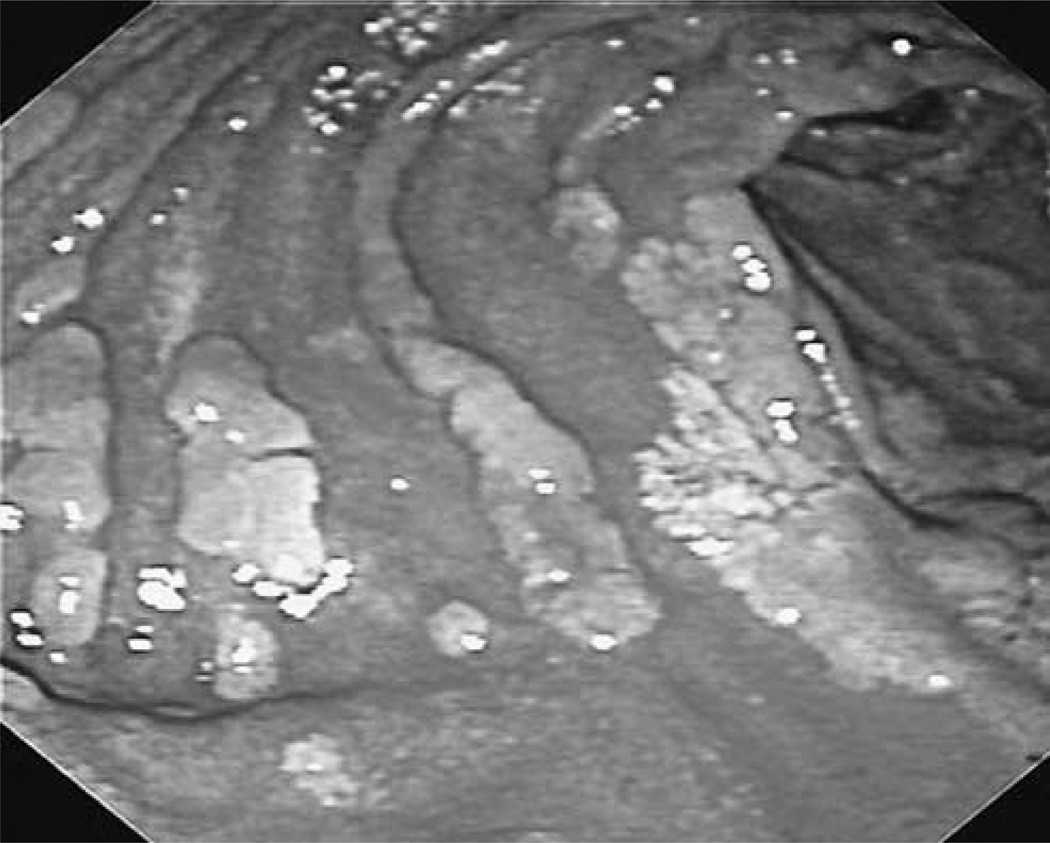 Endoscopic view of confluent adenomatous plaques in the duodenum of a familial adenomatous polyposis patient.
Endoscopic view of confluent adenomatous plaques in the duodenum of a familial adenomatous polyposis patient.
The presence of polyps in the periampullary region is clinically significant due to potential complications. These polyps can obstruct the pancreatic duct or biliary duct, leading to pancreatitis or biliary obstruction, complications observed more frequently in FAP patients. Furthermore, periampullary carcinoma, a severe cancer type, occurs in approximately 4% of individuals with FAP. Notably, it stands as the leading cause of cancer-related deaths in FAP patients who have undergone colectomy, highlighting the critical need for early Familial Adenomatous Polyposis Diagnosis and management of duodenal neoplasia. In contrast to the distal duodenum, adenomas proximal to the ampulla are less frequent and typically manifest as multiple small, discrete adenomas (1–10 mm) or as flat confluent plaques. The average age at diagnosis for duodenal cancer in FAP patients is around 50 years, approximately a decade after the typical onset of colonic cancer in untreated FAP individuals. Compared to the general population, individuals with FAP face dramatically elevated relative risks for duodenal adenocarcinoma and ampullary carcinoma, estimated to be 331 and 124 times higher, respectively.
The frequency of esophagogastroduodenoscopy (EGD) for surveillance is determined by the severity of duodenal adenomatosis, assessed using the Spigelman staging criteria. This classification system categorizes the severity into five stages (0–IV) based on a point system considering the number, size, histology, and dysplasia grade of duodenal adenomas. A previous decade-long study correlated Spigelman stages with duodenal cancer risk, revealing a 2.3%, 2.4%, and 36% risk for stage II, III, and IV disease, respectively. These findings underscore the importance of the Spigelman stage in tailoring surveillance intervals and guiding treatment decisions for FAP patients with duodenal adenomatosis. During endoscopic procedures, a side-viewing endoscope is recommended for optimal visualization of the duodenal papilla. Biopsy specimens should be taken even if polyps are not visually apparent but the papilla appears enlarged. Endoscopic retrograde cholangiopancreatography (ERCP) might be necessary to investigate potential adenomas in the common bile duct.
| No. of points | 1 | 2 | 3 |
|---|---|---|---|
| No. of polyps | 1–4 | 5–20 | >20 |
| Polyp size (mm) | 1–4 | 5–10 | >10 |
| Histology | Tubulous | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
Small bowel cancer distal to the duodenum is a rare occurrence in FAP, with limited reported cases of jejunal and ileal carcinoma. Consequently, the clinical significance of small bowel polyps beyond the duodenum appears to be less pronounced. Push enteroscopy (PE) has been utilized for screening high-risk FAP individuals; however, it often provides incomplete small intestine visualization. The optimal screening method for small bowel polyps in FAP patients remains an area of ongoing research and clinical evaluation.