The landscape of obesity treatment and Medicare coverage has shifted significantly with a recent decision by the Food and Drug Administration (FDA). Wegovy (semaglutide), a prominent anti-obesity medication, has received Fda Approved Diagnosis For Wegovy to reduce the risk of serious cardiovascular events such as heart attack and stroke in adults who are overweight or obese and have existing cardiovascular disease. This crucial approval not only marks a new therapeutic avenue for Wegovy but also fundamentally alters the potential for Medicare coverage for millions of Americans struggling with obesity and related health issues.
Wegovy belongs to the class of drugs known as GLP-1 (glucagon-like peptide-1) agonists. Initially developed and approved for the treatment of type 2 diabetes, these medications have demonstrated remarkable efficacy in weight management. Semaglutide, under the brand name Ozempic, is already covered by Medicare for diabetes treatment. However, prior to this new fda approved diagnosis for wegovy, Medicare was legally restricted from covering Wegovy and similar drugs when prescribed specifically for obesity. This new indication for cardiovascular risk reduction bypasses this restriction, paving the way for broader access to this medication for a significant patient population.
Medicare Coverage Implications Following FDA Approval for Cardiovascular Risk Reduction
The FDA’s approval of this new indication is a game-changer for Medicare beneficiaries. Immediately following the announcement, the Centers for Medicare & Medicaid Services (CMS) released a memo clarifying that Medicare Part D plans now have the authority to include Wegovy on their formularies. This is because the fda approved diagnosis for wegovy for cardiovascular risk reduction qualifies as a “medically-accepted indication” that is not explicitly excluded under Medicare guidelines. As Wegovy is administered via self-injection, coverage falls under Medicare Part D, the outpatient prescription drug benefit, offered through private standalone drug plans and Medicare Advantage plans, rather than Part B which covers drugs administered in physician offices.
Who Qualifies for Medicare Coverage of Wegovy Under the New Indication?
The new fda approved diagnosis for wegovy targets individuals with established cardiovascular disease – encompassing a history of heart attack, stroke, or peripheral arterial disease – who are also classified as obese or overweight. A Kaiser Family Foundation (KFF) analysis of 2020 Medicare data estimates that approximately 7% of Medicare beneficiaries, or 3.6 million individuals, meet these criteria. This substantial group represents over a quarter (26%) of the 13.7 million Medicare beneficiaries diagnosed with obesity or overweight in 2020. Therefore, the fda approved diagnosis for wegovy potentially extends access to this treatment to one in four Medicare beneficiaries struggling with obesity or overweight.
It’s important to note that among these 3.6 million individuals, approximately 1.9 million also have type 2 diabetes (excluding type 1). This subgroup may have already had access to GLP-1 medications like Ozempic for diabetes management, even before this new fda approved diagnosis for wegovy.
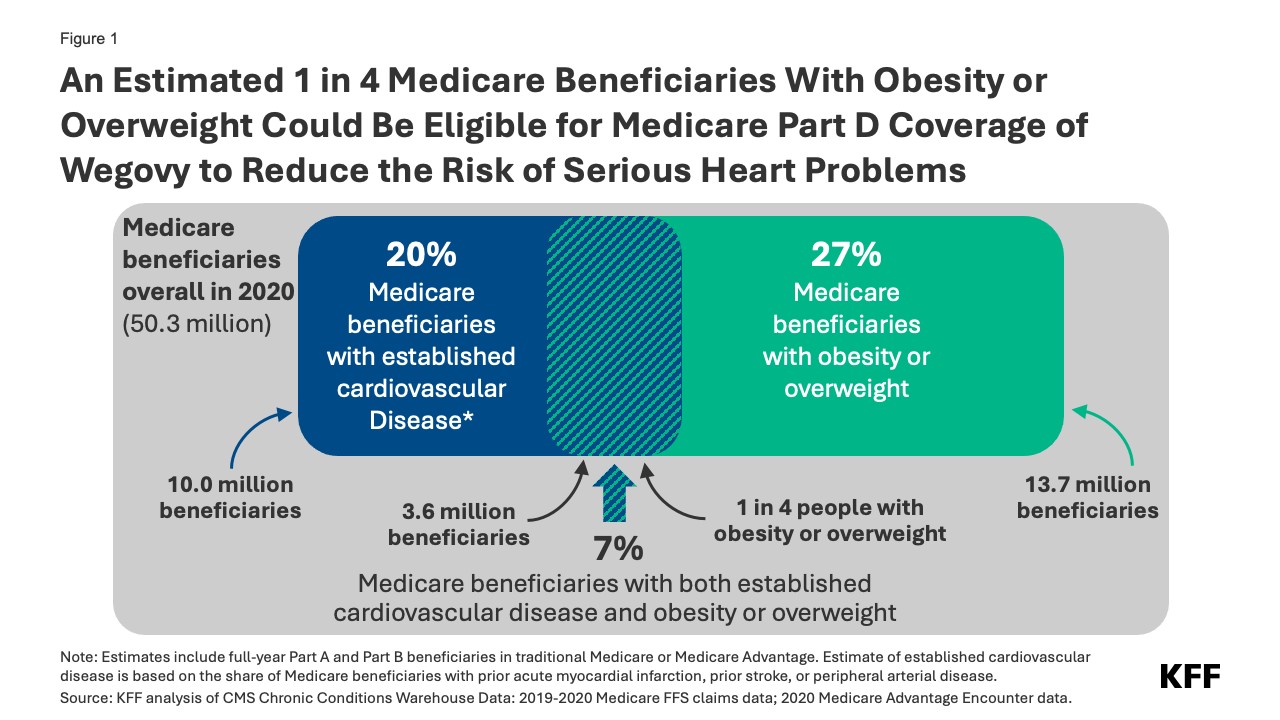 Figure 1: An Estimated 1 in 4 Medicare Beneficiaries With Obesity or Overweight Could Be Eligible for Medicare Part D Coverage of Wegovy to Reduce the Risk of Serious Heart Problems
Figure 1: An Estimated 1 in 4 Medicare Beneficiaries With Obesity or Overweight Could Be Eligible for Medicare Part D Coverage of Wegovy to Reduce the Risk of Serious Heart Problems
However, it’s crucial to acknowledge that eligibility does not guarantee uptake. Potential side effects, as detailed in the fda approved diagnosis for wegovy documentation, and the anticipated out-of-pocket expenses could deter some beneficiaries. Wegovy’s list price is around $1,300 per month, and under Part D specialty tier drug coverage, beneficiaries might face coinsurance of 25% to 33%. This translates to monthly costs ranging from $325 to $430, potentially before reaching the annual out-of-pocket spending cap set by the Inflation Reduction Act. Even with the cap, these costs can be significant for Medicare recipients with limited incomes. Ultimately, the actual cost for beneficiaries will hinge on individual Part D plan coverage and Wegovy’s placement within the formulary tiers.
Furthermore, access to Wegovy might be further influenced by Part D plans implementing utilization management tools such as prior authorization and step therapy. These measures, while intended to manage costs and ensure appropriate utilization, could create hurdles for beneficiaries seeking access, even within the target population defined by the fda approved diagnosis for wegovy.
Timeline for Medicare Part D Plans to Cover Wegovy
While some Medicare Part D plans have already announced intentions to cover Wegovy in the current year, the widespread availability of coverage in 2024 remains uncertain. Medicare drug plans are permitted to add new drugs to their formularies throughout the year to reflect new approvals and expanded indications like this fda approved diagnosis for wegovy. However, they are not mandated to cover every new drug entering the market. Plans are obligated to cover at least two drugs in each therapeutic category and substantially all drugs within six protected classes.
Considering Wegovy’s high price and the large potential patient pool defined by the fda approved diagnosis for wegovy, many Part D plans may be hesitant to expand coverage immediately, particularly as they cannot adjust premiums mid-year to accommodate the increased costs. Therefore, more comprehensive coverage is more likely to materialize in 2025 as plans adjust their formularies and pricing strategies for the coming year.
Potential Impact on Medicare Spending
The financial implications for Medicare stemming from expanded Wegovy coverage are multifaceted. They depend on the number of Part D plans that opt to include Wegovy, the extent of utilization management restrictions they employ, the actual number of eligible beneficiaries who initiate and continue treatment, and the negotiated prices secured by these plans.
For illustrative purposes, if plans secure a 50% rebate on the $1,300 monthly list price, the net annual cost per patient could be approximately $7,800. If just 10% of the estimated 3.6 million eligible individuals (360,000 people) utilize Wegovy for a full year, the added expenditure for Medicare Part D could reach $2.8 billion annually for this single medication.
Interestingly, semaglutide, the active ingredient in Wegovy, may become eligible for Medicare drug price negotiation as early as 2025. This is based on the earliest FDA approval date of Ozempic in late 2017. For small-molecule drugs like semaglutide, a seven-year period post-FDA approval is required before negotiation eligibility. If selected for negotiation in 2025, any negotiated price would take effect in 2027. This could potentially mitigate Medicare and beneficiary out-of-pocket spending on semaglutide products, including Wegovy, Ozempic, and Rybelsus (oral semaglutide for type 2 diabetes). In 2022, Medicare’s gross spending on Ozempic alone ranked sixth among top-selling Part D drugs, reaching $4.6 billion annually. This figure doesn’t account for rebates, which are estimated to be substantial.
Broader Implications for Medicare Coverage of Anti-Obesity Medications
Currently, Medicare law continues to exclude coverage for GLP-1 medications when used solely for obesity treatment. However, the fda approved diagnosis for wegovy for cardiovascular risk reduction represents a significant shift. It establishes a precedent for covering GLP-1s for indications beyond diabetes, opening potential pathways for broader Medicare coverage. Further expansions could arise if these drugs gain FDA approval for other obesity-related conditions. For example, Eli Lilly recently reported positive clinical trial results for its GLP-1 agonist, Zepbound (tirzepatide), in reducing sleep apnea severity in overweight and obese individuals. If the FDA approves this indication, Zepbound could become the first pharmaceutical treatment for sleep apnea, creating another avenue for Medicare coverage for GLP-1s in the context of obesity-related comorbidities.
Expanding Medicare access to GLP-1s based on these newly fda approved diagnosis for wegovy and potential future indications could influence the cost of proposed legislation aimed at lifting the statutory ban on Medicare coverage for anti-obesity drugs. The Congressional Budget Office (CBO) would likely factor in the costs associated with these expanded indications into their baseline Medicare spending projections. This could mean that the incremental cost of removing the ban on anti-obesity drug coverage would be lower than previously estimated. Ultimately, the extent to which Medicare Part D coverage for GLP-1s expands, driven by approvals like this fda approved diagnosis for wegovy, will have profound implications for individuals affected by obesity and for the overall trajectory of Medicare expenditures.
Authors: Juliette Cubanski, Tricia Neuman, Nolan Sroczynski, and Anthony Damico (KFF)
Funding: Arnold Ventures
Methodology: (Summary of original methodology section – Detailed methodology section omitted for brevity as per instructions, but key aspects are mentioned in the main text where relevant, e.g., KFF analysis of Medicare data).
Topics: (Topics from original article – Omitted as per instructions)
Tags: (Tags from original article – Omitted as per instructions)